Traditional methods of butter preservation in Dire Inchini and Ambo districts were presented in Table 1. In the study areas, 100, 98.33, 29.17 and 11.67% of interviewed smallholder producers commonly preserve butter in the form of traditional ghee, spiced, melted and salted butter, respectively. This finding is in agreement with previous reports in Arsi Negele, Oromia, Walayita, Southern Ethiopia, North Western Ethiopia and East Wollega, Ethiopia by Lemma et al. (2004), Mekdes (2008), Eyassu (2014) and Alganesh and Yetenayet (2016), respectively. In the study areas in wet season and during Ethiopian Orthodox fasting time, demand for butter is low and during such occasions, surplus butter is preserved using traditional preservation techniques. In the present study, spiced, melted and salted butter are used as raw materials for traditional ghee making and they are optional butter preservation techniques.
Spices used for ghee making and spicing butter (spiced butter)
The result for the spices used for spiced butter and traditional ghee making in the study areas were indicated in Table 2. For traditional ghee making smallholder producers in the study areas mainly use Trachyspermum ammi, Aframomum angusti-folium, Nigella sativa, Trigonella foenum, Zingiber officinale, Allium sativum and Curcuma domestica is used for coloring purpose. While for making spiced butter, they use Trigonella foenum and Nigella sativa. Similar study by Joe et al. (2009) stated that spices are used to enhance aroma, flavor and for preservation of food substances.
Microbial properties of butter preserved using traditional preservation methods
Aerobic mesophilic bacterial count
The mean total bacterial count (log cfu/g) for the treatments and preservation time is presented in Table 3. The aerobic mesophilic bacterial counts of butter preserved using traditional ghee, salted and spiced butter at 0, ends of 1, 2 and 3 months of preservation did not show significant difference at P<0.05. While aerobic mesophilic bacterial counts of melted, untreated, frozen and butter stored in refrigerator at 4°C showed significant difference among treatments at 0, ends of 1, 2 and 3 months at P<0.05 level of significance. The total aerobic mesophilic bacterial counts for traditional ghee, salting, spicing, melted, untreated, frozen and refrigerated butter (4
0c) showed significant difference among the treatment means at P<0.05 level of significance. At initial preservation time, greater counts of aerobic mesophilic bacterial counts was observed in salted (9.58 log cfu/g) and melted butter (9.65 log cfu/g), which were significantly different (P<0.05) from other treatments. This might be due to the poor hygiene of the salt used in preserving the butter. In the case of melted butter, the increase in the aerobic mesophilic bacterial count might probably be as a result of post melting contamination. At initial preservation period, the mean aerobic mesophilic bacteria in untreated butter were 8.71 log cfu/g of butter samples.
The current result is far beyond the maximum tolerable limit of 6 log cfu/g of aerobic mesophilic bacteria counts set by Standards Authority of Ethiopia (QSAE, 2009).
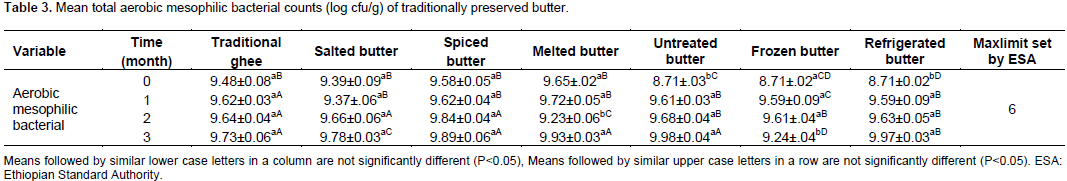
The sensory attributes used to evaluate butter samples in this experiment were odor, texture, color and overall acceptability. So there was no risk on the health and safety of the panelists since they evaluated the butter by smelling to check for the odor, checked the texture using smoothness, solidity and appropriate degree of firmness by their hands and visual observation of the color of the butter samples. The current result is also similar to a report from Wolayita in Southern Ethiopia of 8.10 log cfu/g of butter samples of total bacterial count (Mekdes, 2008). After one month of preservation, aerobic mesophilic bacterial counts of 9.37 log cfu/g was observed and this was significantly different (P<0.05) from the other treatments. Relatively lower mean aerobic mesophilic bacterial counts were observed in frozen butter, traditional ghee and spiced butter. This might be due to the antagonistic effects of active ingredients of spices, heat treatment and low temperatures in spiced butter, traditional ghee, melted butter, butter kept at 4 and -20°C, respectively on bacterial growth and survival. Similar studied by Kilcast and Subramaniam (2000) confirmed that shelf life of products can be extended by the use of processing treatments such as heat and radiation which kills the microorganisms or control of microbial growth by chilling, freezing, reducing the water content and addition of preservatives.
Lactic acid bacterial count
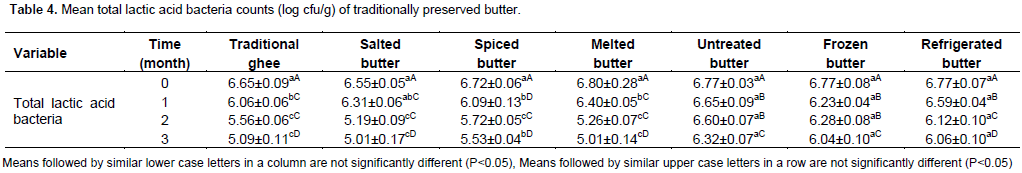
The mean total lactic acid bacteria counts (log cfu/g) of treatments and preservation time are described in Table 4. There were significant differences (P<0.05) between total lactic acid bacterial counts in traditional ghee, salted, spiced and melted butter at 0, ends of 1, 2 and 3 months of preservation. While the total lactic acid bacterial counts of butter preserved using untreated, frozen (-20°C) and refrigerated (4°C) butter showed no significant difference at P<0.05 among the means at 0, 1, 2 and 3 months of preservation, respectively. Total lactic acid bacterial counts at 0 and 1 month of preservation showed no significant (P<0.05) difference for traditional ghee, salted, spiced, melted, untreated, frozen and refrigerated (4°C) butter samples. While, the total lactic acid bacterial counts at the end of the second month of preservation did not show significant (P<0.05) difference among traditional ghee, salted, spiced, melted and refrigerated butter (4°C) except for untreated and frozen butter (-20°C). Whereas, at the end of the third month of preservation, the mean total lactic acid bacterial counts in traditional ghee, salted , spiced, melted and refrigerated butter samples did not show significant (P<0.05) difference among their mean counts except for untreated and frozen butter which did not significantly (P<0.05) differ from each other. The mean total lactic acid bacterial counts of the treatments at initial time of preservation ranged from 6.55 to 6.80 log cfu/g. This could be due to prior fermentation of composite sample as local butter is usually made of spontaneously fermented whole milk. At initial time of preservation, the mean lactic acid bacterial count in traditional ghee was 6.06 log cfu/g and it significantly differed at P<0.05 from other treatments; except for salted butter which was 6.09 log cfu/g of butter samples. The current result is also similar with the finding of Mekdes (2008) who reported mean lactic acid bacterial count of 7.51 log cfu/g for butter sample collected from Wolayita in Southern region. From the second to third months of preservation time, mean lactic acid bacterial count of spiced, melted butter and traditional ghee were relatively lower and significantly differed (P<0.05) from other treatments. This might be due to heat treatment and antimicrobial effects of spices used in melted butter, traditional ghee and spiced butter, respectively.
Yeast and mold counts
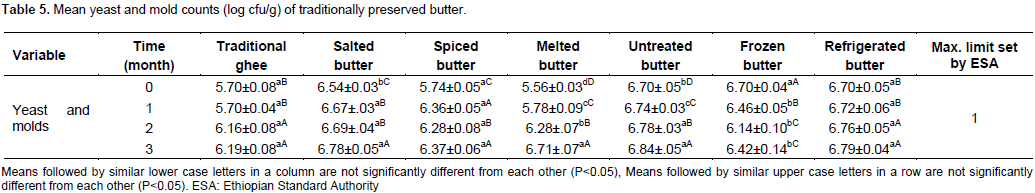
The result for the mean yeast and mold counts (log cfu/g) of treatments and preservation time is indicated in Table 5. The mean yeast and mold counts during initial 0, 1, 2 and 3 months of preservation for traditional ghee, spiced and butter refrigerated at 4°C did not significantly (P<0.05) differ from each other. The mean yeast and mold counts (log cfu/g) of salted, spiced, melted, untreated and frozen butter samples significantly differed (P<0.05) from each other. The mean yeast and mold counts (log cfu/g) of traditional ghee, salted, spiced, melted, untreated, frozen and refrigerated butter samples did not show significant difference (P<0.05) between the treatment means. Mean yeast and mold counts of butter at initial preservation time for traditional ghee (5.70 log cfu/g), salted butter (5.74 log cfu/g) and melted butter (5.56 log cfu/g) significantly differed (P<0.05) from spiced, untreated, frozen (-20°C) and refrigerated (4°C) butter. This might be attributable to the effect of heat treatment; antimicrobial properties of spices used to treat butter samples and reduced water activity in salted butter. The temperature range for yeast and mold growth is 0 to 47°C, out of which, the growth of yeast and mold can be hampered. This is in agreement with Seriler (2003) who revealed the possibility of reducing mold growth on the surface of butter by salting. The mean yeast and mold count of untreated butter sample at initial time of preservation was 6.70 log cfu/g. The current finding is beyond the maximum tolerable limit of 1 log cfu/g of yeast and mold count of butter sample recommended by the Ethiopian Standards Authority (QSAE, 2009). The present result is also higher than the mean yeast and mould count of 5.58 log cfu/g of butter samples reported in Wolayita Southern Ethiopia (Mekdes, 2008). Increasing trends of yeast and mold counts have been observed from one month of preservation period to the end in untreated, refrigerated (at 4°C), spiced and melted butter and these results were significantly different (P<0.05) from other treatments. In the case of spiced butter poor hygiene of spices purchased from open market might have contributed to the high rate of contamination. In untreated and refrigerated butter samples water activity might have been high and have created favorable environment for the growth of yeast and molds.
Total coliform counts
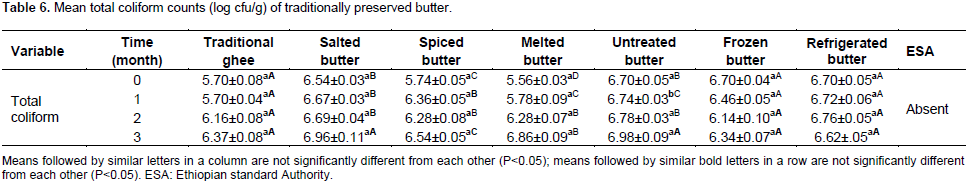
Mean total coliforms count (log cfu/g) for the treatments and time of preservation is presented in Table 6. There were no significant difference (P<0.05) between the mean total coliforms bacterial counts of butter preserved using traditional ghee, salted, frozen and refrigerated butter at 0, 1, 2 and 3 months of preservation, while, the total coliforms bacterial counts of spiced, melted and untreated butter showed significant (P<0.05) difference among treatments at 0, 1, 2 and 3 months of preservation, respectively. Mean total coliform counts at initial time of preservation for traditional ghee, spiced, melted, untreated, frozen and refrigerated butter samples did not differ (P<0.05) significantly except for salted butter. The mean total coliforms counts at the end of one month of preservation for traditional ghee, spiced, melted, frozen and refrigerated butter samples were not significantly different at P<0.05 except for salted and untreated butter. While the mean total coliform counts at the end of one month of preservation for salted and untreated butter samples did not significantly (P<0.05) differ from each other. At the end of second month of preservation, the mean total coliforms counts of traditional ghee, spiced, melted, frozen and refrigerated butter samples did not significantly (P<0.05) differ from each other except for salted and untreated butter. At the end of the third month of preservation, the mean total coliforms counts of traditional ghee, spiced, melted, frozen, refrigerated, salted and untreated butter samples did not significantly (P<0.05) differ from each other. Mean total coliform count of untreated butter at initial preservation period was 5.62 log cfu/g of butter sample. The current result is by far beyond the mean total coliform count of 2 log cfu/g of butter samples collected from Wolayita zone (Mekdes, 2008). From two months of preservation period and onwards, relatively decreasing trends of total coliform count were observed in traditional ghee, frozen and salted butter compared to other treatments. This might be associated with the inhibitory effects of heat treatment, low storage temperature and addition of salt in ghee, butter kept at-20°C and in salted butter, respectively.
Enterobacteriaceae count
Mean Entrobacteriaceae count (log cfu/g) for the treatments and preservation time is presented in Table 7. There were no significant difference (P<0.05) between mean Entrobacteriaceae counts of traditional ghee, salted, spiced, melted, frozen and refrigerated butter except for untreated butter at 0, 1, 2 and 3 months of preservation, respectively. The mean Entrobacteriaceae counts of butter samples for traditional ghee, spiced, melted and frozen butter at 0 month of preservation did not significantly (P<0.05) differ from each other. While the mean Entrobacteriaceae counts of butter samples for salted, untreated and refrigerated butter at initial preservation time significantly (P<0.05) differed from other treatments but the mean counts for untreated and refrigerated butter did not significantly differ from each other. The mean Entrobacteriaceae counts of samples for traditional ghee, spiced, melted and frozen butter at the end of one month did not significantly (P<0.05) differ from each other. While, the mean Entrobacteriaceae counts of samples for salted, untreated and refrigerated butter at initial preservation time significantly (P<0.05) differed from other treatments. But the mean counts for untreated and refrigerated butter did not significantly differ from each other. The mean Entrobacteriaceae counts of butter samples at the end of second months of preservation for traditional ghee, spiced, untreated and refrigerated butter did not significantly (P<0.05) differ from each other. While, the mean Entrobacteriaceae counts of butter samples for salted, melted and frozen butter at the end of second months of preservation significantly (P<0.05) differed from other treatments. However, at the end of third months of preservation, the mean Entrobacteriaceae counts of traditional ghee, spiced, untreated, melted, refrigerated and frozen samples did not significantly differ (P<0.05) except for salted butter. During the initial preservation time, relatively smaller mean Enterobacteriaceae count of 4.26 log cfu/g of butter was observed in traditional ghee compared to other treatments. This might be ascribed due to the heat treatment and moisture removal from butter during ghee making.
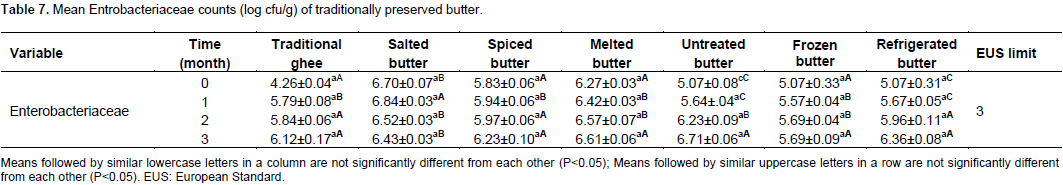
A report by Mattick et al. (2001) stated that some thermo tolerant Enterobacteriaceae comprising a sub-group of mesophiles are capable of growth at up to 44°C, with an optimum growth temperature of 37°C. Fellows (2008) also reported that ghee is preserved by a combination of heat which destroys enzymes and contaminant microorganisms by removing moisture from the butter oil to prevent microorganisms growing during storage. Samaraweera et al. (2001) confirmed that lowering moisture content substantially reduces the growth rate of some Enterobacteriaceae. During the initial preservation time, relatively higher counts of Entero-bacteriaceae of 6.70 log cfu/g was observed in spiced butter than in other treatments. This might be attributable to poor hygienic status of spices purchased from open market. Throughout the preservation period, relatively smaller mean Enterobacteriaceae count was observed in frozen, refrigerated butter and traditional ghee compared to the other treatments. This could be explained in terms of the inhibitory effects of low storage temperatures in refrigerated and frozen butter and heat treatment in traditional ghee. Mattick et al. (2001) confirmed that cooling of food to normal refrigeration temperatures of 0 to 8°C inhibits Enterobacteriaceae growth in storage facilitates.
Rhea (2009) also reported that deep freeze retards the growth of undesirable microorganisms and proper salting of butter removes moisture droplets and negatively affects the growth of undesirable microorganisms.
Organoleptic quality of butter preserved using traditional preservation techniques
Organoleptic quality of butter at the beginning of preservation
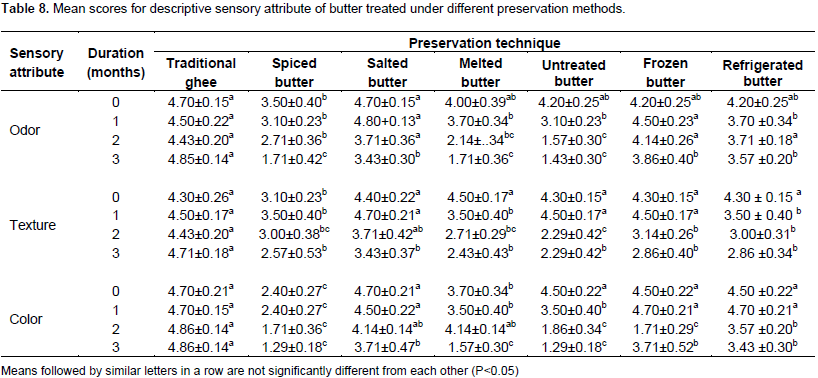
The hedonic rating scale at the end of one month of preservation for color, texture and odor is presented in Table 8. The hedonic rating scale of color, texture and odor of butter samples preserved using traditional ghee; salted, spiced, melted, untreated, frozen and refrigerated butter is presented in Figure 1. At the initial time of preservation, color, texture and odor of traditional ghee, spiced, salted, melted, untreated and butter stored in deep freeze and in refrigerator at 4°C were in acceptable range except for spiced butter which was relatively lower compared to others. This might be attributable to darkening of the color of spiced butter due to mixing with spices such as black cumin. At initial time of preservation, traditional ghee and salted butter were extremely liked compared to untreated, butter stored in refrigerator at 4°C and in deep freezer which were liked moderately. The color and odor of traditional ghee and salted butter were extremely liked. Except in spiced butter the color of butter in the other treatments were extremely liked.
Organoleptic quality of butter at the end of one month of preservation
The hedonic rating scale at the end of one month of preservation for color, texture and odor is presented in Table 8. At the end of one month of preservation, the color, texture and odor of butter samples for traditional ghee, spiced, salted,melted, untreated and butter stored in deep freeze and in refrigerator at 4°C were in acceptable range except for the color of spiced butter which ranged between dislike slightly to neither like nor dislike. At the end of one month of preservation, the sensory panelists extremely liked traditional ghee, salted, refrigerated and butter kept in deep freeze. While, spiced butter was neither liked nor disliked by the sensory panelists. Melted and untreated butter samples were moderately liked.
Organoleptic quality of butter at the end of two months of preservation
The hedonic rating scale result of color, texture and aroma of the treatments is presented in Table 8. At the end of two months of preservation, the sensory acceptability of spiced, melted and untreated butter highly deteriorated compared to others. Traditional ghee and salted butter were rated as extremely liked for their color, aroma and texture. This is in agreement with a report of Illingworth et al. (2009) that revealed that application of heat during preparation of ghee and removal of moisture and solid non-fat contribute to a product of unique color, flavor and texture.
Organoleptic quality of butter at the end of three months of preservation
The hedonic rating scale of the butter samples at the end of three months of preservation based on color, texture and odor is presented in Table 8. The color, texture and odor of traditional ghee and salted butter were extreme and moderate likeness, respectively. While frozen and refrigerated butter were moderately liked by the sensory panelists. Whereas, the odor and texture of spiced butter is rated as neither like nor disliked. The color and aroma of melted and untreated butter were slightly disliked except for their texture.
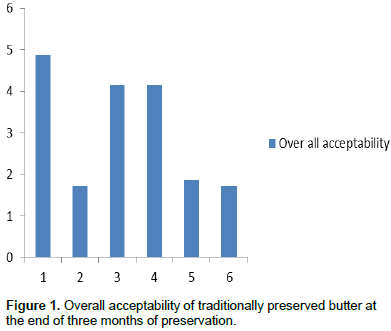
Overall acceptance of traditionally preserved butter at the end of three months
The hedonic rating scale on the overall acceptance of color, texture and odor of butter samples at the end of three months of preservation is presented in Figure 1. Among all the treatments traditional ghee was extremely liked followed by salted butter which was rated between moderate and extreme likeness. At the end of three months, refrigerated and frozen butter were moderately liked by the sensory panels. The relative reduction in likeness in the overall acceptability of refrigerated and frozen might be attributable to the change in odor as a result of the metabolic and enzymatic activities of psychrophilic bacteria that can multiply under low temperature.