Full Length Research Paper
ABSTRACT
Bambara groundnut is the main food legume after cowpea, especially in rural areas in Burkina Faso. Due to inappropriate production and storage conditions, the seeds are susceptible to contamination by several fungi. Therefore, the present study was conducted to isolate and identify the postharvest fungi associated with Bambara groundnut seeds produced in Burkina Faso. To do this, a total of 99 seed samples were collected in the three agro ecological zones of Burkina Faso. The sanitary analysis of the seeds was carried out using the blotting paper method with slight modifications. The isolation and purification of the isolates was performed on Potato Dextrose Agar medium while their identification was done through macroscopic and microscopic phenotypical characterization using different culture media (Malt Extract Agar and Czapeck Dox Agar) and different identification keys. A total of 421 fungal strains were isolated and the predominant genera were Aspergillus belonging to section flavi (66.84%), nigri (59.04%), and Macrophomina (26.49%). This study shows that Bambara groundnut seeds produced in Burkina Faso are contaminated by several fungal strains and that seed infection rates by these fungi differ according to the agro-ecological zones. Post-harvest and storage techniques need to be improved to limit crop losses.
Key words: Agro-ecological zone, Bambara groundnut, seeds, fungal strains, macroscopic and microscopic characteristics, Burkina Faso.
INTRODUCTION
Bambara groundnut is the second most important food legume after cowpeas for numerous populations, especially during the dry season in Burkina Faso (Ouoba et al., 2016). In order to have their crops available throughout the year, rural farmers use several traditional storage techniques. Unfortunately, these technics do not provide the expected protection to the seeds that are damaged due to several agents including insects and fungi (Ouoba et al., 2016; Kpatinvoh et al., 2017). Fungal contamination of food commodities can cause considerable economic losses through the reduction of their organoleptic and nutritional qualities. According to the Food and Agriculture Organization of the United Nations (FAO), about one quarter of the world's production is annually lost due to uncontrolled fungal growth, representing an economic loss of 5-10% (FAO, 2010). These fungi make food products dangerous because of the production of toxic metabolites such as mycotoxins which are detrimental to human and animal health (Pereira et al., 2013; Olagunju et al., 2018; Okayo et al., 2020). The identification of post-harvest fungi impli-cated in food spoilage and the production of mycotoxins is an essential step in achieving food security.
During its production, Bambara groundnut can be contaminated by toxinogenic fungi present in the soil (Olagunju et al., 2018), which may lead to accumulation of mycotoxins in the seeds. Studies conducted in South Africa revealed that the seeds were contaminated with fungi and mycotoxin (aflatoxins) at concentrations ranging from 0.01 to 0.1 ppm (Shabangu, 2009; Olagunju et al., 2018). In Burkina Faso, the research on Bambara groundnut has mainly consisted in making agronomic characterizations (Ouedraogo et al., 2008), assessing in a real environment the best technical options to increase its productivity and the pathogens contaminating the vegetative system (Ouoba et al., 2019). However, data on fungal and mycotoxinogenic contamination of the seeds and the risks associated with them remain very limited. The contamination, the development of fungi and the production of mycotoxins can vary depending on the environmental conditions such as temperature, humidity etc. and the storage conditions (Njoroge et al., 2019; Baddi et al., 2021). Burkina Faso is divided isn three agro-climatic zones with different environmental con-ditions and traditional storage practices that could impact the mycoflora of stored seeds. Therefore, this study was conducted to isolate and characterize the fungal strains associated with the seeds of Bambara addressed two important questions: (i) what are the main fungal strains contaminating the seeds of Bambara groundnut in Burkina Faso? (ii) How do agro-ecological conditions affect the distribution and the infection rates of the fungi?
MATERIALS AND METHODS
Collection sites and seeds sampling
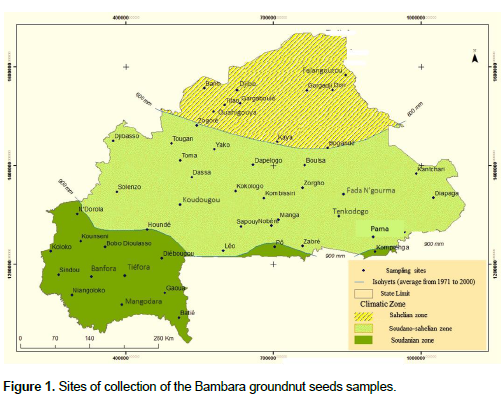
Samples of Bambara groundnut (seeds or pods) were collected in 2020 from 47 sites throughout the three agro-ecological zones (Sahelian, Sudano-Sahelian and Sudanian) of Burkina Faso (Figure 1). Sample locations were randomly selected in each of the three agro-ecological zones from a list initially drawn up according to accessibility, the level of Bambara groundnut production and the need to cover the study area. Accordingly, 9 sites were considered in the Sahelian zone, 25 sites in the Sudano-Sahelian zone, and 13 sites in the Sudanian zone. Seed samples (1,000 to 2,000 g) were randomly collected from farmers in each agro-ecological zone (25, 51 and 23 from the Sahelian, Sudano-Sahelian and Sudanian zones, respectively) making a total of 99 samples. Each sample was placed in a sterile plastic bag and transported immediately to the laboratory. The samples were further divided into two equal parts; the first portion was used for the study while the other was stored (refrigeration) for further analyses.
Seeds health testing
The standard blotter method described byMathur and Kongsdal (2003)was used with slight modifications, to detect fungi growth from seeds in the presence of humidity. Two hundred (200) untreated seeds from each sample were placed on moistened blotters in Petri dishes at the rate of 10 seeds per dish and incubated for 7 days at 20-25°C under alternating cycles of 12 h near Ultraviolet Light (NUV) and 12 h darkness. Then, the individual seeds were examined for the presence of fungi under a stereo-microscope. A preliminary identification of each fungus developed on the seeds was made by examining the mycelium and/or conidia under a compound microscope and the different strains present on each seed were recorded. Then, the infection rate of each fungus and the percentage of infected samples were computed using the following formulae (Marasas et al., 1988):

Isolation and purification of the developed fungal strains
Each visible mycelial growth on the seeds was isolated by collecting a fragment of the mycelium using a sterilized needle that was placed in the center of a Petri dish containing PDA medium for growth. During the collection, precautions were taken to avoid the contact with other neighbouring mycelia from the same seed; then, successive subcultures were performed on PDA medium in order to purify the isolated fungus. The subculturing was carried out by placing a fragment of the mycelium in the center of a new Petri dish using a sterilized loop. The purified strains obtained were kept on PDA at 4°C.
Identification
Three culture media (potato dextrose agar, malt extract agar and czapeck dox agar) were used to identify the purified strains based on their phenotypical and cultural characteristics. Furthermore, the fragments of the mycelium stained with methylene blue were observed under a microscope. The identification keys used were those described by Samson et al. (1996), Pitt and Hocking (1997), Botton et al. (1999)andMathur and kongsdal (2003).
Data analyses
Seed-borne fungi of Bambara groundnut and infection rates were determined using equations 1 and 2. The distribution of fungal strains on the samples and between agro-ecological zones was compared by the Analyses of Variance (ANOVA); it was done with the Duncan’s Multiple Range (DMR) test at the significance level of p < 0.05 using Statistical Analysis System, version 8.
RESULTS
Mycoflora of Bambara groundnut seeds
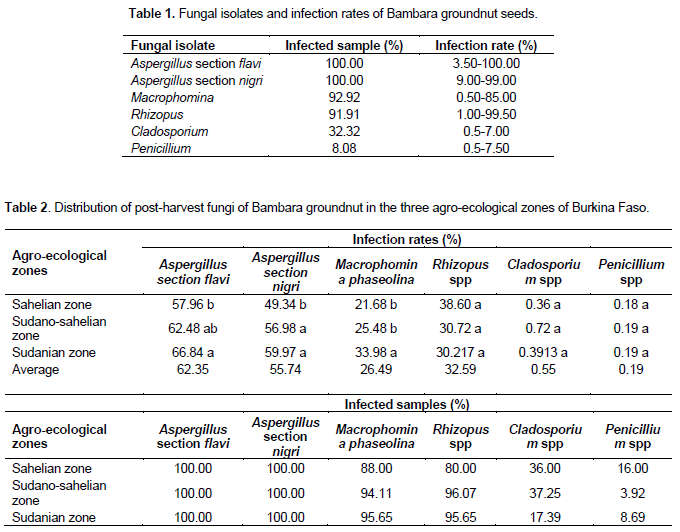
A total of 421 fungal strains belonging to five genera were detected from the seeds ollected in the three agro-ecological zones: Aspergillus, Macrophomina, Rhizopus, Cladosporium and Penicillium (Table 1). The health testing of the seeds revealed that all of the tested seed samples were infected by at least two fungal strains. The mycoflora can be split into three groups based on the percentage of infected samples: (i) Aspergillus belonging to section flavi and section nigri infected all samples with high infection rates (3.50-100% and 9.00-99%, respectively); (ii) Macrophomina and Rhizopus were isolated from 92.92 and 91.91% of the samples with infection rates varying from 0.50 to 85% and 1 to 99.50% respectively; (iii) Cladosporium and Penicillium contaminated 32.32 and 8.08% of the samples with infection rates of 0.5-7.00 and 0.50-54.50%, respectively.
Distribution of fungal strains in the three agro-ecological zones
The results show that all fungal isolates were detected in all of the three agro-ecological zones. Aspergillus of the sections flavi and nigri contained all of the samples regardless of the agro-ecological zone. Macrophomina phaseolina and Rhizopus spp. were detected in more than 80% of the samples in the three agro-ecological zones (Table 2). Cladoporium spp. and Penicillium spp. were isolated from less than 40% of the samples. The statistical analysis (ANOVA at 5% level) revealed a positive effect of the agro-ecological zone on the infection rates of several fungi (Table 2). Three strains including Aspergillus belonging to section flavi and section nigri and M. phaseolina presented significant differences in relation to their distribution in the three agro-ecological zones. Indeed, the mean infection rate of Aspergillus belonging to section flavi was significantly higher in the Sudanian zone (66.84%) than the Sahelian zone (57.96%); regarding the distribution of Aspergillus belonging to section nigri, the mean infection rate obtained in the Sahelian zone (49.34%) was significantly lower than that of the Sudanian (59.97%) and the Sudano-sahelian (56.98%) zones. As for M. phaseolina, its mean infection rate was significantly higher in the Sudanian zone (33.98%) compared to the other zones (25.48 and 21.68% for Sudano-sahelian and sahelian zones respectively). For a given fungus, infection rates with different letters are significantly different.
Characteristics of the isolated strains
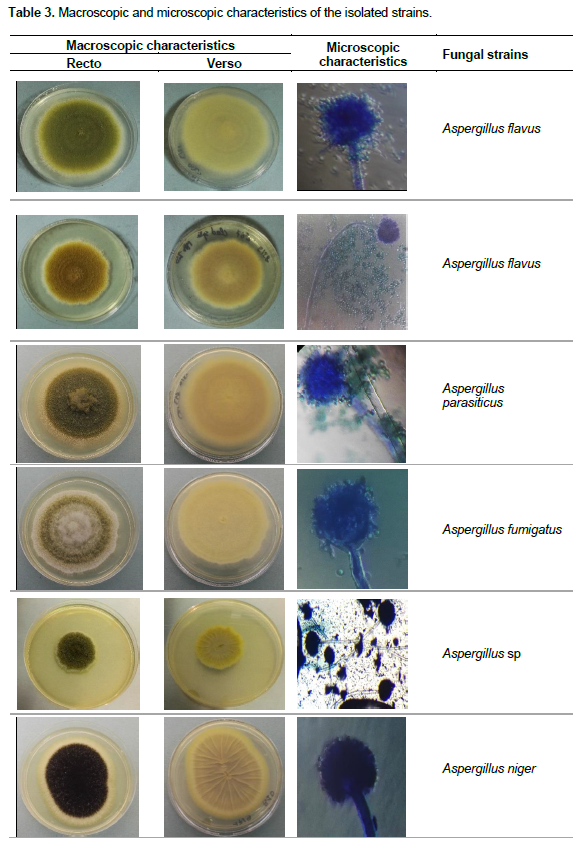
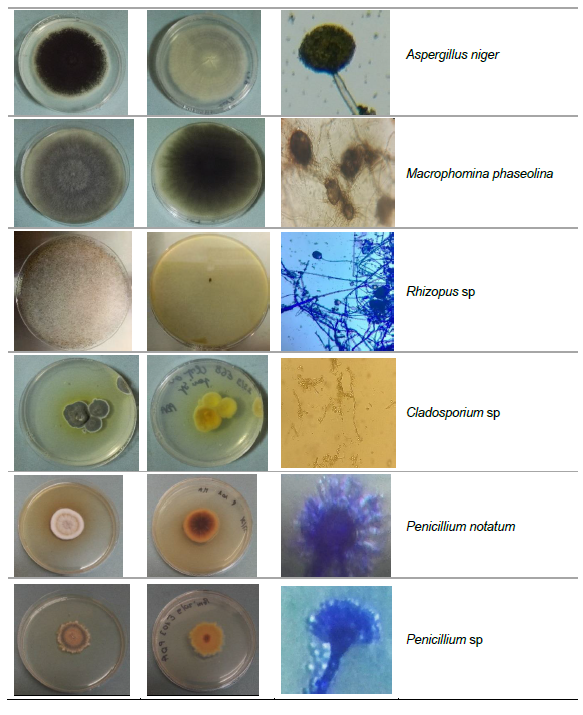
The fungal isolates were identified based on the determination of macroscopic (cultural) and microscopic (morphological) characteristics. The macroscopic identification was performed through the observation of the fungal colonies on specific media. The color, form, size, appearance and apical growth of each fungal strain were determined. The microscopic identification was mainly based on the determination of the morphological characteristics of the mycelium (presence/absence of septa, color, differentiation etc.) and spores (shape, color, wall texture etc.). The following fungal strains were identified: Aspergillus flavus, Aspergillus niger, Aspergillus parasiticus, Aspergillus fumigatus, Aspergillus sp., Macrophomina phaseolina, Rhizopus sp., Cladosporium sp., Penicillium notatum, Penicillium sp. (Table 3).
DISCUSSION
This study presents the contamination of Bambara groundnut seeds produced in Burkina Faso based on the determination of phenotypical characteristics. The post-harvest mycoflora contains species of the genus Aspergillus, Macrophomina, Rhizopus, Cladosporium and Penicillium. These fungi are very common in the soil and air through their spores (Abdoullahi et al., 2019)and frequently isolated from poorly stored dried food. Strains of the genus Aspergillus were isolated from all of the seed samples in the three agro-climatic zones with very high infection rates (66.84% for section flavi and 59.04% for section nigri). However, these values were higher in the Sudanian zone than in the Sahelian and Sudano-Sahelian zones. In fact, drying has always been a challenge for farmers. In some areas like the Sudanian zone of Burkina Faso where rainy season can last up to 6 months, frequent rain events can make the weather humid and the sky almost cloudy most of the time. As the sun is the main source of energy used for drying, insufficient light intensity in this area can last the drying time of the pods. As mentioned by Olagunju et al. (2018), insufficiently dried seeds are prone to mould, especially Aspergillus, and to rot during storage. In general, Aspergillus has a wide geographical distribution, but is more often associated with warm climate regions (Hedayati et al., 2007; Perrone et al., 2020). The optimal growing temperature for most Aspergillus species is between 25 and 40°C. For this reason, they grow very well in the so-called 'dry' food products like Bambara groundnut.
The predominance of genus Aspergillus in the mycoflora of Bambara groundnut seeds has been reported by previous studies (Shabangu, 2009). Our results are similar to those of Olagunju et al. (2018)who isolated the genus predominantly from Bambara groundnut seeds produced in South Africa, with infection rates of 52.5%. The high infection rates obtained from our study could be explained by the common presence of spores of Aspergillus in the air, soil and materials used to remove the pods. Hocking (2006)has also reported that fungi of the genus Aspergillus easily colonize food crops when storage conditions are not adequate; that could also explain their high presence. In addition, several studies conducted using other food samples including olives (Roussos et al., 2006), stored wheat (Belkacem-Hanfi et al., 2014), coffee bean (Djossou et al., 2015), dried fishes (Abdoullahi et al., 2019), groundnut kernels (Okayo et al., 2020), wheat and sorghum silages (del Palacio and Pan, 2020)and cassava (Ono et al., 2021)have revealed the presence of strains of Aspergillus. Besides the presence of these strains, aflatoxins B1, B2, G1, G2 and ochratoxins A were detected in the samples.
Species of the genus Penicillium are very common in soils, organic substances and food commodities. These fungi proliferate mainly during storage (Kpatinvoh et al., 2017). Penicillium was detected in all the agro-climatic zones with low infection rates. Its presence in Bambara groundnut seeds could be the result of inadequate storage conditions. Its low infection rates could be explained by the significant growth of fungi such as Aspergillus sections flavi and nigri, Rhizopus sp. and M. phaseolina which rapidly invade the seeds inhibiting the growth of other fungi. Indeed, these invasive fungi are able to colonize the environment in a very short period of time, considerably reducing the space necessary for the growth of other fungal species. Studies have highlighted the presence of Penicillium species in the seeds of Bambara groundnut (Olagunju et al., 2018)as well as in cowpea seeds during the storage (Kpatinvoh et al., 2017). Penicillium strains are responsible for food spoilage. In addition, several mycotoxins are produced by a variety of Penicillium species during food storage including cyclopiazonic acid (P. chrysogenum), penicillic acid (P. cyclopium), patulin or clavacin (P. expansum, P. griseofulvum), citrinin (P. expansum) and ochratoxin A (P. verrucosum) (Pitt, 2000)
Macrophomina conidia are common in soils (Iqbal et al., 2010). Since Bambara groundnut seeds develop underground, the fungus could colonize the pods, leading to infection. Its average infection rate was significantly higher in the Sudanian zone than in the Sahelian and Sudano-sahelian zones. This could be explained by the relatively high rainfall and humidity in this zone compared to the other zones. It can cause seeds to rot during storage, destruction of seedlings during emergence and complete wilting of the plant (Zida et al., 2008; Ouoba et al., 2017). Under high temperatures (30-35°C) and low soil humidity (below 60%), this fungus can cause substantial yield losses in crops such as soybean and sorghum (Kaur et al., 2012).
Fungi of the genus Rhizopus were detected in all three climatic zones with approximately equal average infection rates. Since Rhizopus is a common air- and soil-borne fungus, it can infect Bambara groundnut seeds during harvest and post-harvest operations. It can rapidly colonize decaying plants and fruits and cause soft rot in some crops with high humidity content such as sweet potato (Pang et al., 2021).
Fungi of the genus Cladosporium were also found in all three climatic zones at relatively low infection rates. These low values could be explained by the important competitive development of fungi such as Aspergillus section flavi and nigri, Rhizopus spp. and M. phaseolina. The genus includes common saprophytic, phytopathogenic and human pathogenic species. With their small conidia, usually formed in branched chains, they are well adapted to easily spread in large numbers over long distances. Some of the species are responsible for food spoilage while others can cause allergy or even plant or animal disease with sometimes significant environmental impact (Bensch et al., 2012). Studies have shown Cladosporium to be pathogenic through the appearance of lesions on inoculated leaves of Vicia faba (faba bean) (El-Dawy et al., 2021). The existence of the fungus in pods can cause capillary spread of the inner tissue, resulting in the formation of white felted spots extending into the pod cavity (El-Dawy et al., 2021).
The presence of these fungi in the seeds of Bambara groundnut can negatively impact its agricultural production and consumers’ health. Thus, the improvement of storage conditions to prevent seeds attacks by insects, rodents, fungi and their metabolites is necessary (Adetunji, 2007). The sorting and drying practices applied by farmers to have good quality seeds with low water content before storage should help reduce the risk of fungal infection of Bambara groundnut as suggested by Bankole and Adebanjo (2003). However, the high infection rates we obtained from the sun-dried seeds suggest the need for more research and investigations for adequate storage.
CONCLUSION
This study allows the determination of the major fungal strains associated with Bambara groundnut seeds in Burkina Faso and also highlighted the influence of the three agro-ecological conditions on the distribution of several post-harvest fungi. It showed that Bambara groundnut seeds produced in Burkina Faso are contaminated by a wide range of fungi, including species incriminated in human and plant pathology. The analysis of the infection rates and frequency of occurrence of the fungi showed a clear predominance of strains of the genus Aspergillus. These fungi could be the cause of mycotoxin production in the seeds, which are involved in human and animals’ diseases. Therefore, further studies should be conducted to test the mycotoxin production potential of these fungal isolates. In addition, a variety of potential pathogenic fungal species have been detected including Rhizopus sp. and M. phaseolina. The abundance and high frequency of these fungi in the samples can lead to a reduction in the nutritional value and the germinative capacity of the seeds. Inappropriate harvesting, transport, drying and storage techniques could explain the high presence of the fungal strains in the seeds. Therefore, initiatives to improve the storage techniques of Bambara groundnut seeds should be undertaken to limit their contamination and prevent post-harvest losses in order to contribute to food security.
CONFLICT OF INTERESTS
The authors have not declared any conflict of interest.
REFERENCES
|
Abdoullahi HO, Tidjani A, Sawadogo A, Tarnagda B, Abakar LI, Cissé H, Traoré Y, Savadogo A (2019). Isolement et caractérisation de souches fongiques à partir de poissons fumes/séchés du lac Fitri au Tchad. American Journal of Innovative Research and Applied Sciences 2(4):155-160. |
|
|
Adetunji MO (2007). Economics of Maize Storage Techniques by Fanners in Kwara State, Nigeria. Pakistan Journal of Social Sciences 4(3):442-445. |
|
|
Baddi M, Nassik S, Alali S, EL Hraiki A (2021). L'impact économique et sanitaire des mycotoxines entre aujourd'hui et demain. Revue Marocaine des Sciences Agronomiques et Vétérinaires 9(3):339-347. |
|
|
Bankole SA, Adebanjo A (2003). Mycotoxins in food in West Africa: current situation and possibilities of controlling it. African Journal of Biotechnology 2(9):254-263. |
|
|
Belkacem-Hanfi N, Fhoula I, Semmar I, Guesmi A, Perraud-Gaime I, Ouzari HI , Boudabous A, Roussos S (2014). Lactic acid bacteria against post-harvest moulds and ochratoxin A isolated from stored wheat. Biological Control 76:52-59. |
|
|
Bensch K, Braun U, Groenewald JZ, Crous PW (2012). The genus Cladosporium. Studies in Mycology 72:1-401. |
|
|
Botton B, Breton A, Fevre M, Gauthier S, Guy P, Larpent JP, Reymond P, Sanglier JJ, Vayssier Y, Veau P (1999). Moisissures utiles et nuisibles. Importance Industrielle. Masson, Paris. |
|
|
del Palacio A, Pan D (2020). Occurrence and toxigenic potential of Aspergillus section Flavi on wheat and sorghum silages in Uruguay. Mycology 11(2):147-157. |
|
|
Djossou O, Roussos S, Isabelle P G, Macarie H, Germain K, Yoan L (2015). Fungal population, including Ochratoxin A producing Aspergillus section Nigri strains from Ivory Coast coffee bean. African Journal of Agricultural Research 10(26):2576-2589. |
|
|
El-Dawy EGAEM, Gherbawy YA, Hussein MA (2021). Morphological, molecular characterization, plant pathogenicity and biocontrol of Cladosporium complex groups associated with faba beans. Scientific Reports 11(1):1-13. |
|
|
Food and Agricultural Organization of the United Nations (FAO) (2010). Reducing post-harvest losses in grain supply chains in Africa: Lessons learned and practical guidelines. FAO/World Bank Work. FAO Headquarters, Rome, Italy. 18-19 March 2010. |
|
|
Hedayati MT, Pasqualotto AC, Warn PA, Bowyer P, and Denning DW (2007). Aspergillus flavus: human pathogen, allergen and mycotoxin producer. Microbiology 153(6):1677-1692. |
|
|
Hocking AD (2006). Aspergillus and related teleomorphs. Food Spoilage Microorganisms pp. 451-487. |
|
|
Iqbal J, Jilani G, Aslam M (2010). Growth inhibiting effects of plant extracts against the grain moth, Sitotroga cerealella (Oliv.) (Gelechiidae: Lepidoptera). Pakistan Journal of Zoology 42(5):597-601. |
|
|
Kaur H, Ganguli D, Bachhawat AK (2012). Glutathione degradation by the alternative pathway (DUG pathway) in Saccharomyces cerevisiae is initiated by (Dug2p-Dug3p)2 complex, a novel glutamine amidotransferase (GATase) enzyme acting on glutathione'. Journal of Biological Chemistry 287(12):8920-8931. |
|
|
Kpatinvoh B, Adjou ES, Edwige DA, Konfo TRC, Atrevi B, Soumanou MM, Sohounhloue DCK (2017). Efficacité des huiles essentielles de trois plantes aromatiques contre la mycoflore d'altération du niébé (Vigna unguiculata L., Walp) collecté dans les magasins de vente du Sud-Bénin. Journal of Applied Biosciences 109(1):10680-10687. |
|
|
Marasas WFO, Jaskiewicz K, Venter VJ, Van Schalkwyk DJ (1988). Fusarium moniliforme contamination of in oesophageal cancer areas in Transkei. South African Medical Journal 74(3):110-114. |
|
|
Mathur SB, Kongsdal O (2003). Common laboratory seed health testing methods for detecting fungi. First edition. International Seed Testing Association, P.O. Box 308, 8303 Bassersdorf, CH-Switzerland 425 p. |
|
|
Njoroge AW, Baoua I and Baributsa D (2019). Postharvest Management Practices of Grains in the Eastern Region of Kenya. Journal of Agricultural Science 11(3):33-42. |
|
|
Okayo RO, Andika DO, Dida MM, K'Otuto GO, Gichimu BM (2020). Morphological and Molecular Characterization of Toxigenic Aspergillus flavus from Groundnut Kernels in Kenya. International Journal of Microbiology 10 p. |
|
|
Olagunju O, Mchunu N, Durand N, Alter P, Montet D, Ijabadeniyi O (2018). Effect of milling, fermentation or roasting on water activity, fungal growth, and aflatoxin contamination of Bambara groundnut (Vigna subterranea (L.) Verdc). LWT-Food Science and Technology 98:533-539. |
|
|
Ono LT, Silva JJ, Doná S, Martin LM, Iamanaka BT, Fungaro MHP, Pitt JI, Taniwak MH (2021). Aspergillus section Flavi and aflatoxins in Brazilian cassava (Manihot esculenta Crantz) and products. Mycotoxin Research 37(3):221-228. |
|
|
Ouedraogo M, Ouedraogo TJ, Tignere BJ, Didier B, Dabire BC, Konate G (2008). Characterization and evaluation of accessions of Bambara groundnut. Sciences and Nature 5(2):191-197. |
|
|
Ouoba A, Ouedraogo M, Sawadogo M, Nadembega S (2016). Aperçu de la culture du voandzou (Vigna subterranea (L.) Verdcourt) au Burkina Faso: enjeux et perspectives d'amélioration de sa productivité. International Journal of Biological and Chemical. Sciences 10(2):652-665. |
|
|
Ouoba A, Zida PE, Soalla W R, Bangratz M, Essowè P, Konaté MN, Nandkangré H, Ouédraogo M and Sawadogo M (2019). Molecular characterization of the main fungi associated to Bambara groundnut foliar diseases in Burkina Faso. Journal of Applied Biosciences 133(1):13574. |
|
|
Ouoba A, Zida SF, Ouédraogo M, Nandkangré H, Ouédraogo HM, Nanéma RK, Sawadogo N, Zida EP, Konaté MN, Congo AK, Soalla RW, Sawadogo M (2017). Assessment of genetic diversity in Bambara groundnut (Vigna subterranea (L.) Verdcourt) landraces in Burkina Faso using microsatellite markers (SSR). Agricultural Science Research Journal 7(3):96-100. |
|
|
Pang LJ, Adeel M, Shakoor N, Guo KR, Ma DF, Ahmad MA, Lu GQ, Zhao MH, Li SE, Rui YK (2021). Engineered nanomaterials suppress the soft rot disease (Rhizopus stolonifer) and slow down the loss of nutrient in sweet potato. Nanomaterials 11(10):1-16. |
|
|
Pereira E, Santos A, Reis F, Tavares RM, Baptista P, Lino-Neto T, Almeida-Aguiar C (2013). A new effective assay to detect antimicrobial activity of filamentous fungi. Microbiological Research 168 (1):1-5. |
|
|
Perrone G, Ferrara M, Medina A, Pascale M, Magan N (2020). Toxigenic fungi and mycotoxins in a climate change scenario: Ecology, genomics, distribution, prediction and prevention of the risk. Microorganisms 8(10):1-20. |
|
|
Pitt JI (2000). Toxigenic fungi: which are important? Medical Mycology 1(1):17-22. |
|
|
Pitt JI, Hocking AD (1997). Fungi and Food Spoilage, 2nd Edition. Chapman and Hall, Cambridge P 593. |
|
|
Roussos S, Zaouia N, Salih G, Tantaoui-Elaraki A, Lamrani K, Cheheb M, Hassouni H, Verhé F, Perraud-Gaime I, Augur C and Ismaili-Alaoui M (2006). Characterization of filamentous fungi isolated from Moroccan olive and olive cake: Toxinogenic potential of Aspergillus strains. Molecular Nutrition and Food Research 50(6):500-506. |
|
|
Samson RA, Hoekstra ES, Frisvad JC, Filtenbor O (Eds.) (1996). Introduction to Food-Borne Fungi. Centraal Bureau voor Schimmelcultures, Baarn. |
|
|
Shabangu N (2009). The occurrence of fungi and their mycotoxins in maize and bambara nuts and their effects on the health of the rural community in areas of Limpopo province. (MTech), University of Johannesburg, Johannesburg. |
|
|
Zida PE, Sérémé P, Leth V, Sankara P, Somda I, Néya A (2008). Importance of seed-borne fungi of sorghum and pearl millet in Burkina Faso and their control using plant extracts. Pakistan Journal of Biological Sciences 11(3):321-331. |
|
Copyright © 2024 Author(s) retain the copyright of this article.
This article is published under the terms of the Creative Commons Attribution License 4.0