ABSTRACT
Low-viscosity clear banana juice is traditionally produced using a rudimentary mechanical process of kneading a mixture of ripe high-tannin banana and grass. The aim of this study was to come up with a new efficient and hygienic process that does not require use of grass. According to a new process, peeled ripe high-tannin Pisang Awak banana fingers were mashed in a blender, without addition of grass, until pulp agglomeration occurred. The mashed pulp was pressed to separate clear banana juice. The process successfully produced clear banana juice with low-viscosity (1.85 × 10-3 Pa.s), high content of dissolved solids (27 - 28°Brix), and average density of 1120 kg.m-3. Juice yield increased with mashing time up to 60% (w/w), and degree of ripeness until fruit colour was mostly yellow, but juice extraction failed for overripe banana. Condensed tannins decreased with ripening and juice extraction was possible as long as condensed tannin concentration was above 0.68% (w/w) of peeled banana. Therefore, low-viscosity clear banana juice can be produced in a more hygienic condition using the new process.
Key words: Clear banana juice, Pisang Awak, mechanical banana juice extraction, condensed tannins.
Extraction of low-viscosity clear banana juice has always been a challenge. While a standard process of preparing many varieties of low-viscosity fruit juices involves pulping and pressing resulting pulp, the process results in a viscous puree when applied to ripe banana. A number of methods for obtaining low-viscosity clear banana juice such as enzymatic maceration of ripe banana pulp, hot water extraction, as well as a combination of the two have been investigated (Lee et al., 2006; Surendranathan et al., 2003; Minatchy et al., 2007). In most cases banana juice extracted using enzymatic or hot water extraction methods is cloudy, and the processes are expensive due to high cost of enzymes and energy requirement (Byarugaba-Bazirake, 2008; Kyamuhangire et al., 2002; Surendranathan et al., 2003).
However, low-viscosity clear banana juice has been extracted using indigenous mechanical method for several centuries, and the process has remained virtually the same in Eastern and Central African countries (Kyamuhangire, 1990; Kyamuhangire et al., 2002; Kyamuhangire and Pehrson, 1999; Kasozi and Kasisira, 2005). The indigenous extraction process entails kneading by hands or feet a mixture of ripe banana fingers and grass, usually spear grass (Imperata cylindrica), until pulp gets entangled within grass matrix, allowing clarified juice to separate from pulp (Kasozi and Kasisira 2005; Kyamuhangire et al. 2002; Wilson et al. 2012). There have been a few improvements to the indigenous process: grass has been replaced by plastic fibres, hand and foot have been replaced by dough kneading machine or new mechanical systems (Kyamuhangire et al. 2002; Kasozi and Kasisira 2005; De Beer and Sigawa 2008). The improvements have not eliminated grass or fibres used during juice extraction.
Mechanism for releasing low-viscosity banana juice remains unclear and is more complicated than simple mastication and pressing. Apparently, low-viscosity banana juice can be extracted from high tannin content bananas (Gensi et al., 1994; Kyamuhangire and Pehrson, 1999; Kyamuhangire et al., 2002), most of which are East African Highland Banana (EAHB) (Musa AAA-EA genotype) and Pisang Awak (Musa ABB genotype) (Kyamuhangire and Pehrson, 1999; Kyamuhangire et al., 2006). Tannins, in particular condensed tannins are known to form insoluble complexes with protein and polysaccharides (McManus et al., 1981; McManus et al., 1985; Hagerman and Butler, 1981; Obreque-Slier et al., 2012; Ozdal et al., 2013; Naumann et al., 2014; Bilgener, 2015). In a microstructure study, juice-producing bananas were found to contain more and larger tannin laticifers embedded within their pulp than non-juice-producing banana (Kyamuhangire et al., 2006). In light of the above, high tannin content in banana was identified as a key factor for the mechanical extraction of low-viscosity clear banana juice. Hence, it was hypothesised that releasing tannins from laticifers and mixing it with the rest of the pulp could facilitate formation of tannin-protein insoluble complexes and consequently juice separation from the pulp. Objective of this study was to extract low-viscosity clear banana juice by mashing ripe peeled Pisang Awak, so as to split and release tannins from laticifers, and mix it with proteins and pectin in order to facilitate formation of insoluble aggregate, resulting in juice separation without using extraction aids such as grass, fibres or enzymes.
Mature Pisang Awak bananas were purchased from a local market in Dar es Salaam, Tanzania and ripened at ambient temperature in an open space until colour of banana finger turned black within 5 days in a Food Laboratory, Department of Chemical and Mining Engineering, University of Dar es Salaam, Tanzania. Chemical reagents used in this study were of analytical grade from Carlo Erba, Milano, Italy.
Juice preparation
Ripe banana fingers were peeled, sliced to approximately 3 cm thick discs. A batch of sliced banana weighing 300 g, was mashed in a blender (Russell Hobbs, Johannesburg, South Africa). A prolonged mashing was carried out while observing for changes of flow characteristics of pulp. Once sliced banana changed to homogeneous viscous puree, followed by a mixture of semi-solid pulp and low-viscosity clear banana juice, the mixture was removed from blender, wrapped in a cotton cloth and hand-squeezed to separate the juice. Juice volume and weight were recorded using a 250 mL measuring cylinder and analytical balance, respectively. Degree of ripeness of banana was visually assessed in terms of colour of unpeeled banana fingers and in terms of firmness of peeled banana fingers using fruit penetrometer (GY-3, Yueqing Handpi Instruments Co., Ltd., Yueqing, Zhejiang, China). Effect of mashing time on juice yield was assessed using bananas whose ripeness was mostly yellow, a degree of ripeness that had been identified to provide the maximum juice yield. Effect of degree of ripeness on juice yield was assessed by mashing banana of various degree of ripeness for 20 min, an extraction time that had been identified to provide more than 98% of the maximum juice yield. Juice dissolved solids were measured with a temperature compensation hand-held refractometer (MT-032ATC, Three-In-One Co., New Taipei City, Taiwan), with a measuring range of 0 to 32° Brix and 0.2% accuracy.
Tannin assay
Condensed tannins in ripe banana, spent pulp and juice were determined according to dry sample Butanol-HCl assay by Makkar (2003), with modification to accommodate wet samples. Condensed tannins were extracted by grinding 200 mg wet samples of fresh banana and spent pulp with laboratory ceramic mortar while slowly adding up to 5 mL of 70% aqueous acetone. The mortar was rinsed with up to 5 mL of 70% aqueous acetone and the mixture was sonicated in a 50 mL beaker suspended in chilled water (10°C) at 30% amplitude and 20 kW for 20 min with ultrasonic processor, UP200St (Hielscher, Teltow, Germany). Banana juice was directly used as a tannin extract. Extract contents were transferred to test tubes, cooled on ice and centrifuged for 10 min at 3000 g with Axiom 800 (Axiom, Bürstadt, Germany). From the tannin extract 6 mL was transferred into a test tube, followed by 3 mL of Butanol-HCl (butanol-HCl 95:5 v/v) and 0.1 mL Ferric reagent (2% ferric ammonium sulphate in 2N, HCl). The tube was vortexed to ensure proper mixing, covered with Teflon-lined plastic screw cup and placed in a boiling water bath for 60 min. For each sample a blank of unheated tannin extract and reagent mixture was prepared. The tube was cooled to room temperature and absorbance was measured at 550 nm using Milton Roy Spectronic 21D spectrophotometer. The absorbance of corresponding blank was subtracted from sample absorbance to eliminate absorbance of unheated mixture. Condensed tannins as leucocyanidin equivalent were calculated using equation (1) Makkar (2003):

Where dilution factor = 0.5 mL/(Volume (in mL) of extract used for assay). Condensed tannins were assayed twice (morning and evening) a day.
Low-viscosity clear banana juice was successfully extracted without using grass, fibres or enzymes. During mashing, sliced banana fingers formed a creamy homogenous viscous puree; with prolonged mashing, the puree was transformed to a semi-solid pulp of two mixed phases: Insoluble agglomerates and a crystal clear liquid juice. Transformation was observed within 5 to 35 min of mashing depending on degree of ripeness. The juice oozed out when the mixture was left undisturbed. Fast juice separation was achieved by manually squeezing the mixture in a clean cotton cloth. Pressing pulp/puree that had not gone through a transformation to the two phases did not release the juice. Properties of the juice are indicated in Table 1. Mashing time and degree of ripeness affected juice yield and overall juice extractability depended on content of condensed tannins in the banana.
Effect of mashing time on juice yield
Effect of mashing time on juice yield was investigated when banana had attained a complete yellow colour, which was equivalent to a fruit firmness of 1.8 × 105 N.m-2. Two phase formation phenomenon accompanied by juice separation commenced within 5 min of mashing (Figure 1). Prolonged mashing resulted in higher juice yield which reached 59% (w/w) after 20 min and 60% (w/w) after 35 min. Kyamuhangire et al. (2002) obtained juice yield of 54.1% after 20 min from Pisang Awak mixed with plastic fibre using dough mixer. De Beer and Sigawa (2008) reported 50% juice yield from Pisang Awak and Kasozi and Kasisira (2005) reported much lower juice yield of 31%. In both cases, mechanical juice extractors were employed. Juice yield was comparable to 55.6 to 64.8% (w/w) for juice produced by mechanical and enzymatic method from Pisang Awak (Kyamuhangire et al., 2002; Byarugaba-Bazirake, 2008).
Effect of degree of ripeness on juice yield
Degree of ripeness was measured in terms of fruit firmness. Within five days or ripeness, fruit firmness decreased from 3.5 × 105 N.m-2 for greenish yellow banana to 0.9 × 105 N.m-2 for overripe, black-coloured banana (Figure 2).
Juice yield increased with degree of ripeness (that is, with decreasing fruit firmness) from 34% (w/w) to maximum yield of 58% as fruit firmness decreased from 3.5 × 105 N.m-2 to 1.2 × 105 N.m-2, respectively as shown in Figure 3. Thereafter, juice yield decreased with fruit firmness and extraction failed when fruit firmness reached 8.5 × 106 N.m-2. Maximum juice yield was obtained when banana fingers were mostly yellow, a ripeness stage that coincided with a release of strong ripe banana sweet flavour.
Variation of condensed tannins and their effect on juice extraction
Condensed tannin, a necessary fruit component for mechanical extraction of banana juice was monitored in peeled banana fingers, spent pulp and juice, and expressed as weight percentage of peeled banana, within fruit firmness of 1.3-0.77 × 105 N.m-2 as shown in Figure 4. Based on the method used, condensed tannins decreased with ripening from 1.09 to 0.68% in peeled banana fingers and 0.57 to 0.25% in spent pulp, but increased in juice from 0.12-0.27%. Decrease of tannins with ripening happens in many fruits and is known to be a result of polymerisation of tannins (Barnell and Barnell, 1945; Goldstein and Swain, 1963; Obreque-Slier et al., 2012). As condensed tannins diminish, so does the extent of tannin-protein complex formation, hence undesirable for mechanical extraction of banana juice. This explains why juice extraction failed when condensed tannins decreased below 0.68% in peeled banana. A similar trend of higher tannins in spent pulp than in juice was observed by Kyamuhangire et al. (2006)during mechanical extraction of banana juice using a dough mixer.
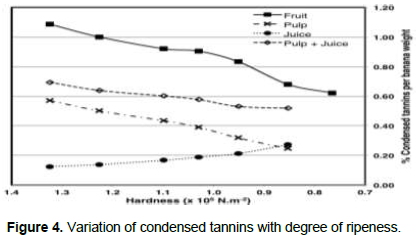
Higher quantities of tannins in spent pulp than in juice tend to support a hypothesis that condensed tannins are involved in formation of insoluble tannin-protein/polysaccharide complexes resulting in juice release. It was not clear why condensed tannins increased in banana juice with ripening; the matter remains the subject for further study. Quantity of condensed tannins in banana fingers was higher than corresponding combined quantities in the spent pulp and the juice. Kyamuhangire et al. (2006), using a different tannin quantification method, obtained similar results whereby tannin quantity in Pisang Awak fruit was more than combined quantities of tannins in spent pulp and juice. The difference is likely due to acceleration of natural tannins reduction process that is usually slow during fruit ripening.
The new banana juice extraction method is better and superior than the indigenous banana juice extraction technology and its mechanical variants. It is now possible to extract low-viscosity clear banana juice exclusively by a mechanical method without addition of grass or fibres. The mechanical banana juice extraction process is possible only if there were sufficient condensed tannins in banana. Mechanism for tannin-protein/polysaccharide interaction during mechanical banana juice extraction will be a subject for future study.
The authors have not declared any conflict of interests.
The authors are grateful to Lake Victoria Research Initiative (VicRes) under Inter-University Council of East Africa and Sida of Sweden for funding the research.
REFERENCES
|
Barnell H, Barnell E (1945). Studies in tropical fruits: XVI. The distribution of tannins within the banana and the changes in their condition and amount during ripening. Ann. Bot. 9(33):77-99.
Crossref
|
|
|
|
Bilgener M (2015). Kinetics of tannin binding with natural polymers-1 overview.
|
|
|
|
|
Byarugaba-Bazirake GW (2008). The effect of enzymatic processing on banana juice and wine. PhD Thesis, Stellenbosch University, South Africa.
|
|
|
|
|
De Beer Z, Sigawa A B (2008) Juice Production in South Africa. In International Conference on Banana and Plantain in Africa: Harnessing International Partnerships to Increase Research Impact 879:233-238.
|
|
|
|
|
Gensi R, Kyamuhangire W, Carasco J (1994). Traditional production and characteristics of banana juice in Uganda. In African Crop Science Conference Proceedings, Kampala Uganda, 1993. 1, 356-359).
|
|
|
|
|
Goldstein JL, Swain T (1963). Changes in tannins in ripening fruits. Phytochemistry 2(4):371-383.
Crossref
|
|
|
|
|
Hagerman AE, Butler LG (1981). The specificity of proanthocyanidin-protein interactions. J. Biol. Chem. 256(9):4494-4497.
|
|
|
|
|
Kasozi G, Kasisira LL (2005) Design and performance of a banana juice extractor. In African Crop Science Conference Proceedings, 7:1381-1384.
|
|
|
|
|
Kyamuhangire W (1990). Banana juice extraction and processing. MSc Thesis, Univesrity of New South Wales, Australia.
|
|
|
|
|
Kyamuhangire W, Krekling T, Reed E, Pehrson R (2006). The microstructure and tannin content of banana fruit and their likely influence on juice extraction. J. Sci. Food Agric. 86 (12):1908-1915.
Crossref
|
|
|
|
|
Kyamuhangire W, Myhre H, Sørensen HT, Pehrson R (2002). Yield, characteristics and composition of banana juice extracted by the enzymatic and mechanical methods. J. Sci. Food Agric. 82(4):478-482.
Crossref
|
|
|
|
|
Kyamuhangire W, Pehrson R (1999). Conditions in banana ripening using the rack and pit traditional methods and their effect on juice extraction. J. Sci. Food Agric. 79(2):347-352.
Crossref
|
|
|
|
|
Lee W, Yusof S, Hamid N, Baharin BS (2006). Optimizing conditions for hot water extraction of banana juice using response surface methodology (RSM). J. Food Eng. 75(4):473-479.
Crossref
|
|
|
|
|
Makkar HP (2003). Quantification of tannins in tree and shrub foliage: A laboratory manual. Kluwer Academic Publishers: Dordrecht, The Netherlands.
Crossref
|
|
|
|
|
McManus JP, Davis KG, Beart JE, Gaffney SH, Lilley TH, Haslam E (1985). Polyphenol interactions. Part 1. Introduction; some observations on the reversible complexation of polyphenols with proteins and polysaccharides. J. Chem. Soc. Perkin Trans. 2(9):1429-1438.
Crossref
|
|
|
|
|
McManus JP, Davis KG, Lilley TH, Haslam E (1981). The association of proteins with polyphenols. J Chem Soc. Chem. Comm. (7):309 -311.
|
|
|
|
|
Minatchy N, Escudier JL, Mikolajczak M (2007). Method for obtaining banana-derived products, in particular for liquefying banana to obtain pure juice.
|
|
|
|
|
Naumann H, Hagerman A, Lambert B, Muir J, Tedeschi L, Kothmann M (2014). Molecular weight and protein-precipitating ability of condensed tannins from warm-season perennial legumes. J. Plant Interact 9(1):212-219.
Crossref
|
|
|
|
|
Obreque-Slier E, Pe-a-Neira Á, López-Solís R (2012). Interactions of enological tannins with the protein fraction of saliva and astringency perception are affected by pH. Food Sci. Technol-LEB, 45(1):88-93.
|
|
|
|
|
Ozdal T, Capanoglu E, Altay F (2013). A review on protein–phenolic interactions and associated changes. Food Res. Intl. 51(2):954-970.
Crossref
|
|
|
|
|
Surendranathan K, Ramaswamy N, Radhakrishna P, Nair J (2003). Value added products from ripe banana: Banana juice and ripe banana powder. BARC, Founders Day Special Issue.
|
|
|
|
|
Wilson P, David T, Sam B (2012). Microbial and biochemical changes occurring during production of traditional Rwandese banana beer "Urwagwa". Ferment Technol. 1(3):104.
Crossref
|
|