ABSTRACT
Ready-to-eat (RTE) meats are products sold to consumers which do not require significant further processing except re-heating or completion of cooking process. These meats may constitute a likely potential hazard to human health due to non-compliance with food safety regulations by food handlers. This study was aimed at evaluating the bacteriological safety of RTE roasted meats sold by selected food vendors in Lukaya and Najembe highway markets. Bacteriological analyses were conducted on 20 samples for each of the three meat products which included chicken, beef and goat meat during dry and wet seasons. ISO standard methods were used in the laboratory to test for presence of coliforms, Escherichia coli and Staphylococcus aureus. Most samples (68.3%) were contaminated and exceeded the recommended microbial contaminant limit (MCL). S. aureus was high in beef and chicken where it appeared in 85% of the samples for each product. S. aureus was also in 75% of goat meat samples. E. coli was high in chicken (50%), followed by beef (45%) and goat meat (35%) samples. Contamination was slightly higher in the wet season. S. aureus was the main contaminant. Most RTE meats that are sold in highway markets were highly contaminated. This result should draw the attention of relevant authorities to ensure that adequate hygienic standards and regular monitoring of the quality of RTE meats are improved and practiced to avoid possible foodborne infections.
Key words: Ready-to-eat meats, contamination, coliforms, Staphylococcus aureus, highway markets.
Due to the vital role of food in human existence, it is imperative to maintain high level of food safety in order to ensure that human beings are safe from diseases or other related health hazards associated with food (Adolf and Aziz, 2012). The food vending industry plays a very important role in meeting food requirements of travelers and local dwellers in Uganda as is the case in many developing countries. It feeds thousands of people daily, with a large range of foods that are relatively cheap and easily accessible (Tambekar et al., 2008). However, food borne illnesses of microbial origin are a major health risk associated with vended foods (Mensah et al., 2002). The American CDC reported that about 77% of food poisoning occurs in restaurants, 20% in homes and 3% from commercial foods relating to non-compliance with food standards and secondary pollution (Tavakoli, 2008).
RTE foods can be defined as foods and beverages prepared and/ or sold by vendors on the street and in other public places for immediate consumption or consumption at a later time without further processing or preparation (Cho et al., 2011; Tsang, 2002). Due to socio-economic changes characterized by increased mobility, resulting in more RTE foods taken outside the home, food vendors’ services are on the increase and responsibility for the food safety have been transferred from individuals/families to the food vendors who rarely enforce good manufacturing practices (Musa and Akande, 2002). Diseases that result from foods are one of the major health problems in developing and developed countries (Razavilar, 2010). Conditions of food safety include efforts to avoid contamination from biological, chemical agents and other substances that can endanger human health (Adolf and Aziz, 2012).
Microbiological food contamination refers to the presence in food of harmful microorganisms which can cause illness (Ahmad et al., 2013). Microbiological safety of food involves assurance that food will not cause microbial harm to the consumer when it is prepared and/or eaten according to its intended use (O’Brien, 2008). There are many risk factors in food vending markets that potentially expose food to microbial contamination. The traditional processing methods that are used in the preparation of vended food, inappropriate holding temperature and poor personal hygiene of food handlers are some of the main causes of contamination of RTE foods (Mensah et al., 2002).
In Uganda, highway vended foods are mostly prepared and sold to travelers at various highway markets by the roadsides. Despite the high potential for outbreaks of food borne illnesses associated with food vending such as cholera, diarrhea, hepatitis and dysentery; improved food safety systems have not been widely implemented in Uganda’s food vending markets which raises concern about the role food vending plays in food poisoning (Sebudde et al., 2012). Street foods are frequently linked with gastrointestinal diseases such as diarrhea and typhoid fever due to improper handling and serving practices (Barro et al., 2006; Tambekar et al., 2011). According to Nkere et al. (2011), poor environmental sanitation is largely responsible for much of the contamination and poor personal hygiene among the food handlers. These bacteria can come in contact with the foods when they are prepared especially in unhygienic environments and contaminated cooking utensils (Shamsuddeen and Ameh, 2008; Kawo and Abdulmumin, 2009).
Gastrointestinal illnesses continue to be a serious public health challenge in Uganda (Muyanja et al., 2011). At least 1.4 million Ugandans are diagnosed with food borne illnesses in Uganda annually (MOH, 2012). This represents 14% of all diseases treated each year (UBOS, 2011). However, this figure is definitely very low for Uganda considering that majority of illnesses in developing countries is treated outside hospitals (at home) and is not recorded at the health centers (MOH, 2013). Therefore, good estimates could put the food borne illnesses beyond 95% of all illnesses in Uganda. The Uganda Demographic Health survey of 2011 resported that 6 in every 10 households experiences a diarrhea episode every month and over 72% of these are attributed to consumption of contaminated food that is accessed from different sources (UBOS, 2011). Markets could be one of the potential sources of microbial contamination. For example in 2013 the Ministry of Health in Uganda reported that 1,357,165 Ugandans were diagnosed with acute diarrhea attributed to consumption of contaminated food. This represented 4.1% of the total population in Uganda and the 5th largest illness diagnosed. In addition, intestinal worms which are also attributed to consumption of contaminated food contribute 5.5% to the disease burden in Uganda. This means that over 2,403,712 Ugandans suffer from intestinal worms annually (MOH, 2013). Cholera and dysentery are also reported by the ministry of health among gastrointestinal disorders that contribute over 730,973 cases every year (MOH, 2012).
According to Karamaji (2012), millions of Ugandans suffer from food poisoning annually. For example in 2012, WHO recorded cholera epidemic in 6 districts across the country. A cumulative figure of 358 cases with 18 deaths were recorded in 5 of the 6 districts. In one district Kasese (in western region) alone, a cumulative figure of 366 cases including 10 deaths were recorded (WHO, 2012). Bwire et al. (2013) noted that Uganda has reported cholera cases to the World Health Organization every year since 1997. They estimated that an average of about 61 to 182 deaths occur in Uganda each year. All these cases have been mainly attributed to eating contaminated food. At the beginning of 2015, a typhoid outbreak was reported in central region of Uganda in which 1940 suspected cases were reported (WHO, 2015). In 2010, there was an outbreak of dysentery in one district (of Kanungu) located in South Western region of Uganda where 12 people were affected (URN, 2010). All these outbreaks are an indicator of the gastrointestinal frequencies and a clear demonstration of the food contamination problem in Uganda.
Several studies showed that different pathogens have been isolated from RTE foods in different countries which include: Staphylococcus aureus, Escherichia coli, Salmonella sp., Shigella sp., Klebsiella sp., Pseudomonas sp., Vibrio sp., Campylobacter sp. and Listeria monocytogenes (Adolf and Aziz, 2012; Tavakoli, 2008; Tambekar et al., 2010, Oghene et al., 2014; Makelele et al., 2015; Akusu et al., 2016). The aforementioned observations confirm the risk posed by consuming these vended foods. According to Codex Alimentarius Commission (CAC) classification, cooked RTE foods are among the high risk foods (Tavakoli, 2008). Similarly, Stewart and Humphrey (2002) attributed the cases of food infection and intoxication to poor and inadequate sanitary condition observed in processing of many locally made foods. E. coli and S. aureus are commonly associated with poor hygiene and sanitation and are usually implicated in the outbreak of food borne illnesses (Odu, 2013). S. aureus is capable of producing a highly heat stable protein toxin that causes illness in humans. Onset of symptoms of food poisoning occurs between 1 and 7 days, usually 2 to 4 h after the ingestion of food containing staphylococcal enterotoxins (ICMSF, 2011). The most common symptoms are nausea, vomiting, retching, abdominal cramps and diarrhoea. In severe cases, headache and collapse may occur.
RTE meats are especially a concern since these may be consumed without further cooking and are known to be good growth substrates for pathogenic microorganisms (Zhu et al., 2005). Ensuring good quality raw materials, adequate lethality treatment and effective sanitation of both the equipment and processing environment are crucial in preventing contamination of RTE meats. The presence of pathogens on surfaces of equipment or the environment particularly in post-cooking areas, serves as one of the most important routes for contamination of RTE meats (Zhu et al., 2005). These conditions do exist in Uganda because the state of sanitation and hygiene in Ugandan highway markets is poor. The vendors in these markets lack inadequate cleaning and sanitization materials. They have insufficient knowledge about good hygiene practices. They also lack adequate protective gear such as mouth covers, hair/beard nets and aprons to enhance their personal hygiene. Most highway markets have inadequate hand washing facilities (Winnie, 2005). There are insufficient personnel and therefore a situation of counting money by food handlers during food display and service cannot be avoided. The vendors lack adequate requisite materials and awareness to cover wounds during food handling. The markets do not have adequate latrine/toilet facilities, waste disposal pits/bins and sewage systems. The vending stalls and food storage facilities are also poor(ULRC, 2013). This could expose the meats that are sold in these markets to contamination from E. coli and S. aureus which are associated with poor sanitation and hygiene.
This study was conducted to evaluate the bacteriological contamination of street-vended RTE roasted meats in highway markets in Uganda. The work will benefit the unsuspecting consumers, government health agencies and the vendors on minimizing any health risk such food might pose.
Description of the study area
The study was conducted in Lukaya and Najembe highway markets. Najembe market is located approximately 45 km on Kampala-Jinja highway in the central region district of Buikwe leading to the eastern part of Uganda; and on-ward to Kenyan border. Lukaya market is located approximately 100 km on Kampala-Masaka highway in the central region district of Kalungu leading to the southern and western parts of Uganda; and on-ward to the Rwandan and Democratic Republic of Congo (DRC) borders. These markets were also selected for the study because they had a large population of vendors when compared to other highway markets.
Najembe and Lukaya markets have populations of about 320-350 and 350-400 vendors respectively, who are involved in the sale of various foods. Their main activities include mainly roasting and selling of RTE food stuffs to travellers both leaving out of, and coming into Kampala city, Uganda. The food products sold include meats such as chicken, beef and goat meat. Other RTE products sold include cassava, potatoes, plantain “gonjja”, chapattis, fruits, vegetables, and drinks such as water, soda and fruit juice.
Selection of products and parameters studied
The study concentrated on high risk RTE products that are sold in highway markets. The high risk foods (chicken, beef and goat meat) were selected basing on the U.S. Food and Drug Administration (FDA) guidelines for identifying high risk foods (FDA, 2013).
Sample collection
Sample collection equipment
A sample collection kit containing an insulated cooler box that was sterilized with ethanol (70%) 10 ice bags and 60 sterile stomacher bags were used to collect and preserve the samples during transportation to the laboratory.
Vendor selection and sample purchase
A total of sixty (60) samples of chicken, beef and goat meat were randomly procured from 30 vendors, in Najembe and Lukaya markets (typically 15 vendors from each market). Twenty (20) samples of each vended meat product (chicken, beef and goat meat) were procured from both markets where 10 samples of chicken, 10 samples of beef and 10 samples of goat meat were obtained each from Najembe and Lukaya markets. Samples were obtained at two separate times, one during the dry season (February 2014) and the other in the wet season (late March 2014); where 5 samples for each food product were obtained in each sampling session from each of the markets. Each market was visited twice and samples of the second visit (wet season) were picked from the same vendors that had provided the samples
during the first visit (dry season). The actual samples were picked randomly from the roasted meats on display for sell/service to customers since these were regarded as RTE meats/products that required no further processing or preparation. Manual collection was used to pick roasted chicken, beef and goat meats that were found on roasting sticks of selected vendors in the highway markets.
Packaging and delivery
Each category of the procured products was packaged separately. Details of sample history were taken. The sample history captured covered the details on means of delivery of raw food stuffs, time taken after delivery of raw meat before roasting the meat, storage conditions, roasting method, status of water used, time taken by meat out of the recommended temperature range, reheating method and conditions of the surrounding environment. The samples were properly coded, packaged separately in sterile containers and transported in cold pack to the Microbiology Laboratory of the School of Food Technology, Nutrition and Biosciences in Makerere University in Kampala. The transportation time between sample collection and arrival at the Laboratory was approximately 2 hours. Upon arrival, the general physical condition of sampling containers and the samples was noted. The cooler box and the stomacher bags were carefully inspected for tears, pinholes, puncture marks, fractures and loose enclosures before they were accepted. The samples were immediately prepared for analysis.
Sample preparation, culture and bacterial count
The modified method of ISO 4832 and ISO 6888-1 was used for the preparation of the samples. Twenty five grams of each food sample was weighed and homogenized by blending in 225 mL of sterile quarter strength ringer’s solution. Thereafter, one milliliter of each food sample homogenate was mixed into 9 mL of the buffered peptone water in a test tube. Serial dilution was made to 105 in five other test tubes comprising 10-2, 10-3, 10-4, 10-5 and 10-6. A 0.1 aliquot portion of each of the diluted samples was spread onto duplicate sterile plates of Baird Parker agar (BPA) and Violet Red Bile Lactose agar (VRBLA) for total aerobic count, S. aureus and E. coli, respectively. The inoculated plates were incubated aerobically at 37°C for 24 h. After incubation time, the different culture plates were examined for bacterial growth and discrete colonies were counted using the colony counter (Gallenkamp, England) and expressed as colony forming units per gram (CFU /g) of sample homogenate.
To confirm E. coli, five (5) colonies of each type were inoculated into tubes of the peptone broth and the tubes were incubated at 37°C for 24 ±2 h. Two to three (2-3) drops of kovac’s reagent were added, and formation of a pink ring was a confirmation for E. coli.
Statistical analysis
Statistical analysis of data was carried out using one-way analysis of variance (ANOVA) and post-hoc Scheffe tests were used to analyse the level of contamination according to type and source of RTE meats at P≤0.05 level of significance using SPSS version 20 (SPSS Inc., USA). The obtained results for total coliforms, E. coli and S. aureus were compared with the Microbial Contaminant Limits (MCLs) which are recommended by Codex Alimentarius Commission (CAC) and European Commission (EU). This was intended to determine the samples that were within the recommended limits and those that had exceeded the limits.
Bacterial isolates in chicken, beef and goat meat
Status of contamination of roasted chicken
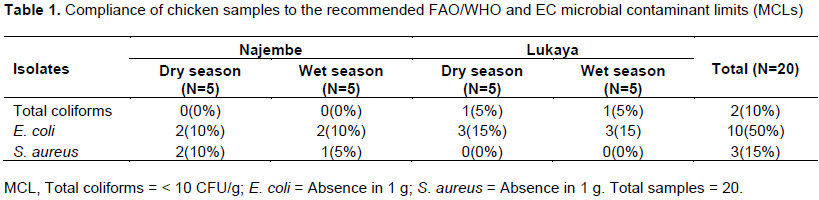
Overall 10% of all the chicken samples from Lukaya and Najembe that were tested had coliforms which were within the recommended MCL of < 10 CFU/g. None of the samples from Najembe met the recommended MCL while 20% of the samples from Lukaya were below the recommended MCL. Generally, chicken samples obtained from Lukaya market were more compliant than those obtained from Najembe market (Table 1).
Fifty (50%) percent of the chicken samples tested for E. coli were within the required MCLs of (absence in 1 g of a sample). Sixty (60%) percent of the samples from Lukaya were within the recommended MCLs, while for Najembe market only 40% of the samples were within the recommended MCL
For S. aureus, only 15% of the chicken samples from Lukaya and Najembe markets were within the recommended MCLs. Only 30% of the samples from Najembe were within the recommended MCLs. No chicken samples taken from Lukaya were within the recommended MCL.
Status of contamination of roasted beef
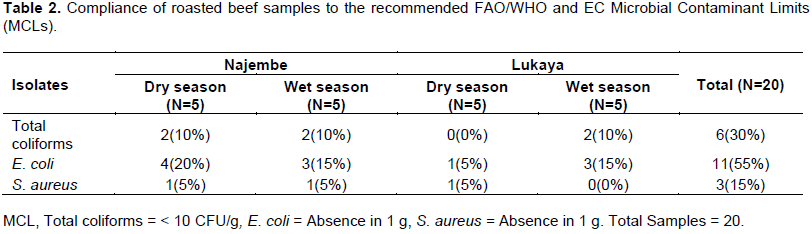
Of all the samples of beef that were taken from both markets, only 30% were found to contain coliforms that were within the recommended MCLs of < 10 CFU/g. Samples from Lukaya market were more compliant than those from Lukaya market. Forty (40%) percent of the beef samples taken from Najembe market were below the recommended MCLs compared to 20% from Lukaya market (Table 2).
For E. coli, 55% of the beef samples from Lukaya and Najembe markets were within the recommended MCLs. Seventy 70% of the beef samples from Najembe were within the recommended MCLs whereas 40% of the samples from Lukaya were within the recommended MCL.
As for S. aureus; only 15% of the beef samples from Lukaya and Najembe markets were within the recommended MCLs and 20% of the beef samples from Najembe were within the recommended MCLs. Only 10% of the beef samples from Lukaya were within the recommended MCLs.
Status of contamination of roasted goat meat
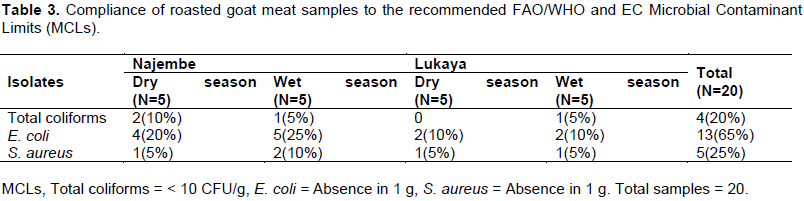
Overall, 20% of the goat meat samples from Lukaya and Najembe markets were within recommended MCLs of <10 cfu/g of a sample for coliforms. Thirty (30%) percent of the goat meat samples from Najembe were within the recommended MCLs while only 10% of the goat meat samples from Lukaya market were within the recommended MCLs (Table 3).
For E. coli, 65% of goat meat samples from Lukaya and Najembe markets were within the recommended MCLs. Ninety (90%) percent of the goat meat samples from Najembe market were within the recommended MCLs whereas only 40% of goat meat samples from Lukaya were within the recommended MCLs.
Concerning S. aureus, only 25% of the goat meat samples from Lukaya and Najembe markets were within the recommended MCLs. Thirty (30%) percent of goat meat samples from Najembe market were within the recommended MCLs and 20% of the samples from Lukaya were within the recommended MCLs. In general, Najembe market had more goat meat samples that complied with the recommended MCLs than Lukaya market.
Difference in Coliforms and S. aureus contamination of meat products
S. aureus contamination between chicken, beef and goat meat
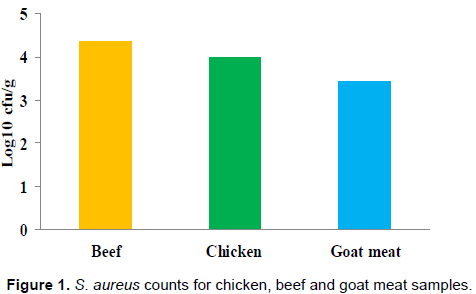
Goat meat samples had the lowest mean counts for S. aureus (3.44 Log10 cfu/g), these were followed by counts of chicken samples (3.99 Log10 cfu/g), and beef meat samples (4.37 Log10 cfu/g) in that order (Figure 1). The difference within the mean counts of the meat products was not significant (P>0.05).
Coliform contamination between chicken, beef and goat meat
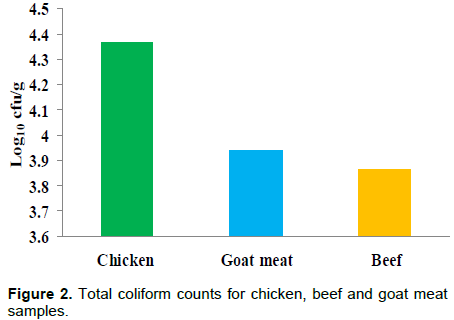
Beef samples had the lowest mean counts for coliforms (3.86 Log10 cfu/g), these were followed by goat meat samples (3.9 4Log10 cfu/g), and chicken samples (4.37 Log10 cfu/g) in that order (Figure 2). The difference within the mean counts of the meat products was not significant (P>0.05).
Comparison between samples obtained in the dry season and wet season
Total coliforms
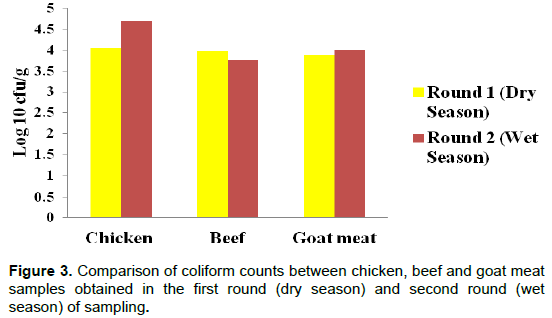
During the dry season, chicken samples had lower mean counts (4.05 log10 cfu/g) than wet season (4.68 log10 cfu/g). As for beef samples, dry season had higher mean counts (3.96 log10 cfu/g) than wet season (3.766 log10 cfu/g). For goat meat, dry season had lower mean counts (3.88 log10 cfu/g) than the second round of sampling -wet season (4.02 log10 cfu/g) (Figure 3). Generally, the dry season had lower counts than the wet season especially for chicken and goat meat although the difference was not significant between the dry and wet seasons (P>0.05).
S. aureus
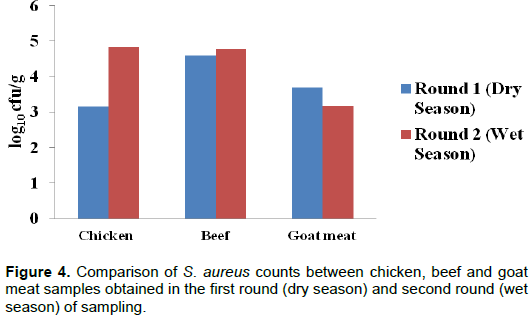
During the dry season, chicken samples had lower mean counts for S. aureus (3.15 log10 cfu/g) than the wet season (4.82 log10 cfu/g). For beef samples, the samples of dry season had lower mean counts (4.59 log10 cfu/g) than the wet season (4.78 log10 cfu/g). As regards goat meat samples, the samples of dry season had higher mean counts (3.69 log10 cfu/g) than wet season (3.18 log10 cfu/g) (Figure 4). Generally, the dry season had lower counts than the wet season although the mean counts of S.aureus were not significantly different (P>0.05).
Bacterial contamination of roasted chicken, beef and goat meat samples as compared to the recommended CODEX and EC Microbial Contaminant Limits (MCLs)
Roasted chicken samples
The occurrence of E. coli and S. aureus in all the samples may be as a result of poor handling and storage methods used by the food vendors. Similar results have been obtained in previous studies (Wogu et al., 2011; Bukar et al., 2010). The high coliform count in roasted chicken samples could be attributed to use of dirty items by the vendors to serve or to store the RTE products, overcrowding of markets, long stay of RTE products in the temperature danger zone (5 to 57°C) and the poor hygiene conditions of markets. A comparable study on the risk factors for contamination of RTE street vended poultry dishes in Dakar, Senegal found out that most of the vendors used dirty buckets, sinks, dishes and tongs to serve food to their customers. This exposed the consumers to a risk of eating poultry products that are contaminated with coliforms (Cardinale et al., 2005).
The presence of E. coli in chicken samples is an indication of faecal contamination probably at one stage of preparation or from the materials used (Adu-Gyamfi et al., 2012). Handling food with unwashed hands may result in cross-contamination, hence leading to introduction of microbes on safe food (CAC, 1997).
Defective hand washing can facilitate the transmission of pathogenic bacteria found in the environment and on people's hands via food to humans (Chirag, 2013). These scenarios could have been the cause of E. coli contamination in the chicken products obtained from the markets.
S. aureus contamination in RTE poultry normally results from excessive handling, man's respiratory passages, skin and superficial wounds which are common sources of S. aureus (Pointon et al., 2008). S. aureus is a normal flora of the human skin, nasal passage and throat of most healthy people and may have entered the food chain through such sources which suggests poor hygiene practices of the operators. When S. aureus is permitted to grow in foods, it can produce a toxin that causes illness. Although it may be destroyed by heat, its toxin is heat stable (Hazariwala, 2002). The presence of these organisms could cause mild to severe symptoms of diseases such as diarrhea, typhoid and cholera (Miriam et al., 2012; Mbah et al., 2012). Contamination of RTE chicken with S. aureus could lead to food poisoning and this could be attributed first to non-adherence to standard hygiene practices employed during food preparation and second, the type of water used in mixing the food which is often not clean (Ahmad et al., 2013). Open-air markets have been implicated in direct transfer of S. aureus during handling between traders and consumers of RTE foods (Amusan et al., 2010). These factors could as well have been responsible for the S. aureus contamination in the chicken samples obtained from the markets.
Roasted beef
Keeping of beef at ambient temperature aids the growth of coliforms to unacceptable levels hence causing the meat’s quality to deteriorate (Soyiri et al., 2008). This scenario applies to the current study as well. Same explanation was given for microbial contamination of beef in Kigali, Rwanda where most beef vendors stored beef at ambient temperature for the next day’s sale. Most of them did not wash their food stuffs (Eugène et al., 2013). Given the presence of such poor practices among beef vendors in the markets involved in the current study; this could explain the low level of compliance of the beef samples that were tested for coliforms.
A study on street vended foods in Atbara City in the Naher Elneen state of Sudan showed that the most prevalent bacteria contaminating RTE food of beef category was E. coli (Abdalla et al., 2009). The isolation of this pathogen in raw and RTE foods such as beef has also been reported by Soyiri et al. (2008), in food vending markets in Accra, Ghana where it was pointed out that it is highly likely that beef vending markets will usually be implicated in shortage of hand washing facilities. From these comparable studies it is noted that having functional hand washing facilities is critical to the control of E. coli. Given the limited availability of such facilities in the highway markets sampled during the study; these factors could as well be responsible for the presence of E. coli in the beef samples.
The presence of S. aureus in beef samples could have been due to vendors spending long hours with their beef products before they are sold. This increased the risk of excessive human handling and consequent multiplication of S. aureus before sale (Eugène et al., 2013). Another related study conducted by Adu-Gyamfi and Nketsia-Tabiri (2007), in Ghana showed that lack of good personal hygiene practices was the major factor contributing to the contamination of beef meat with S. aureus. S. aureus can be introduced when beef products are exposed to excessive human handling (Adzitey et al., 2011). These factors could also be responsible for the contamination of beef products with S. aureus in the highway markets in Uganda given that poor hygiene practices were observable in these markets during the study.
Roasted goat meat
Improper sanitation and hygienic practices such as poor storage of meat products and use of unclean equipment’s/utensils during vending can considerably increase the contamination of goat meat with coliforms (Hirwa, 2010). Such practices could have been responsible for the contamination of goat meat products with coliforms.
Mensah et al. (2001) found out that E. coli in goat meat was largely due to infestation of flies. Another study by Haque et al. (2008) observed that high levels of contamination from E. coli was due to the growth of the existing microorganisms encouraged by the warm temperatures and the cross-contamination from utensils during goat meat preparation. According to CAC (1997), cross-contamination from pests such as flies is a high risk factor to meat handlers because they can facilitate E. coli to go directly into the food thus reducing its microbial quality. This could have been the case for goat meat vendors in the markets studied given that goat meat vendors had similar sanitation and hygiene challenges.
A study of bacterial contamination in goat meat from metropolitan Accra, Ghana found out that S. aureus was among the most prevalent bacteria contaminating goat meat products (Mensah et al., 2001). The presence of this pathogen in goat meat was found to be due to congestion of food vending stalls and poor personal hygiene practices among the vendors such as irregular hand washing, and absence of proper dressing gear such as gloves, mouth and hair covers and aprons. From this study, it is noted that ensuring personal hygiene and decongesting meat preparation areas are critical to the control of S. aureus in meat vending markets. Given that goat meat vendors in the highway markets studied had congested stalls and did not observe proper personal hygiene practices, the contamination of their goat meat products with S. aureus could be attributed to this as well.
Difference in coliforms and S. aureus contamination of meat products
The difference between the mean counts of chicken, beef and goat meat samples that were obtained from highway markets was not significant. Related studies testing S. aureus in chicken, beef and goat meat at different vending outlets of Lahore, Pakistan also showed that the difference between the mean counts of the three products was not significant (P>0.05) (Ahmad et al., 2013). That study indicated that the slight difference was due to excessive human handling of beef products and limited knowledge about personal hygiene practices among the beef meat sellers as compared to the traders of other types of meat products (goat meat, chicken, mutton and turkey). The current study also found out that beef was supplied at ambient temperature long before the start of business which could have favoured S. aureus to grow. Given that these poor hygiene practices were also observed among beef vendors in the areas sampled (highway markets); these factors could also be responsible for the slight differences in S. aureus contamination between chicken, beef and goat meat samples.
The coliform counts were higher in chicken samples than in beef and goat meat. A related study on prevalence of different microbial contaminants in meat products from the greater Washington, D.C area also found high coliform contamination of chicken compared to the other meat products (Cuiwei et al., 2001). The study indicated that chicken products were more vulnerable to contamination because chicken contains more fluid than beef and goat meat which could facilitate the quick multiplication of coliforms. This factor could have been responsible for the slightly higher coliform counts found in chicken samples that were obtained from the highway market that were studied.
Comparison between samples obtained in the dry and wet season
The coliform counts during the wet season especially for
chicken and goat meats were higher than those of the dry season. During the wet season, the conditions of the surrounding environment and the observed appearance (especially colour) of water used for food preparation had changed when compared with what was observed in dry season. Insect vectors were observed at most stalls. The environment was filled with mud, stagnant water and the smell in some areas of the markets was more intense in the wet season than in dry season. In addition, most food contact surfaces were observed to be wet. This could explain why the coliform counts during the wet season, especially for chicken and goat meats, were higher than those of the dry season. It is already known that in such scenarios the pathways for contamination include distinguishable vectors such as insects and environmental conditions such as rains and winds (Barro et al., 2007). Use of contaminated water could aid contaminants to enter into the food, hence putting the health of consumers at risk (Chirag, 2013). Unhygienic surroundings and inadequate supply of clean water attract all kinds of flies which further increases food contamination (Chumber et al., 2007).
Results from the samples of chicken, beef and goat meat showed an increment in the counts during the wet season. A comparable study on the bacteriological status of street vended foods of Buldana District, MS, India where samples were picked during the initial rainy season indicates that almost 70% of the food samples collected from street vendors had high bacterial load compared to samples which had been picked during the dry season (Garode, 2012). According to FDA (2016), although food handlers are the main source of contamination from microbial food poisoning outbreaks; wet, muddy and dirty environmental surfaces can also be sources of contamination with various microbes. This means that the rainy season which is characterised by environmental conditions described by FDA could have had an incremental impact on the S. aureus counts of the products obtained from highway markets.
The study results indicated that the RTE meats were contaminated with bacterial pathogens such as S. aureus and E. coli and did not meet the required safety levels recommended by Codex Alimentarius and European Commission (EC). The presence of these food pathogens in the foods could pose a serious public health hazard to unsuspecting consumers as all these bacterial pathogens have been implicated in food borne illnesses.The detection of these organisms in all the RTE meats investigated portends danger that could be associated with poor personal hygiene, poor food preparation, lack of good manufacturing practices,as well as and non-compliance to Hazard Analysis and Critical Control Points (HACCP) principles during the preparation, packaging and serving of these foods to consumers. S. aureus contamination was higher in beef meat samples, followed by chicken and goat meat samples, respectively. E. coli contamination was higher in chicken samples, followed beef and goat meat samples respectively although in both cases the differences were not significant. As expected, samples taken during the wet season were more contaminated than those that were picked during the dry season; although the difference was insignificant. Generally the study findings indicate that the meats that are vended in highway markets are not safe and could pose health risks to consumers. This therefore calls for installation of proper facilities, awareness of vendors in good sanitation and hygiene practices and enforcement of sanitation and hygiene. More so, the following primary food safety measures should be effectively observed by food handlers and vendors: Proper hand washing practices, preparation and selling of foods in hygienic premises, proper covering of prepared foods, washing of utensils and dish with soap, use of portable water as well as proper disposal of wastes among others.
The authors have not declared any conflict of interests.
REFERENCES
|
Abdalla MA, Suliman SE, Bakhiet AO (2009). Food safety knowledge and practices of street-food vendors in Atbara city (Naher Elneel State Sudan). Afr. J. Biotechnol. 8:6967-6971.
|
|
|
|
Adolf JNP, Azis BS (2012). Microbiological status of various foods served in elementary school based on social economic status differences in Karawachi Region, Tangerang District-Indonesia. Int. Food Res. J. 19(1):65-70.
|
|
|
|
Adu-Gyamfi A, Nketsia-Tabiri J (2007). Microbiological studies of macaroni and vegetable salads in Waakye, a local street food. Ghana J. Sci. 47:3-9.
|
|
|
|
Adu-Gyamfi A, Torgby-Tetteh W, Appiah V (2012). Microbiological quality of chicken sold in Accra and determination of D10-value of E. coli. J. Food Nutr. Sci. 3:693-698.
Crossref
|
|
|
|
Adzitey F, Teye G A, Kutah W N, Adday S (2011). Microbial quality of beef sold on selected markets in the Tamale Metropolis in the Northern Region of Ghana. J. Livestock Res. Rural Dev. 23:5-10.
|
|
|
|
Ahmad MUD, Sarwar AM, Najeeb I, Nawaz M, Anjum A A, Ali MA, Mansur N (2013). Assessment of microbial load of at abattoirs and retail outlets. J. Anim. Plant Sci. 23:745-748.
|
|
|
|
Amusan E, Oramadike CE, Abraham-Olukayode AO, Adejonwo OA (2010). Bacteriological quality of street vended smoked Blue Whiting (Micromesistus poutasou). Int. J. Food Saf. 12:122-126.
|
|
|
|
Akusu OM, Kiin-Kabari D B, Wemedo SA (2016). Microbiological quality of selected street vended foods in Port Harcourt metropolis, Rivers State, Nigeria. Sky J. Food Sci. 5(2):008-011.
|
|
|
|
Barro N, Bello A, Itsiembou Y, Sevadogo A, Ouattara C (2007). Street-vended foods improvement: Contamination mechanisms and application of food safety objective strategy: Critical review. Asian Network for Scientific information Pak. J. Nutr. 6:1-10.
Crossref
|
|
|
|
Barro N, Bello AR, Savadogo A, Ouattara CAT, Ilboudo AJ, Traore AS (2006). Hygienic status assessment of dish washing waters, utensils, hands and pieces of money from street food processing sites in Ouagadougou (Burkina Faso). Afr. J. Biotechnol. 5(11):1107-1112.
|
|
|
|
Bukar A, Uba A, Oyeyi TI (2010). Occurrence of some enteropathogenic bacteria in some minimally and fully processed ready-to-eat foods in Kano metropolis, Nigeria. Afr. J. Food Sci. 4(2):032-036
|
|
|
|
Bwire G, Malimbo M, Maskery B, Kim YE, Mogasale V, Levin A (2013). The burden of cholera in Uganda. PLoS. Negl. Trop. Dis. 7(12):e2545.
Crossref
|
|
|
|
CAC (1997). Regional guidelines for the design of control measures for street-vended foods (Africa) - Codex Alimentarius Supplement. Joint FAO/WHO food standards program. Agriculture and consumer protection department, FAO, Rome.
|
|
|
|
Cardinale E, Perrier JD, Tall F, Gueye EF, Salvat G (2005). Risk factors for contamination of ready-to-eat street vended poultry dishes in Dakar, Senegal. Int. J. Food Microbiol. 103:157-165.
Crossref
|
|
|
|
CDC (2005). Food-borne illness: Frequently asked questions. Centers for Disease Control and Prevention, USA.
|
|
|
|
Chirag G (2013). Study of hygienic practices of street food vendors in Allahabad city, India and determination of critical control points for safe street food. The Allahabad Farmer 68:1-13.
|
|
|
|
Cho JI, Cheung CY, Lee SM, Ko SI, Kim KH, Hwang, IS, Kim SH, Cho SY, Lim CJ, Lee KH, Kim KS, Ha SD (2011). Assessment of microbial levels of street-vended foods in Korea. J. Food Saf. (31):41-47.
Crossref
|
|
|
|
Chumber SK, Kaushik K, Savy S (2007). Bacteriological analysis of street foods in Pune. Indian J. Pub. Health 51:114-6.
|
|
|
|
Cuiwei Z, Beilie G, Juan D V, Robert S, Emily Y, Shaohua Z, David W, Jianghong M (2001). Prevalence of Campylobacter spp., Escherichia coli, and Salmonella Serovars in retail chicken, turkey, pork, and beef from the Greater Washington DC Area. J. Appl. Environ. Microbiol. 67:5431-5436.
Crossref
|
|
|
|
Eugène N, Divine B, Martin P (2013). Assessment of beef meat microbial contamination during skinning, dressing, transportation and marketing at a commercial abattoir in Kigali city, Rwanda. Pak. J. Food Sci. 23:79-112.
|
|
|
|
FDA (2016). Food Safety Survey. Consumer Studies Branch, Center for Food Safety and Applied Nutrition, FDA.
|
|
|
|
FDA (2013). Draft methodological approach to identifying high-risk foods under section 204(d)(2) of the FSMA, Food and Drug Administration.
|
|
|
|
Garode AM, Waghode SM (2012). Bacteriological status of street-vended foods and public health significance: A case study of Buldana District, MS, India. ISCA J. Biol. Sci. 1:69-71.
|
|
|
|
Haque M A, Siddique M P, Habib M A, Sarkar V, Choudhury KA (2008). Evaluation of sanitary quality of goat meat obtained from slaughter yards and meat stalls at late market hours. Bangl. J. Vet. Med. 6:87-92.
|
|
|
|
Hazariwala A (2002). Distribution of staphylococcal enterotoxin genes among Staphylococcus aureus isolates from poultry and human with invasive Staphylococcal disease. Avian Dis. 46:132-136.
Crossref
|
|
|
|
Hirwa N (2010). The study of the microbiological quality of beef sold in Nyarugenge District. Unpublished MSc. Kigali Institute of Science and Technology, Kigali, Rwanda.
|
|
|
|
ICMSF (2011). Microorganisms in Foods 8: Use of Data for Assessing Process Control and Product Acceptance. View
|
|
|
|
Karamaji P (2012). Millions of Ugandans suffer from food poisoning annually.
View
|
|
|
|
Kawo AH, Abdulmumin FN (2009). Microbiological quality of pre-packaged sweets sold in metropolitan Kano, Nigeria. Bayero J. Pure Appl. Sci. 2(1):154-159.
|
|
|
|
Makelele LK, Kazadi ZA, Oleko RW, Foma R, Rosette KM, Koto- te-Nyiwa N. and Bongo NG. (2015). Microbiological quality of food sold by street vendors in Kisangani, Democratic Republic of Congo. Afr. J. Food Sci. 9(5):285-290.
Crossref
|
|
|
|
Mbah M, Ogban GI, Konlack GD, USEH MF, Asuquo AE (2012). The bacteriological status of five selected street vended cooked foods in Calabar, Nigeria. IOSR J. Pharm. Biol. Sci. (IOSRJPBS) 2(4):25-29.
|
|
|
|
Mensah P, Amar-Klemesu M, Hammond A, Haruna A (2001). Bacterial contamination on lettuce, tomatoes, beef and goat meat from metropolitan Accra. Ghana Med. J. 35:1-6.
|
|
|
|
Mensah P, Yeboah-Manu D, Owusu-Darko K, Ablordey A (2002). Street foods from Accra, Ghana: How safe? Bulletin of World Health Organ, 80:546-54.
|
|
|
|
Miriam EN, Collins, EO, Nicoline FT, Ezekiel G, Roland NN (2012). Foodborne pathogens recovered from ready-to-eat foods from roadside cafeterias and retail outlets in Alice, Eastern Cape Province, South Africa: Public Health Implications. Int. J. Environ. Res. Public Health 9:2608-2619.
Crossref
|
|
|
|
MOH (2012). Annual Health Sector Performance Report. Ministry of Health Kampala, Uganda.
|
|
|
|
MOH (2013). Annual Health Sector Performance Report. Ministry of Health Kampala, Uganda.
|
|
|
|
Musa OL, Akande TM (2002). Effect of health education intervention or food safety practices among food vendors in Ilorin. Sahel Med. J. 5:120-124.
|
|
|
|
Muyanja C, Nayiga L, Namugumya B, Nasinyama G (2011). Practices, knowledge and risk factors of street food vendors in Uganda. Food Cont. 22:1551.
Crossref
|
|
|
|
Nkere CK, Ibe NI, Iroegbu CU (2011). Bacteriological quality of foods and water sold by vendors and in restaurants in Nsukka, Enugu State, Nigeria: A comparative study of three microbiological methods. J. Health Popul. Nutr. 29(6):560-566.
|
|
|
|
O' Brien SJ (2008). Foodborne disease outbreaks in healthcare setting. In: Lund, B.M. & Hunter, P.R. (Eds.). The microbiological safety of food in healthcare settings, pp. 251-289. Oxford: Blackwell Publishing Ltd.
Crossref
|
|
|
|
Odu N (2013). Microbiological quality of street-vended-ready-to-eat "Bole" fish. In: Port Harcourt Metropoplis. New York Sci. J. 6:532-544.
|
|
|
|
Oghene BO, Oyarekua MA, Edeh AN. (2014). Bacteriological status of commonly consumed foods and vegetables from food vendors in a market in Enugu, Nigeria. Int. J. Curr. Microbiol. Appl. Sci. 3(11):151-156.
|
|
|
|
Pointon A, Sexton M, Dowsett P, Saputra T, Kiermeier A, Lorimer M, Holds G, Arnold G, Davos D, Combs B, Fabiansson S, Raven G, McKenzie H, Chapman A, Sumner J (2008). A baseline survey of the microbiological quality of chicken portions and carcasses at retail in two Australian states (2005 to 2006). J. Food Prot. 71:1123-1134.
Crossref
|
|
|
|
Razavilar V (2010). Pathogenic bacteria in food. 3rd. ed. Tehran: Tehran University Publication.
|
|
|
|
Sebudde S, Kabagambe R, Muganwa M (2012). Hygiene and sanitation in public eating places in one municipal health system of Uganda. Kampala: Erudite J. Med. Med. Sci. Res. (EJMMSR). 1:1-8.
|
|
|
|
Shamsuddeen U, Ameh JB (2008). Survey of the possible critical control points during the production of Balangu in Kano. Bayero J. Pure Appl. Sci. 1(1):76-79.
|
|
|
|
Soyiri I, Agbogli H, Dongden J (2008). A pilot microbial safety of beef sold in Ashiaman market a suburb of Accra. Afr. J. Dev. 8:91-103.
|
|
|
|
Stewart WL and Humphrey K (2002). Ready to eat food and foodborne infection, 5th ed., CBS Publisher and Distributors, New Delhi, India: pp. 240-245.
|
|
|
|
Tambekar DH, Kulkarni RV, Shirsat SD, Bhadange DG (2011). Bacteriological quality of street vended food panipuri: A case study of Amravati city (MS) India. Biosci. Discov. 2(3):350-354.
|
|
|
|
Tambekar D, Jaiswal V, Dhanorkar D, Gulhane P, Dudhane M (2008). Identification of microbiological hazards and safety of ready-to-eat food vended streets of Amravati city, India. J. Appl. Biosci. 7:195 - 201.
|
|
|
|
Tavakoli HR (2008). Food microbiology and control of food production and distribution centers. 2nd ed. Tehran: Marz-e-Danesh publication.
|
|
|
|
Tsang D (2002). Microbiological guidelines for ready-to-eat food. Road and Environmental Hygiene Department Hong Kong, 115-116.
|
|
|
|
UBOS (2011). Uganda Demographic and Health Survey. Uganda Bureau of Statistics (UBOS) and Macro International Inc Calverton, Maryland, USA.
|
|
|
|
ULRC (2013). Review of the Markets Act, Cap 94 in Uganda. Market Review report, Uganda Law Reform Commission.
|
|
|
|
URN (2010). Dysentery outbreak in Kanungu.
View
|
|
|
|
WHO (2012). Uganda Cholera (Situation as of 31 March, 2012). Accessed on 15th January 2017 at http://who.int/csr/don/17-march-2015-uganda/en
|
|
|
|
WHO (2015). Typhoid fever in Uganda.
View
|
|
|
|
Winnie V M (2005). Street vending in African cities: A synthesis of empirical findings from Kenya, Cote D'Ivoire, Ghana, Zimbabwe, Uganda and South Africa. Background Paper for the 2005 World Development Report, Institute for Development Studies University of Nairobi. pp. 4-30.
|
|
|
|
Wogu MD, Omoruiy MI, Odeh HO, Guobadia, JN (2011). Microbial load in ready-to-eat rice sold in Benin city. J. Microb. Antimicro. 3(2):29-33.
|
|
|
|
Zhu M, Du M, Cordray J, Ahn DU (2005). Control of Listeria monocytogenes contamination in ready-to-eat meat products. Compr Rev Food Sci. Food Saf. 4:34-42.
Crossref
|