ABSTRACT
Moin-moin (steamed cowpea food; MM) is one of the most popular local dishes in Bahia-Brazil. The present study was carried out to improve the knowledge of type of cowpea used in the preparation of moin-moin and its influence on mineral content and antinutritional factors. MM samples were collected from 30 out of the sales points located in the city of Salvador-Bahia. The mineral contents of MM showed a wide variation: K: 917.4 ± 166.2 mg 100 g-1 DM, P: 400.0 ± 59.8 mg 100 g-1 DM, Mg: 97.9 ± 16.4 mg 100 g-1 DM, Ca: 94.0 ± 70.4 mg 100 g-1 DM, Fe: 57.3 ± 16.9 µg g-1 DM, Zn: 22.4 ± 4.2 µg g-1 DM, Cu: 6.3 ± 3.3 µg g-1 DM, Mn: 6.0 ± 2.8 µg g-1 DM and V: 4.3 ± 2.0 µg g-1 DM. AF in MM were: 0.60 ± 0.77 µmol g-1 DM (InsP4); 1.34 ± 1.07 µmol g-1 DM (InsP5); 4.77 ± 3.53 µmol g-1 DM (InsP6); 1.69 ± 0.03 mg eq. CE g-1 DM (tannins); 6.37 ± 0.12 mg g-1 DM (polyphenols). Fe bioavailability might be negatively affected. Information on cowpea cultivars used for MM preparation was obtained from a structured questionnaire. ‘Olho de Pombo’ showed the highest concentrations for AF and minerals. MM was identified as a good source of minerals.
Key words: Antinutritional factors, cowpea (Vigna unguiculata), crude palm oil, minerals, moin-moin.
Originally introduced from East and West African countries, moin-moin (moyi-moyi, olèl) is a steamed pudding made of beans, sold in the streets by typically clothed women called baianas de acarajé. The preparation of moin-moin (MM) starts with cowpea soaking in cold water until they can be easily dehulled. Different varieties of cowpeas such as fradinho, olho de pombo and macáçar or a ready-to-use cowpea paste are used to prepare moin-moin at different sales points (Dos Santos et al., 2013; Rogério et al., 2014). The dehulled cowpeas are ground to a fine paste and crude palm oil, grated onions, dry shrimps, salt and other seasonings (chestnut, peanut, ginger) are added to obtain the typical taste. The paste is formed to balls which are steamed and wrapped in banana leaves. There are some variations in the methods applied to prepare MM, such as the soaking time for the cowpeas, the amount and type of ingredients, as well as steaming time.
Cowpea (Vigna unguiculata), also called black-eyed pea or southern pea, is the main ingredient of MM, it is widely cultivated in the Northern and Northeastern regions of Brazil (Carvalho et al., 2012). Cowpeas contain high-quality protein (23 to 25%), carbohydrates (57%), vitamin B, minerals, dietary fiber (3.9%), and are low in fat (1.3%) (Carvalho et al., 2012). However, its nutritional value is frequently reduced by the presence of antinutrients such as phytates, trypsin inhibitors, lectins, tannins and polyphenols, affecting mineral bioavailability (Dahdouh et al., 2019; Almeida et al., 2008; Samtiya et al., 2020).
Food processing and preparation techniques may have effects on the content of minerals and antinutritional factors of the final foods (Samtiya et al., 2020). So far, there is only limited information about the content of minerals and antinutritional factors of moin-moin. Therefore, the objective of this study was to improve the knowledge of type of cowpea used in the preparation of moin-moin and its influence on mineral content and antinutritional.
Study site and sampling
Moin-moin (MM) samples were collected from 30 out of the 168 MM sales points (18%) located in the city of Salvador-Bahia. The samples collected at each sales point consisted of four MM balls wrapped in banana leaves. Directly after collection, the moin-moin samples were packed in freezer storage bags and transported to the laboratory in thermal insulated boxes. After arrival in the laboratory, the samples were stored at minus 80°C for 24 h. Thereafter, the samples were freeze-dried (Freeze-dryer LS 3000 D, Terroni, Brazil) and ground in a domestic stainless-steel food processor (DCG-20 Cuisinart Coffee Grinder). The ground material was stored in amber bottles at minus 20°C until further use.
While collecting the samples, the sales women (baianas de acarajé) were invited to complete a structured questionnaire
about the selection of ingredients and the mode of preparation. Each participant in the questionnaire gave informed consent, and it was previously approved by the Ethics Committee of the School of Nutrition of Federal University of Bahia (Protocol 730.192/2014).
Antinutritional factors
Myo-Inositol phosphates
Myo-inositol phosphates were quantified in duplicate (two independent extractions per sample) by High-performance liquid chromatography (HPLC) ion-pair chromatography, using an Ultrasep ES 100 RP18 (2 x 250 mm), as described by Greiner and Konietzny (1998). A mixture of the individual myo-inositol phosphate esters (InsP3 - InsP6) was used as a standard.
Haemagglutinating activity
Haemagglutination assays, using trypsin-treated rabbit erythrocytes, were carried out by serial dilution, as described by Grant et al. (1983). One unit of haemagglutinating activity (HU) was defined as that contained in the amount of sample in the last dilution which caused 50% agglutination of the blood cells.
Condensed tannins
Condensed tannins were extracted with HCl:methanol (1:100 v/v) for 2 hours with mechanical shaking at 25°C and centrifuged at 5,000 g at 15°C for 15 min. Aliquots were immediately analyzed for tannins using the 0.5% vanillin assay (Broadhurst and Jones, 1978).
Polyphenols
Total phenols were extracted with water. An internal standard curve was prepared by adding 10 ml of 0 - 0.01% tannic acid to the flasks. The contents were heated for 30 min at 70°C with constant shaking. Clear supernatants were collected after centrifugation at 2500 g for 15 min, followed by filtration. Polyphenols were determined using the Folin-Denis reagent (King and Health, 1967).
Trypsin inhibitor
Trypsin inhibitor activity was determined as described by Kakade et al. (1974), using alpha-N-benzoyl-DL-arginine-p-nitroanilide hydrochloride as the substrate for trypsin. One trypsin unit was defined as the increase by 0.01 absorbance unit at 410 nm.
Minerals
The following reagents and standard solutions were used: 65% (w/w) HNO3 (Merck, Germany), 30% (v/v) H2O2 (Vetec, Brazil) and 1000 mg L-1 standard solutions (Specsol, Brazil) of Ca, Cd, Co, Cr, Cu, Fe, K, Mg, Mn, Ni, P, Pb, V and Zn. All solutions were prepared with ultrapure water with a specific resistivity of 18.2 MΩ cm, provided by a Milli-Q® Purification System (Millipore, Bedford, MA, USA). All glass and plasticware was decontaminated in a 10% (v/v) HNO3 bath for at least 24 hours and extensively washed with ultrapure water.
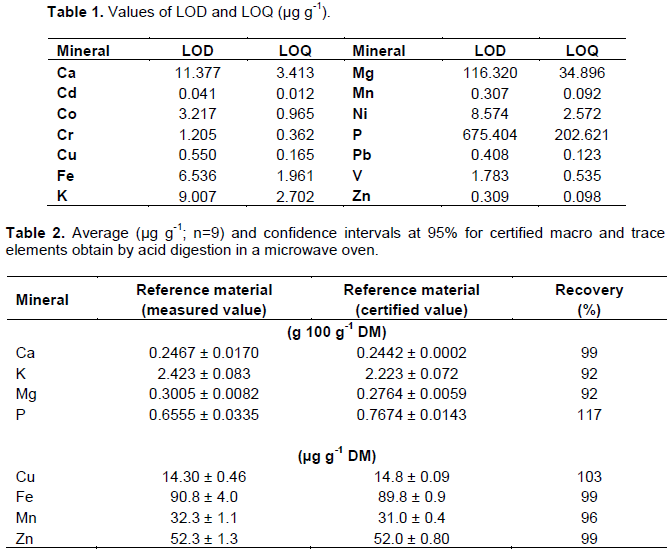
For mineral quantification, an inductively coupled plasma optical emission spectrometer, ICP OES, (OPTIMA 7300 DV - PerkinElmer, USA) was used. The ICP OES operating conditions were: measured power: 1300 W; signal integration time: 1 s; plasma gas flow: 15 L min-1; auxiliary gas flow: 1.5 L min-1; nebulization gas flow: 0.70 L min-1; sample pump flow: 0.70 ml min-1. For sample introduction, a cyclonic chamber and a GemConeTM nebulizer - Low Flow were used. The chosen nuclear (I) and ionic (II) lines (nm) were: Ca II 422.673; Cd II 228.802; Co II 238.892; Cr II 267.716, Cu I 324.752; Fe II 238.204; K 766.490; Mg II 279.077; Mn II 257.610; Ni II 231.604; P I 213.617; Pb II 220.000; V II 309.310 and Zn II 213.857. The samples were decomposed by acid digestion in a microwave oven with cavity (Dos Santos et al., 2013). Briefly, 9.00 ml of 65% (w/w) HNO3, 1.00 ml of 30% (v/v) H2O2 were added to 500 mg of the ground sample. The following heating program was applied: step 1 (6 min ramp to 90°C); step 2 (4 min at 90°C); step 3 (18 min ramp to 190°C); step 4 (7 min at 190°C). The volumes of the digested mixtures were adjusted to 15.0 ml with ultrapure water and the solutions were kept in 50 ml Falcon® tubes. For calibration, a 40.0 mg L-1 multielement solution of Ca, Cd, Co, Cr, Cu, Fe, K, Mg, Mn, Ni, P, Pb, V and Zn in 2.0 mol L-1 HNO3 was used. All samples were analyzed in triplicate. The estimated limit of quantification for the method (LOQ) was calculated as 3.3-fold of the limit of detection (LOD) (Thomsen et al., 2003), based on the measurement of the analytical signal of the digest blank assays (n=10) (Table 1). The Extreme Studentized Deviate Test was used to remove outliers from the data set. INCT-SBF-4 SOYA BEAN FLOUR was used as a reference material (n = 9) to determine precision and accuracy of the method (Table 2).
Multivariate statistical analysis
Normalized data were uploaded to MetaboAnalyst 2.0, a web-based analytical platform for high-throughput metabolomics studies, as previously described (Ribeiro et al., 2015). ANOVA was performed to assess the overall variation in the levels of minerals, followed by post-hoc analyses (Bonferroni correction, FDR < 0.05). Partial least squares discriminant analysis (PLS-DA) was used to compare the overall variation of minerals and antinutritional factors in moin-moin in respect to the cowpea variety used for preparation. Mineral data were also analyzed using the SPSS 13 software (SPSS Inc, Chicago, Il, USA). Each sample was analyzed in triplicate and values were given as mean ± standard patron (SP).
Minerals
The great variation in the mineral content of MM (Table 3) could be explained by variations in the mineral content of the cowpeas used, as well as differences in the recipes and preparation methods for MM (Dos Santos et al., 2013; Rogério et al., 2014).
Potassium (K) was identified as the most abundant mineral in MM (Table 3). Due to the high potassium content of cowpeas (1121.0 100 g-1 DM) (Franco, 2008), this result was expected. MM exhibited higher potassium levels than akara (545.31 - 613.01 mg 100 g-1 DM), another cowpea product frequently consumed in Salvador, Bahia (Feitosa et al., 2015). This could be explained by differences in the recipe of both cowpea products. Akara is mainly made of grated onions, cowpea and salt (Feitosa et al., 2015), whereas the potassium-rich ingredients peanuts (740 mg 100 g-1 DM) and cashew nuts (620 mg 100 g-1 DM) (Franco, 2008) were used in the preparation of moin-moin. Another study on minerals in abará found lower values of this element (104.32- 652.10 mg/100 g).

Phosphorus (P) was identified as the second most abundant mineral present in moin-moin (Table 3). The P content in moin-moin was higher than the P content of akara (210.55 - 353.99 mg 100 g-1 DM) (Feitosa et al., 2015). This could be explained by the use of ingredients higher in P, such as peanuts (354 mg 100 g-1 DM) and cashew nuts (575 mg 100 g-1 DM) (Franco, 2008) for the preparation of MM and ingredients lower in P, such as grated shrimp heads (209 mg 100 g-1 DM) for the preparation of akara (Franco, 2008).
The calcium (Ca) content in MM varied significantly among the samples (5.0 - 224.9 mg 100 g-1 DM) (Table 3). The high variation of Ca content in MM might be due to the variation already reported in Ca content for cowpeas (29 - 51 mg 100 g-1 DM) (Carvalho et al., 2012). Besides cowpeas, ingredients such as dry shrimp, peanuts, cashew, ginger (164, 42, 10, 51 mg 100 g-1 DM, respectively) (Franco, 2008) add to the Ca content of moin-moin. The mean magnesium (Mg) content in MM was very comparable to those observed for akara (106.98 - 125.74 mg 100 g-1 DM) (Feitosa et al., 2015). With 334 ± 0.71 mg 100 g-1 DM, African moin-moin was reported to contain twice the amount of magnesium (Adeyeye et al., 2012). The higher magnesium content is very likely due to the use of fish and eggs for moin-moin paste preparation in Africa.
The iron (Fe) and zinc (Zn) contents of MM were found to be low (Table 3). A lower Fe and Zn content compared to cowpeas (Fe: 51 µg g-1 DM, Zn: 39 µg g-1 DM) (TACO, 2011) could be explained by the removal of the mineral-rich tegument prior to preparing the paste for moin-moin. Higher values, especially for iron, might be due to the use of iron-fortified wheat flour (42 µg g-1 DM Fe) (ANVISA, 2005) for the preparation of MM paste and/or the transfer of these minerals from the banana leaves used to wrap the moin-moin balls. Olayiwola et al. (2012) reported that 11.1 ± 0.12 mg kg-1 DM of iron were derived from banana leaves while preparing moin-moin.
Manganese (Mn), copper (Cu) and vanadium (V) contents in MM are shown in Table 3. Manganese content was found to be lower and copper content was in the same range, compared to Nigerian moin-moin (Mn: 17.4 µg g-1 DM, Cu: 0.85 and 0.98 mg 100 g-1 DM) (Madukorsiri and Adoga, 2009). Values for vanadium could not be found in the scientific literature. The trace elements Cd, Co, Cr, Ni and Pb were not detected in MM. In fact, these minerals are found at very low concentrations in cowpea and other ingredients used to prepare MM. Dos Santos et al. (2013) reported that cowpeas contain 2.1 µg g-1 DM Co, 0.6 -1.7 µg g-1 DM Ni and 0.29 µg g-1 DM Pb.
In Table 4, the contribution of one moin-moin serving (186.6 g) to the daily dietary reference intake (DRIs) for minerals in adults is given. The daily requirements for K, P, Cu, Fe and Mn could be fully covered by one MM serving, whereas the daily requirements for Mg and Zn could be covered at least by 50%. Since the content of macro-and micronutrients differed considerably between the different MM samples analyzed, the contribution of one MM serving to the daily requirements might be sometimes significantly lower. A study analyzing the data obtained in a Household Budget Surveys in 2008 and 2009 demonstrated the prevalence of inadequate intakes of Ca, Cu, K, Mg, Mn, P, Fe and Zn among men and women aged between 20 and 59 years old, mainly in the Northeast region of Brazil (Araújo et al., 2013). Therefore, moin-moin could play an important role in the supply of the population in Salvador, Bahia, with these macro-and micronutrients (Table 4).
Content of antinutrients in moin-moin
Cowpeas have been reported to contain antinutrients such as phytates, polyphenols, condensed tannins, trypsin inhibitors and haemagglutinating activity (Almeida et al., 2008; Carvalho et al., 2012; Bolade, 2016; Rogério, et al., 2014). The processing of cowpeas, however, was reported to result in a reduction in the initial content of antinutrients. A 40-65% reduction in phytate by fermentation of cowpeas and a 50-80% reduction by germination of cowpeas was shown by Dahdouh et al. (2019). In respect to MM, Bolade (2016) revealed that every unit operation involved in its preparation contributed to varying degrees to the overall reduction in trypsin inhibitor activity, phytate and tannins. Soaking and steaming resulted in a complete absence of trypsin inhibitor activity in MM. Furthermore, Feitosa et al. (2015) reported that the cowpea paste used for the preparation of akara still contained trypsin inhibitors and haemagglutinating activity but, in the final food, none was detectable. This is in good agreement with the data on trypsin inhibitor activity obtained in this study. All moin-moin samples analyzed were free of trypsin inhibitor and haemagglutinating activity.
The tannin content of cowpeas was reported to be highly dependent on the variety. For example, 0.34; 4.20; 3.26 and 6.88 mg eq. CE g-1 DM condensed tannins were found in black-eyed, light brown, mottled and maroon-red cowpeas (Plahar et al., 1997). Dehulling reduced the content of condensed tannins to 0.23; 0.08; 0.08 and 0.46 mg CE g-1 DM. Bolade (2016) reported a 96.4 - 97.5% reduction in the tannin content during the preparation of moin-moin. Dehulling was the unit operation identified to contribute the most to the reduction in tannins (39.7 - 47.6%), followed by steaming (19.6 - 24.7%), soaking (9.8 - 15.9%) and wet milling (9.5-13.1%). Feitosa et al. (2015) obtained condensed tannin contents between 1.67 and 1.69 mg eq. CE g-1 DM in akara. The tannin contents in moin-moin (Table 5) were comparable to those reported for akara. While preparing MM, other sources for condensed tannins than cowpeas might need to be considered. Megat Rusydi and Azrina (2012) identified peanuts as a source of tannins (32.69 ± 2.392 mg gallic acid equivalent/mg DM). In addition, a reduction in the amount of tannins leaching into the cooking water due to the wrapping of the MM balls in banana leaves also need to be considered when evaluating effects of processing on the tannin content of MM (Asogwa and Onweluzo, 2010). The tannin content of MM was not expected to impose a significant negative effect on their nutritional quality, once contents up to about 4.2 mg eg. CE g-1 DM were reported not to have adverse effects in humans (Plahar et al., 1997) (Table 5).

The cowpea varieties used to prepare moin-moin were reported to contain 6.42 ± 0.18 mg kg-1 DM total polyphenols (Almeida et al., 2008). The content of polyphenols (6.15 and 6.60 mg g-1 DM) in MM was very comparable to the polyphenol content of the cowpeas used for its preparation (Table 5), as well as the polyphenol content of akara (6.26 to 6.30 mg g-1) (Feitosa et al., 2015). While preparing MM, other sources for polyphenols than cowpeas might need to be considered. Cushnie and Lamb (2005) reported a migration of polyphenols from banana leaves into the wrapped product and Megat Rusydi and Azrina (2012) found 43.79 ± 2.38 mg gallic acid equivalent/mg DM of total phenolic compounds in peanuts, another MM ingredient rich in polyphenols.
According to Rogério et al. (2014), akara paste contained 12.63 µmol g-1 DM phytate (InsP6), 2.21 µmol g-1 DM myo-inositol pentakisphosphate (InsP5) and 0.14 µmol g-1 DM myo-inositol tetrakisphosphate (InsP4). The corresponding values for akara were reported to be 7.72, 1.95 and 0.27 μmol g-1 DM for InsP6, InsP5 and InsP4, respectively. The myo-inositol phosphate contents of moin-moin (Table 5) were found to be comparable to those of akara. Besides cowpeas, peanuts and cashew nuts may contribute to the myo-inositol phosphate content of moin moin. Schlemmer et al. (2009) reported cashew nuts to contain 0.19 - 4.9% (w/w DM) phytate and peanuts 0.17 - 4.47% (w/w DM). Once the mean content of InsP6 and InsP5 were lower compared to akara, steam cooking can be assumed to have a greater efficiency than deep frying in the reduction in myo-inositol phosphates.
To evaluate the effect of phytate on the bioavailability of Ca, Fe and Zn, the phytate: mineral molar ratio was calculated (Table 5). According to Gibson et al. (2006), iron bioavailability is reduced at phytate:Fe molar ratios above 1, whereas phytate: Zn molar higher than 15 is associated with low estimated bioavailability, and Phy:Zn between 5 and 15 and below 5 is associated with moderate and high estimated bioavailability, with a zinc absorption corresponding to 15, 30 and 50%, respectively. In only 10% of MM samples analyzed, the phytate:Fe molar ratio was calculated to be 1. Thus, a low Fe bioavailability from MM is expected. In respect to Zn, 16.7, 56.7 and 26.7% of the MM samples exhibited phytate:Zn molar ratios below 5, between 5 and 15 and above 15 (Gibson and Ferguson, 1998). A significant reduction in Zn bioavailability from MM due to phytate is therefore very likely. The dietary phytate x Ca:Zn molar ratio has been reported to be a more useful parameter to assess Zn bioavailability compared to the phytate:Zn molar ratio. Molar ratios above 200 have been reported to result in a significant reduction in Zn bioavailability; 36.7% of the MM samples showed values below 200, the threshold value for a significant negative effect on Zn bioavailability (Fordyce et al., 1987). Furthermore, the phytate:Ca molar ratio may be used as an indicator for calcium bioavailability in humans (Fordyce et al., 1987). Values above 0.24 point to a reduction in Ca bioavailability. The phytate:Ca molar ratio was shown to be below 0.24 for 76.7% of the MM samples. Therefore, Ca bioavailability should not be affected by phytate for the majority of the MM samples.
Multivariate statistical analysis of the compositional data obtained for the different moin-moin samples
The information on the cowpea cultivars used by the saleswomen (baianas de acarajé) to prepare moin-moin was obtained from a structured questionnaire. Principal component 1 (PC1) accounted for 38.6% of total variance, whereas 15.2% were explained by principal component 2 (PC2) (Figure 1). A clear separation of moin-moin samples prepared with different cowpea cultivars was observed along principal component 1. This result is not very surprising, since cowpeas are the major ingredient used for moin-moin preparation and studies aiming at assessing the mineral composition of cowpea seeds have shown a wide variation in mineral content among different cultivars (Carvalho et al., 2012). The MM samples prepared with the ‘Olho de Pombo’ (OP) cowpea exhibited clear differences in respect to their content of minerals and antinutritional factors compared to moin-moin samples prepared with ‘fradinho’ (F) and ‘maçácar’ (MA) cowpeas. Curiously, moin-moin samples prepared with F and MA cluster together, indicating that both cultivars were characterized by very similar mineral and antinutrient profiles. Additionally, important insights into the origin of the cowpea mixture (CM) or ready-to-use cowpea paste (RCP) used for MM preparation can be obtained from the PLS-DA plot. It was obvious that the mineral and antinutrient composition of MM prepared with RCP was very comparable to the mineral and antinutrient composition of moin-moin prepared with cultivars F and MA, whereas CM might also include OP cowpeas.

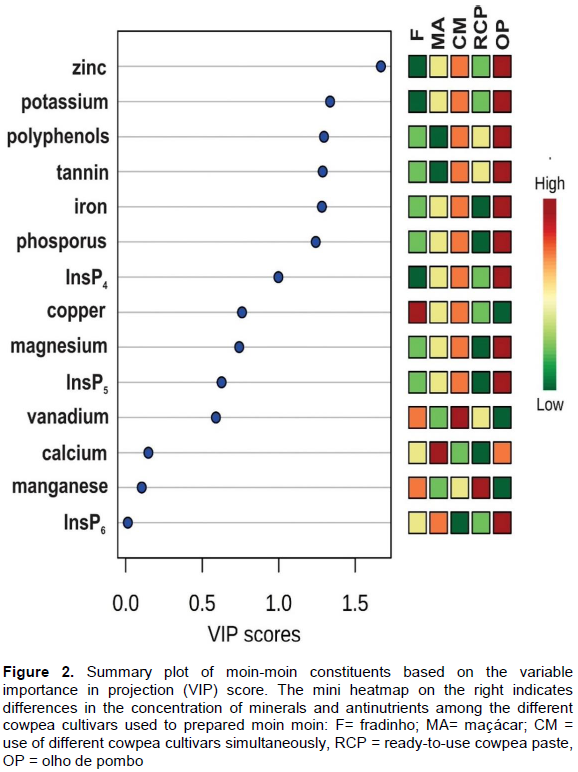
To obtain a possible contribution of each individual mineral and antinutritional factor to the observed differences in the cowpea cultivars used for moin-moin preparation, the variable importance in projection (VIP) threshold was set to 1 (Xia and Wishart, 2011). Zinc potassium, polyphenols, tannins, iron, phosphorus and InsP4 are the moin-moin constituents identified to contribute the most to the differentiation of cowpea cultivars used for MM preparation (Figure 2). Moin-moin samples prepared with the OP cultivar showed the highest concentrations for all these constituents. The lowest concentrations for these constituents were observed for MM samples prepared with RCP and the F cultivar. Moin-moin samples prepared with CM were found to have intermediate concentrations for these constituents, supporting the hypothesis that CM might also include cowpeas of the OP cultivar. Therefore, multivariate statistical analysis was shown to be a tool to identify the cowpea cultivars used for the preparation of moin-moin.
The content of minerals and antinutritional factors varied considerably between the different moin-moin samples. The cowpea cultivar used for moin-moin preparation had the most significant effect on their mineral and antinutrient composition. The daily requirements for K, P, Cu, Fe and Mn could be fully covered by one moin-moin serving, whereas the daily requirements for Mg and Zn could be covered at least by 50%. Thus, moin-moin could play an important role in the supply of the population of Salvador, Bahia, with macro-and micronutrients.
The authors have not declared any conflict of interests.
The authors are grateful for the financial support received from (Fundação de Amparo à Pesquisa do Estado da Bahia (FAPESB), Process INT0012/2014, and Baianas de acarajé, for all cooperation.
REFERENCES
|
Adeyeye EI, Orisakeye OT, Oyarekua, MA (2012). Composition, mineral safety index, calcium, zinc and phytate interrelationships in four fast-foods consumed in Nigeria. Bulletin of the Chemical Society of Ethiopia 26(1):43-54.
Crossref
|
|
|
|
Almeida DT, Greiner R, Furtunado DMN, Trigueiro INS, Araújo, MDPN (2008). Content of some antinutritional factors in bean cultivars frequently consumed in Brazil. International Journal of Food Science and Technology 43:243-249.
Crossref
|
|
|
|
|
ANVISA - Ministério da Saúde. Brasil. Agência Nacional de Vigilância Sanitária (2005). Resolução RDC nº 269, de 22 de setembro de 2005. Regulamento técnico sobre Ingestão Diária Recomendada (IDR) para proteína, vitaminas e minerais. Diário Oficial da União, Brasília, Seção 1, p. 372, 23, setembro 2005.
View
|
|
|
|
|
Araújo MC, Bezerra IN, Barbosa FS, Junger WL, Yokoo EM, Pereira RA, Sichieri R (2013). Consumo de macronutrientes e ingestão inadequada de micronutrientes em adultos. Revista Saúde Pública 47(1):177-189.
Crossref
|
|
|
|
|
Asogwa IS, Onweluzo, JC (2010). Effects of processing methods on the chemical composition of flour, moinmoin and akara from Mucuna pruriens. Journal of Tropical Agriculture, Food, Environment and Extension 9(3):200-208.
Crossref
|
|
|
|
|
Bolade MK (2016). Individualistic impact of unit operations of production, at household level, on some antinutritional factors in selected cowpeaâ€based food products. Food Sciences and Nutrition 4(3):441-455.
Crossref
|
|
|
|
|
Broadhurst RB, Jones WJ (1978). Analysis of condensed tannins using acidified vanillin. Journal of the Science of Food and Agriculture 29(9):788-792.
Crossref
|
|
|
|
|
Carvalho AFU, Sousa NM, Farias DF, Rocha-Bezerra LCB, Silva RMP, Viana MP, Gouveia ST, Sampaio SS, Sousa MB, Lima GPGL, Morais SMM, Barros CC, Filho RF (2012). Nutritional ranking of 30 Brazilian genotypes of cowpeas including determination of antioxidant capacity and vitamins. Journal of Food Composition and Analysis 26(1-2):81-88.
Crossref
|
|
|
|
|
Cushnie TPT, Lamb AJ (2005). Antimicrobial activity of flavonoids. International Journal of Antimicrobial Agents 26(5):343-356.
Crossref
|
|
|
|
|
Dahdouh S, Grande F, Espinosa SN, Vincent A, Gibson R, Bailey K, King J, Rittenschober D, Charrondière UR (2019). Development of the Fao/Infoods/Izincg global food composition database for Phytate. Journal of Food Composition and Analysis 78:42-48.
Crossref
|
|
|
|
|
dos Santos, WPC, Santos, DCMB, Fernandes, AP, Castro JT, Korn MGA (2013). Geographical Characterization of Beans Based on Trace Elements After Microwave-Assisted Digestion Using Diluted Nitric Acid. Food Analytical Methods 4(6):1133-1143.
Crossref
|
|
|
|
|
Feitosa S, Korn MGA, Pinelli MS, Oliveira TRS, Boffo E, Greiner R, Almeida DT (2015). Content of minerals and antinutritional factors in akara (Fried Cowpea Food). International Journal of Food Processing Technology 2:42-50.
Crossref
|
|
|
|
|
Fordyce EJ, Forbes RM, Robbins KR, Erdman Jr. JW (1987). Phytate × Calcium/Zinc Molar Ratios: Are They Predictive of Zinc Bioavailability? Journal of Food Science 52(2):440-444.
Crossref
|
|
|
|
|
Franco G. (2008). Tabela de Composição Química de Alimentos, 9th Edition. São Paulo, SP: Editora Atheneu 307p.
|
|
|
|
|
Gibson R, Perlas L, Hotz C (2006). Improving the bioavailability of nutrients in plant foods at the household level. Proceedings of the Nutrition Society 65(2):160-168.
Crossref
|
|
|
|
|
Gibson RS, Ferguson EL (1998). Food processing methods for improving the zinc content and bioavailability of homeâ€based and commercially available compllementary foods. Micronutrient interactions: Impact on child health and nutrition. Washington, DC: International Life Science Institute Press pp. 50-57.
|
|
|
|
|
Grant G, More L, McKenzie N, Stewart J, Pusztai A (1983). A survey of the nutritional and haemagglutination properties of legume seeds generally available in the UK. British Journal of Nutrition 50(2):207-214.
Crossref
|
|
|
|
|
Greiner R, Konietzny U (1998). Endogenous phytate-degrading enzymes are responsible for phytate reduction while preparing beans (Phaseolus vulgaris). Journal of Food Process Preservation 22:321-331.
Crossref
|
|
|
|
|
Institute of Medicine (IOM) (2006). Dietary reference intakes. The essential guide to nutrientrequirements. National Academy of Sciences, Washington, DC. Available from: View
|
|
|
|
|
Kakade ML, Rackis JJ, Mcghee JE, Puski G (1974). Determination of trypsin inhibitor activity of soybean products: a collaborative analysis of an improved procedure. Cereal Chemistry 51:376-382.
|
|
|
|
|
King HG, Health GW (1967). The chemical analysis of small samples of leaf material and the relationship between disappearance and composition of leaves. Pedobiologia 7:192-197.
|
|
|
|
|
Madukorsiri CH, Adoga GI (2009). Some Nutrient Composition of Ready-to-Eat Foods Consumed by Pregnant and Lactating Women in Bassa LGA of Plateau State, Nigeria. Pakistan Journal of Nutrition 8(12):1889-1893.
Crossref
|
|
|
|
|
Megat Rusydi MR, Azrina A (2012). A. Effect of germination on total phenolic, tannin and phytic acid contents in soy bean and peanut. International Food Research Journal 19(2):673-677.
|
|
|
|
|
Olayiwola OA, Shittu SA, Adebayo OR (2012). Evaluation of heavy metals in three common Nigerian Cowpea (Vigna unguiculata) paste end product (''Moinmoin'') using different packaging materials. International Journal of Environmental Sciences 3(2):833-840.
|
|
|
|
|
Plahar WA, Annan NT, Nti CA (1997). Cultivar and processing effects on the pasting characteristics, tannin content and protein quality and digestibility of cowpea (Vigna unguiculata). Plant Foods for Human Nutrition 51:343-356.
Crossref
|
|
|
|
|
Ribeiro PR, Willems LAJ, Mutimawurugo MC, Fernandez LG, Castro RD, Ligterink W, Hilhorst HWM (2015). Metabolite profiling of Ricinus communis germination at different temperatures provides new insights into thermo-mediatedrequirements for successful seedling establishment. Source Plant Science 239:180-191.
Crossref
|
|
|
|
|
Rogério WF, Greiner R, Nunes IL, Feitosa S, Furtunato DMN, Almeida DT (2014). Effect of preparation practices and the cowpea cultivar Vigna unguiculata L.Walp on the quality and content of myo-inositol phosphate in akara (fried bean paste). Food Science and Technology 34:243-248.
Crossref
|
|
|
|
|
Samtiya M, Aluko RE, Dhewa T (2020). Plant food anti-nutritional factors and their reduction strategies: an overview. Food Production, Processing and Nutrition 2(6).
Crossref
|
|
|
|
|
Schlemmer U, Frølich W, Prieto RM, Grases F (2009). Phytate in foods and significance for humans: Food sources, intake, processing, bioavailability, protective role and analysis. Molecular Nutrition and Food Research 53:330-S375.
Crossref
|
|
|
|
|
Tabela Brasileira de Composição de Alimentos (TACO) (2011). 4th Edition. Campinas, SP: NEPA-UNICAMP. Available at:
View
|
|
|
|
|
Thomsen V, Schatzlein D, Mercuro D (2003). Limits of detection in spectroscopy. Spectroscopy 18(12):112-114.
|
|
|
|
|
Xia J, Wishart DS (2011). Metabolomic data processing, analysis, and interpretation using metaboanalyst. Current Protocols in Bioinformatics 34(1):14-10.
Crossref
|
|