ABSTRACT
Epidemiological studies have repeatedly shown the apparent association between high fruit intake and lower incidence of chronic degenerative diseases. The chemo protective properties of fruits have been partly attributed to the antioxidants including phenolic compounds and carotenoids. With respect to the latter, no information is available for the smooth cayenne; a common variety of Ananas comosus grown in various counties of Kenya. Of particular concern is how duration under room temperature storage, will affect the carotenoids based on the challenging aspect of the perishability of the fruit. The levels of Lutein were investigated during storage of fruit samples of smooth cayenne sourced from Kiambu, Homabay, Kilifi, Kericho and Nyamira Counties, Kenya and analyzed within 7 days of storage under room temperature (21 to 25°C). High performance liquid chromatography method and one way ANOVA were employed instrumental and statistical analysis, respectively. The lutein levels ranged between 107.52±1.25 and 233.55±5.77 μg/100g being categorized as sufficient. Findings not only showed that the levels differed significantly among samples but as well increased during storage (p<0.05), hence this show the effect of storage on the nutritional value of the fruit. While the fruit consumption should be promoted for the sufficient levels of lutein which can be useful in addressing chronic degenerative diseases, the longer the storage of the fruit will enhance the levels of the carotenoid.
Key words: Ananas comosus, smooth cayenne, lutein, carotenoids, chronic degenerative diseases.
A significant proportion of global deaths are attributed to chronic degenerative diseases (CDDs) which include cancer and cardiovascular disease (Francesco and Monica, 2016). In 2012, CDDs caused approximately 38 million (68%) of the worlds’ 56 million deaths (WHO, 2014). Fundamentally, the diseases are related to life styles which include poor diet thus compromising quality of life and as well may cause premature death and disability (WHO/FAO, 2003). While diet and nutrition is coming to the fore as the major determinant of CDDs,epidemiological studies shows apparent association between high fruit consumption and their low incidence (Francesco and Monica, 2016; Heiner et al., 2012). The underlying reason is that fruits and vegetables contain significant amounts of biologically active phytochemical compounds such as carotenoids (β-carotene, lycopene and lutein) compounds, whose levels generally correlate with their antioxidant activity (Corral-Aguayo et al., 2008; Maria et al., 2011). In other words, fruits including A. comosus L (Pineapple) should be promoted for consumption to address CDDs.
Pineapple (A. comosus L) is a tropical fruit which is a native of southern Paraguay and Brazil (Walter et al., 2014; Annemarie, 2012). Kenya appears in the list of the main pineapple producers in the world and is the leading producer in East Africa (Annemarie, 2012; USAID, 2013). Pineapple fruit is of high economic importance and the commercial variety of pineapple commonly grown in Kenya is the smooth cayenne. Del monte’s farm in Kiambu County is the leading producer in Kenya but small-scale growing is on the increase in Homabay, Kilifi, Nyamira and Kericho counties (Walter et al., 2014).
Depending on the variety, commercially ripe pineapple weighs between 0.9 to 4 kg. Ripe pineapples can be eaten fresh, or processed into jam, juice, canned or dried (Annemarie, 2012). Like any other, the carotenoid levels of this fruit are associated with; the type of cultivar, agronomic conditions, growing season, pH, moisture content, stage of ripeness, postharvest, and storage conditions (Wang and Lin, 2000; Kalyani, 2009).
Studies have concentrated on the tropical fruits in particular on their levels of antioxidants and antioxidant activity. The lack of information for the smooth cayenne A. comosus grown in Kenya presented a gap for study. In addition, carotenoids chemistry is generally challenging as they are sensitive to others like air, heat, acid, oxygen and light. Pineapple fruits when ripe can be stored in bulk for consumption over days; this is a common traditional practice. As to whether storage would have effect on the lutein level this information that is generally not scientifically investigated. Thus, there is an impetus to investigate the effect of storage on the levels of lutein in the smooth cayenne A. comosus grown in Counties of Kenya.
Chemicals and reagents
Calcium carbonate was from Merck chemical supplies (Damstadt, Germany). Lycopene, Lutein and β-carotene standards were obtained from Sigma-Aldrich Chemical Corporation, USA. Acetonitrile, methanol, deionised water and dichloro methane (all HPLC grade) were obtained from Sigma-Aldrich Chemical Corporation. All the other chemicals used including the solvents, were of analytical grade purchased from Sigma-Aldrich Chemical Corporation.
Sampling and sample preparation
Purposive sampling technique was employed to harvest commercially mature fruits which were 120 to125 days from flowering, quarter-yellow in colour and weighing between 3 to 3.5 kg, from farms in Olare (Homabay), Ngoina (Kericho), Kanyoni (Kiambu), Ekonge (Nyamira) and Chamari (Kilifi). The pineapples sampled were ensured to have no external defect or damage (indicated by the secretion of a fluid).
The samples obtained from the five regions were coded appropriately and packed into labeled sisal bags, then transported to Kenyatta university chemistry laboratory within 24 h of collection where the samples were stored on clean shelves under room temperature (21 to 25°C), awaiting determination of changes in the levels of lutein over 7 days of storage. The fruit samples were identified and authenticated by the herbarium curator at National Museums of Kenya, Nairobi. An accession number (NMK/BOT/CTX/1/2) was deposited in the herbarium section of the museum.
Extraction, identification and quantification of carotenoids
One hundred grams (100 g) of sample was homogenized in an electric blender for 1 min, in the dark-room of the research laboratory. 20 g of homogenized paste was measured and 100 mL of acetone:methanol:diethylether mixture (5:3:2) containing 0.5% butylated hydroxytoluene was used to extract carotenoids for 2 min in separating funnel. The extraction was repeated three times to ensure that maximum extraction occur. Calcium carbonate powder was added to neutralize plant acids and avoid cis/trans isomerisation of carotenoids during extraction. The mixture was filtered using Buchner funnel and small portions of the extract were added into 50 ml of diethyl ether and n-hexane mixture (3:1) in a separating funnel for partitioning, then carefully washed six times using distilled water to remove all acetone and methanol.
Anhydrous sodium sulphate was added to the sample potion in other to dry it. Then the samples was concentrated to dryness in rotary evaporator (BUCHI, Rotavapor R-210/R-215, Germany) to near dryness at temperature 30°C under nitrogen environment. The samples were re-dissolved in 6 mL methanol and packed in HPLC vials in nitrogen atmosphere. Identification and quantification was done by HPLC procedure (Matsumoto et al., 2007).
One microgram (1 μg) aliquot of sample was introduced into the HPLC instrument (Prominence LC-20A). The instrument consists of oven (CTO-10ASVP), a LC-20AD pump and a C18 column (250 x 4.6 mm id, 5 μm particle size). Mobile phase are made up of acetonitrile, methanol and dichloromethane (70:10:20) with a flow rate of 20 min. The column temperature was maintained at 30°C. The carotenoids were identified on the basis of retention time, by comparing spectra to standards analyzed under the same conditions. The carotenoids were quantified using single point calibration and the results are expressed in μg/100 g of sample.
Data analysis
Lutein levels were determined daily for 7 days, where experimental measurements were carried out in triplicates and expressed as means of three replicate analyses ± standard deviation, compared by One-way ANOVA test at 95% confidence level using SPSS(Chicago, IL) statistical software package (SPSs for Windows, version XII, 2004). Differences at P<0.05 were considered as significantly different and SNK-test at α=0.05 indicate differences among means.
Lutein content in pineapples
Tables 1 to 5 present the levels of lutein in pineapples obtained from Homabay, Kilifi, Kericho, Nyamira and Kiambu Counties of Kenya, respectively. The trends of lutein concentration in pineapples, from the Counties with storage at room temperature for 7 days are shown in Figure 1.
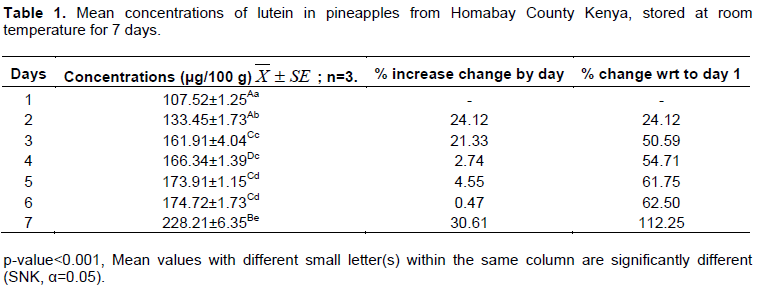
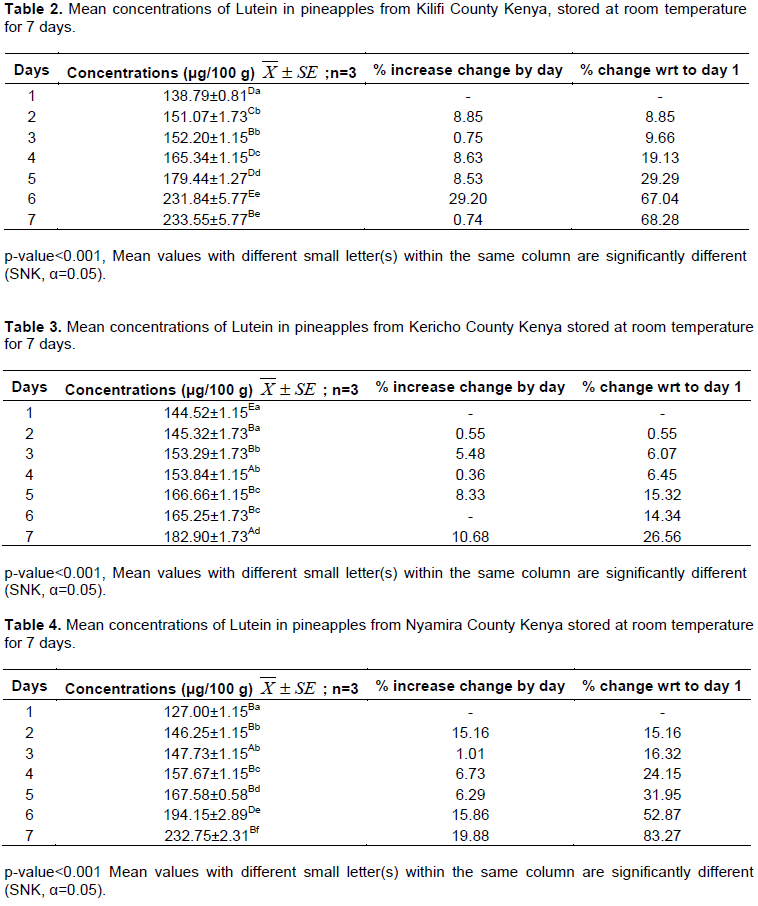
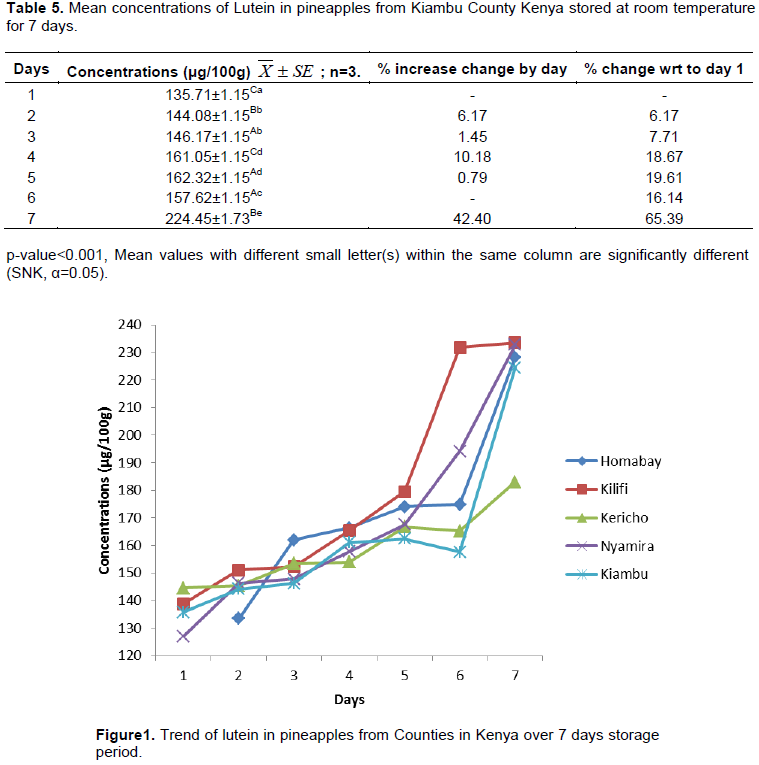
Substantial levels of lutein were found in pineapples obtained from all Counties, ranged between 107.52±1.25 μg/100 g and 233.55±5.77 μg/100 g. There was a significant difference (p<0.001) in the level of lutein in 5 samples of pineapples. On day one of analysis, Kericho sample had the highest lutein content (144.52±1.15 μg/100 g) and Homabay the lowest (107.52±1.25 μg/100 g). On day 7 of analysis, Kilifi sample had the highest content (233.55±5.77 μg/100 g) and Kericho sample the lowest (182.90±1.73 μg/100 g). The differences in content of lutein in common cultivar may be attributed to the presence of difference genes in each of the fruits investigated (Murkovic et al., 2002). In general, Kenyan grown pineapples contain significant levels of lutein though lower than levels reported in broccoli (2358 μg/100 g wet wt), kale (6390 μg/100 g wet wt), carrot (280 μg/100 g wet wt) and spinach (3920 μg/100 g wet wt) (Huck et al., 2000).
It was also observed that the level of lutein increased significantly (p<0.001) as the fruits were stored from day 1 to 7. Similar observations were noted by Kalyani (2009) in fresh pineapples which showed an increase in lutein content of (27.20 to 40.32 μg). Kalyani (2009) attributed this increase to factors such as pH and moisture. Increase in pH during storage of pineapple increases the lutein content in the fruit, while decrease in moisture content increases the lutein levels. The decrease in moisture content lowers the mobility of reactants and catalysts hence reducing the rate of oxidation (Lavelli et al., 2007). The percentage increase in the levels of lutein may also be attributed to the presence of significant levels of phytoene synthase enzyme, which is involved in the biosynthesis of the carotenoid in the pineapple fruits investigated (Bitton and Khachik, 2009; Abdul-Hammed et al., 2014). The overall gain in lutein levels during storage may also be attributed to the conversion of other carotenoids to lutein or to the presence of ε-cyclase and ε-hydroxylase enzymes in the fruits (Bitton and Khachik, 2009). This finding is in agreement with earlier researchers (Hena et al., 2016)
1. Lutein was present in significant amount, with significant difference in levels of lutein in the fruit sample sourced from the five Counties.
2. There was significant increase in the level of lutein as the pineapple fruits were stored from day 1 to 7.
3. Increase in lutein levels, showed that continous ripening of the fruit during storage could be a way of increasing lutein content in the pineapple fruit after harvesting.
These findings propose that, proper storage condition and period should be sought to obtain the maximum nutritional and medicinal benefits of pineapple fruits.
The authors have not declared any conflict of interests.
REFERENCES
|
Abdul-Hammed M, Azeez M, Bello M, Oghenekevwe G (2014). Assessment of nutritional, antioxidant and pro-vitamin A indices of tomatoes under field and postharvest ripening conditions. Res. J. Pharm.Biol. Chem.Sci. 5:649-661.
|
|
|
|
Annemarie G (2012). Business case Pineapple West-Kenya FGL Holding and farmer groups. Centre for Development Innovation.
|
|
|
|
|
Bitton G, Khachik F (2009). Carotenoids in food. In: Britton, G., Liaaen-Jensen, S., Pfander, H. Carotenoids. Volume 5. Nutrition and Health. Springer, New York.
Crossref
|
|
|
|
|
Corral-Aguayo R, Yahia E, Carrillo-Lopez A, Gonzalez-Aguilar G (2008). Correllation between some nutritional components and the total antioxidant capacity measured with six different assays in eight horticulturalcrops. J. Agric. Food Chem. 56:10498-10504.
Crossref
|
|
|
|
|
Francesco S, Monica R (2016) Nutrition and prevention of chronic-degenerative diseases. Agri. Sci. Procedia. 8:713-717.
|
|
|
|
|
Heiner B, Angela B, Achim B, Sabine E, Dirk H, Anja K, Eva L, Manfred J, Helmut O, Matthias S, Peter S, Bernhard W (2012) Critical review: Vegetables and fruit in the prevention of chronic diseases. Euro. J. Nutri. 51:637-663.
Crossref
|
|
|
|
|
Hena J, Andala D, Nyambaka H, Nawiri M (2016). Carotenoid levels, total phenolic content and antioxidant activity variations in varieties of Citrullus lanatus under storage at room temperature. Int. J. Biochem. Res. Rev. 9:1-9.
Crossref
|
|
|
|
|
Huck C, Popp M, Scherz H, Bonn G (2000). Development and evaluation of a new method for the determination of the carotenoid content in selected vegetables by HPLC and HPLC-MS-MS. J. Chromatogr. Sci. 38:441-449.
Crossref
|
|
|
|
|
Kalyani P (2009). Effect of processing on lycopene and lutein content of selected fruits. Thesis.
|
|
|
|
|
Lavelli V, Zanoni B, zaniboni A (2007) Effect of water activity on carotenoid degeneration in dehydrated carrots. Food chem. J. 104:1705-1711.
|
|
|
|
|
Maria M, Paulo H, Angela M, Giovana M, Carlos E, Geraldo A, Telma L (2011). bioactive compounds and antioxidant activity of fresh exotic fruits from northeast Brazil. Food Res. Int. 44:2155-2159.
Crossref
|
|
|
|
|
Matsumoto H, Ikoma Y, Kato M, Kuniga T, Nakajima N, Yoshida T (2007). Quantification of carotenoids in citrus fruit by LC-MS and comparison of patterns of seasonal changes for carotenoids among citrus varieties. J. Agric. Food Chem. 55:2356-2368.
Crossref
|
|
|
|
|
Murkovic M, Muelleder U, Neunteufl H (2002). Carotenoid content in different varieties of pumpkins. J. Food Comp. Anal. 15:633-638.
Crossref
|
|
|
|
|
USAID (2013). The fresh fruit and vegetable markets of East Africa: An assessment of regional value chain actors, activities and constraints in Kenya, Tanzania and Uganda.
|
|
|
|
|
Walter k, Gicuru I, Lawrence K, Evans N (2014). Evaluation of allocative efficiency of small-scale pineapple (Ananas comosus) production in Bureti District, Kenya. Agric. J. 9:61-67.
|
|
|
|
|
Wang S, Lin H (2000). Antioxidant activity of fruits and leaves of blackberry, raspberry, and strawberry varies with cultivar and developmental stages. J. Agric. Food Chem. 48:140-146.
Crossref
|
|
|
|
|
WHO (2014). Global status report on non communicable diseases. Geneva.
|
|
|
|
|
WHO/FAO (2003). Diet, nutrition and the prevention of chronic diseases. WHO technical report series 916. Geneva.
|
|


