ABSTRACT
This study was carried out to evaluate human consumption of the pollen of Scaptotrigona bipunctata colonies stored in pots; it was lyophilized and spray-dried in arrays of arabic gum and hydrolyzed collagen which have been widely applied to get products with best technological features and highest biological activity. After the samples were prepared they were evaluated by physicochemical analysis, and the data were compared. S. bipunctata pollen lyophilized had product with higher antiradical activity than the pollen dried using spray dryer which only quantified the antioxidant activity of the antioxidant compound that was encapsulated and stabilized in the solid matrix material.
Key words: Biological activity, encapsulated pollen, lyophilized pollen, phenolic profile, stingless bees.
Native bees occur in many tropical regions of the world, including all of Latin America and Africa, Southeast Asia and the North of Australia. However, much of the diversity of the species occurs in the Americas, with approximately 400 types described (Villas-Bôas, 2012). Bee breeding is practiced in almost all regions of Brazil, in the North and Northeast of the country; it is a sustainable alternative or additional form of income for small and medium farmers; it is greatly important in the use and sustainable management of the forest environment and preservation of biodiversity of the ecosystems (Venturieri, 2008).
The stingless bee, Scaptotrigona bipunctata (Lepeletier, 1836) belongs to the Meliponinae group popularly known as tubuna bee or straw cane due to the entrance of the colony that has a funnel shape, made of dark cerumen (Costa and Imperatriz-Fonseca, 2000). In the nest, the pots where they store honey and pollen can reach 2.5 up to 3.0 cm in height and the rearing combs can be built helically, with the presence of real cells on the border. They form very populous colonies comprising 2,000 to 50,000 bees, with highly defensive behavior; this often makes humans to destroy their nests, easily found in cavities pre-existing in tree trunks, mainly in the States of Ceará, Minas Gerais, Rio Grande do Sul, Paraná, and São Paulo (Costa and Imperatriz-Fonseca, 2000). There is little knowledge about its management in rational hives, biology and pollen use as a floral resource in the different environments in which they inhabit.
In addition to honey, native stingless bees allow the potential exploitation of products and by-products with a high value, such as propolis, pollen and geopropolis (Silva et al., 2014). The pollen of native stingless bees is deposited in the colony in specific pots, facilitating its exploitation. Natural pollen collected from flowers is processed in these pots by bees, which deposit enzymes in the pollen that initiates its digestion and helps its natural preservation, and then it is called ‘saburá’ in Brazil (Brasil, 2001; DerMardersian et al., 2005; Lima Neto et al., 2017).
Pollen has long been used, especially among natural food supporters, as a supplement to human diet for its richness in relation to proteins, lipids, vitamins and minerals (Komosinska-Vassev et al., 2015; Bogdanov, 2016). Although the chemical composition of a food such as pollen, which is rich in protein and possesses phytotherapeutic properties, is of great interest, it is rarely commercially available. When processed is sold in dehydrated portions, or blended with honey (Villas-Bôas, 2012). Another problem regarding the use by the food industry of natural products such as pollen is the fact that many of the constituents responsible for its biological properties, such as phenolic compounds and pigments with antioxidant activity, are highly susceptible to oxidation and volatilization and unstable in the presence of heat, light, and oxygen (Bobbio and Bobbio, 2001).
The rising of powdered foods has driven the food industry to carry out research activities in technology and innovation aimed at leveraging sales. Thus, it would be possible to convert poorly soluble granular structures into an easily dissolved, attractive fine powder, which preserves the nutritional part of the food, at a low cost and with a short operating time. This can be crucial in the decision to purchase this product for a large number of consumers and, in the short or medium term, can contribute to encouraging the management of stingless bees, the improvement of pollen production techniques and also contribute to the greater appreciation of products of Brazilian native stingless bees. Therefore, this study aimed to develop and characterize a new product from the encapsulation of the pollen of S. bipunctata, in arabic gum and hydrolyzed collagen matrices using spray-dryer and lyophilization drying methods.
The experiment was carried out at Universidade Tecnológica Federal do Paraná (Paraná Federal Technology University), Campo Mourão Campus, Brazil from July 2015 to June 2016. Approximately 10 kg of pollen was collected from different beehives of S. bipunctata in the Mandirituba region of the state of Paraná. After collection, the pollen was manually cleaned and wrapped in polyvinyl chloride (PVC) bags, which were hermetically sealed and stored in a freezer at -20ºC until use. Synth brand arabic gum wall material (treatment I) and Sanavita brand hydrolyzed collagen (treatment II) were used for the production of microcapsules. In treatment III the pollen was lyophilized.
Spray drying method
The production of the microcapsules (Treatments I and II) was carried out according to Rocha et al. (2012). The encapsulating material and the pollen were dispersed in water at a 2:1:3 (m/m/m) ratios, and then filtered a standard fine mesh sieve (150 µm). For the production of microcapsules, the mixture was heated to a temperature of 70°C and mechanically shaken at 4000g for 5 min with a FISATOM model 713D shaker (Brazil). This movement continued during a cooling process in an ice bath until the mixture reached a temperature of 10ºC.
The spray drying process of the samples was performed using a LM-LABMAQ model MSD 1.0 (Brazil) spray dryer with a 1.00 mm diameter atomizer, incoming gas temperature of 130°C, drying air flow of 3.60 and a sample of 0.50 L/h. The samples were packaged in an amber flask and kept at room temperature.
Drying in a lyophilizer method
The pollen was dispersed in water at a 1:1 (m/m) ratio to obtain an ultra-rapid separation, with the minimum degradation of the product to be dried, filtered with a standard fine mesh sieve, placed in Petri plates and frozen in a PANASONIC (Brazil) ultra-freezer to -83ºC. Drying by lyophilization (treatment III) was performed in a LIOTOP lyophilizer; model L101 (Brazil), for 72 h at a temperature ≤ -40°C - compressor temperature and a pressure of approximately 50.00 μHg. The samples were kept in a desiccator and then crushed in a mortar, sieved and packaged in an amber flask and kept at room temperature.
Physical-chemical composition of the resultant products
Each parameter was analyzed in three repetitions: Moisture (%), total sugars, reducing and non-reducing (%), lipids (%), fixed mineral residue (%), proteins (%), total fiber (%), total energy value (TEV), ash (%), pH, water activity, hygroscopicity (g/100g). Apparent density (g/cm3) was measured according to the Instituto Adolfo Lutz (2005) and AOAC (2012). The calories content (Kcal) and energy value (Kj) were calculated according to the National Sanitary Surveillance Agency (Anvisa, 2005).
Total phenols, total flavonoids and antioxidant activity
Obtaining pollen extracts
Using 50.00 mL falcon tubes, 0.40 g of the encapsulated samples was diluted in 10 mL of 80.00% methanol acidulated with 0.20% concentrated hydrochloric acid - 1:25 (w/v). For the lyophilized pollen, a 0.40 g sample was diluted in 20.00 mL of the same diluent, to create the lyophilized pollen extract, with an initial concentration of 1:50 (w/v).
The tubes were sealed, stirred in a QL901 BIOMIXER (Brazil) vortex mixer for 2 min and then placed in a CRISTÓFOLI Ultron2 model (China) ultrasonic bath, for 20 min to rupture the microcapsules. The encapsulated pollen extracts were placed in a NOVATECNICA model NT825 (Brazil) refrigerated centrifuge at 3000 g for 20 min and the supernatant was used for analysis.
Determination of phenolic compounds
The phenolic compounds were determined using the Singleton et al. (1999). The total phenol concentration was determined by interpolating the absorbance of the samples based on a calibration curve constructed with standard gallic acid - GA (Brazil). A calibration curve (0.00 to 1500.00mg GAE/L, r2 = 0.9976) was constructed and the results expressed in mg GAE/g of pollen. Absorbance was measured in quartz cuvettes with a length of 765 nm in an Ocean Optics USB-650 (USA) UV-VIS spectrophotometer.
Total flavonoids
The flavonoid content present in the encapsulated and lyophilized pollen extracts was determined by Alothman et al. (2009). Total flavonoid concentration was determined by interpolating the absorbance of the samples based on a calibration curve constructed with standard quercetin, Sigma-Aldrich, 95.0% purity (USA). A standard calibration curve (50.00 to 500.00 mg QE/L, r2 = 0.9941) was constructed and the results expressed in mg QE/g of pollen. Absorbance was measured in quartz cuvettes at 510 nm in an Ocean Optics USB-650-UV-VIS spectrophotometer.
Antioxidant and antiradical activities
Antioxidant activity was determined according to Roginsky and Lissi (2005). Antiradical effectiveness was evaluated with the DPPH (2,2-diphenyl-1-picryl-hydrazyl) method (Mensor et al., 2001). The scavenging activity of the DPPH free radical was expressed in terms of EC50, which represents the minimum concentration necessary for the antioxidant to reduce the initial concentration of DPPH by 50.00%.
Statistical analysis
The design was completely randomized, with three treatments, and the data were submitted to variance analysis at a 5% significance level. The means were compared by the Tukey test using SAS ver. 9.3 (2012).
Characteristics of the resultant products
The S. bipunctata encapsulated pollen samples in hydrolyzed collagen and arabic gum matrices and lyophilized pollen, while not sensorial evaluated, had a fine texture; it was homogenous, had slightly yellow color, variable aroma and citrus flavor and floral, and had characteristic of natural pollen (Figure 1 a, b, c).
Physicochemical composition of the resultant products
The results of the physicochemical parameters of the S. bipunctata encapsulated pollen samples in hydrolyzed collagen and arabic gum matrices are presented in Table 1.
The means of the physicochemical composition of the dried pollen samples using the spray dryer, hydrolyzed collagen and arabic gum wall material and lyophilized pollen differed statistically (p<0.05) in all tested characteristics (Tables 1 and 2).

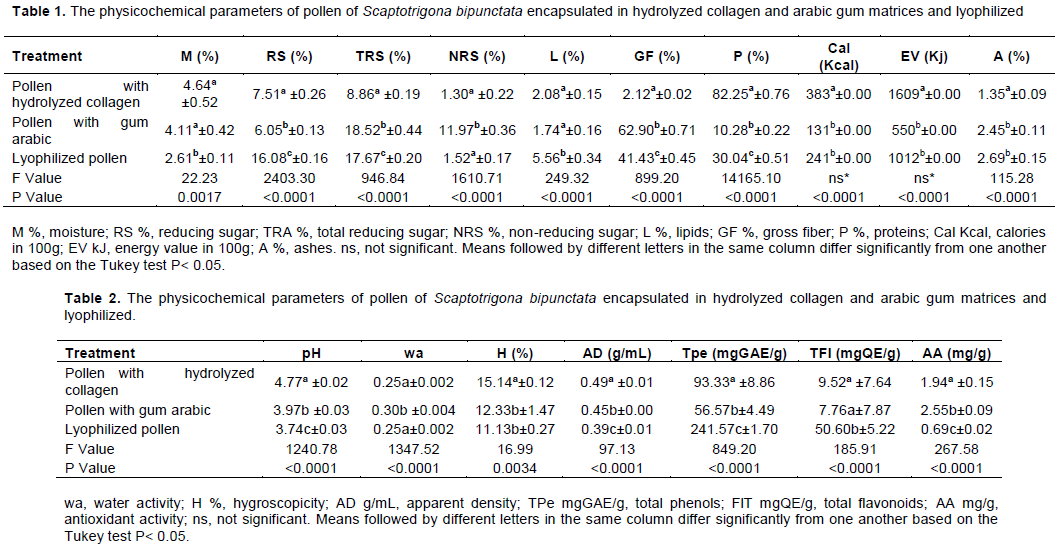
The chemical composition of stingless bee pollen, that quantified its components in a natural form, dried in the oven (Bogdanov, 2016) using a spray dryer or lyophilization is rare, making it difficult to compare with the data obtained in this study. However, despite this lack of information, it was observed that S. bipunctata spray dried using hydrolyzed collagen and arabic gum as capsule wall material had a greater protein and fiber content, respectively, while pollen dried in a lyophilizer presented a more balanced chemical composition in terms of carbohydrates, protein, fiber and lipids, low moisture content and hygroscopicity (Figure 1). These differences are attributed to the chemical drying process employed, and also to the chemical composition of the materials used as walls.
The mixing of hydrolyzed collagen in water resulted in less viscous solutions that were more suitable for the encapsulating process by promoting the efficiency of the atomization in the drying stage; it resulted in a greater opacity of the encapsulating matrix. The use of hydrolyzed collagen considerably increased the protein content of the final product, resulting in nutritional value. This makes it an excellent option as a supplement. arabic gum, meanwhile, is a polysaccharide consisting essentially of D-galactose, L-arabinose, L-ramnose, water and glycoproteins (
Andrade et al., 2013) which possess important technological functions such as acting as a soluble dietary fiber as well as a body or texture agent in industrial preparations.
Rebelo et al. (2016) reported values for moisture from 37.12 to 53.39%; protein from 24.00 to 37.63%; lipids from 6.47 to 10.81%; ashes from 2.74 to 4.03%; crude fiber from 9.30 to 13.65%; carbohydrates from 25.66 to 44.27% energy from 331.33 to 350.47 kcal/%; pH from 3.34 to 3.70; total solids from 46.60 to 62.87% and water activity from 0.85 to 0.91, respectively. Barajas et al. (2012) found a mean protein content of 28.00%; fat from 4.00 to 5.00%; ash, 2.10 and 3.30% in pollen from mountainous and pasture regions of Colombia, but both with a mean daily temperature of 14ºC.
In pollen samples in natura of different species of native stingless bees collected in the Amazon region, average values of 36.90% moisture, 19.50% proteins, 4.00% lipids, 2.10% ash and 37.50% carbohydrate were found (Oliveira et al., 2007). However, Bogdanov (2016) reported that dried bee pollen at 40°C has average 32.8% proteins, including 11.50% essential amino acids, 40.7% reducing sugars, 3.70% sucrose, 12.80% lipids, 0.19% vitamin C, 0.07% β-carotene and a maximum of 6.00% moisture; regardless of its floral origin, it is rich in essential nutrients (Bogdanov, 2016). Melo and Almeida-Muradian (2011) determine the moisture content of bee pollen dried samples by six methods, and reported moisture values of 3.96% with oven drying at 100ºC and 10.02% with lyophilization.
Some countries have official standards for the identification and evaluation of the bee pollen quality, such as: Brazil (Brazil, 2001), Bulgaria (Bulgarian Standard 2567111-91), Poland (PN- R- 78893) “Obnóza pylkowe.” - Polish bee pollen legislation (Campos et al., 2008). These countries have established minimum requirements for the moisture content of dried pollen: Brazil (2001), maximum 4.00%, Bulgaria, maximum 10.00% and Poland, maximum 6.00%. Campos et al. (2008) proposed a classification of the pollen from the A. mellifera for commercial purposes based on its moisture content. The original product had an initial content of 20.00 to 30.00%, which dropped to no greater than 6.00% following drying at temperatures not above 42°C.
Sugars in pollen can vary from 13.0 to 55.0% including glucose, fructose and sucrose (Bogdanov, 2016), 25.70% (Kędzia and Hołderna-Kędzia, 2012), or up to 40.70% (Komosinska-Vassev et al., 2015). In the present study, the reducing sugar, total reducing sugar and sucrose contents were greater in the pollen samples dried with a lyophilizer than those obtained from a spray dryer (Table 2).
Comparing the lipid, fiber, protein, caloric and energetic values (Tables 1 and 2), the final products obtained displayed distinct technological and nutritional characteristics. The fiber and protein grade was significantly greater in pollen samples encapsulated with arabic gum and in collagen, respectively, while lipid content was significantly greater in the lyophilized pollen samples. Szczesna (2006) obtained a mean content of 5.10%, of which 0.40% was represented by long-chain essential fatty acids such as linoleic, linolenic and arachidonic, while Komosinska-Vassev et al. (2015) reported a mean total lipid content of 12.80%.
Mineral content is almost 1.60%, including the macro-nutrients calcium (Ca), phosphorus (P), magnesium (Mg), sodium (Na), potassium (K) and micronutrients iron (Fe), copper (Cu), zinc (Zn), manganese (Mn), silicon (Si) and selenium (Se), the latter in a quantity of 0.02% (Kędzia and Hołderna-Kędzia, 2012). In dried pollen samples taken from different regions of Bulgaria, Dinkov and Stratev (2016) reported the minimum values of 0.91% and maximum values of 2.30% from the Karlovo and Sliven and Lovech regions, respectively. Komosinska-Vassev et al. (2015) described that mean mineral content of 4.00% in pollen. Both the minerals and the type of wall material used can provoke changes in the chemical composition of the final product, reducing its initial pH which is, on average, from 4.00 to 6.00 (Brazil, 2001) (Table 2).
For water activity (Table 2), all values were within the expected range for atomized and lyophilized products, and also within the recommended range to ensure the microbiological, enzymatic, non-enzymatic and oxidative stability of the resultant products (Bobbio and Bobbio, 2001). The pollen extract encapsulated in arabic gum displayed a greater water activity than pollen encapsulated with collagen, and lyophilized pollen. Silva et al. (2013) concluded that the water activity depended on the chemical nature of the hydrocolloids used as wall material. In this case, the chemical structure of the hydrolyzed collagen, due to having a high number of hydrophilic groups along the protein polymer chain, resulted in a higher water binding capacity than the arabic gum encapsulated pollen, significantly reducing the water activity of the pollen encapsulated with collagen. The intensity of this interaction is essential for biocontrol during the storage and marketing of products subjected to drying processes, as moisture content affects water activity, enzymatically and through microbiological stability, and hence the shelf life of the product, as it is the component that causes rapid fermentation and food spoilage (Isengard et al., 2006).
Hygroscopicity and appearance varied similarly in all three treatments (Table 2). The dried particles encapsulated with arabic gum exhibited a lower hygroscopicity tendency than the particles encapsulated in collagen or the lyophilized pollen which, in a saturated environment, can result in the modification of the chemical composition and appearance, favoring microbial multiplication. In studies on the atomization of other types of food, the mean variation in the density values ranged from 0.74 to 0.92g/mL (Botrel et al., 2012).
Total phenols, flavonoids and antioxidant activity
The maximum values of total phenols, flavonoids and antioxidant activity of the lyophilized pollen were 241.57 mg GAE/g , 50.60 mg QE/g and 0.69 mg/g, respectively, which were significantly higher (p <0.05) than the values of the pollen samples encapsulated with hydrolyzed collagen or arabic gum (Table 2). Drying with a lyophilizer in a vacuum was more efficiently concentrated the functional components than drying with a spray drier, in which the particles were formed by the aggregation of substantial quantities of wall material. Bogdanov (2016) reported that pollen should be dried at the lowest temperatures possible, with a maximum of 30°C, with the best alternative being freeze drying.
The results presented a correlation between water content and wall material and the total polyphenol content and antioxidant activity of the pollen preparations. Prelipcean (2012) reported that the storage conditions and the concentration of methanol used as a solvent observed that the total polyphenol content (mg GAE/g) varied from 24.73 to 28.8, and from 21.67 to 26.50, using 96 and 70% methanol, respectively. Stoia et al. (2015) studied that the total phenolic compound content in pollen and propolis in five locations in Romania observed a maximum value of 4.93 ± 0.88 mg GAE/g from dried pollen mass, significantly less than 9.71 ± 0.80 mg GAE/g found for propolis. All these values were significantly lower than those found in the present study for S. bipunctata pollen. Although, it was difficult to compare the results found in this study with the previous studies, as the authors used various solvents and extraction conditions and work with samples from different regions with different concentrations of polyphenols.
Menezes et al. (2010) analyzed that the antioxidant potential of pollen produced by Africanized honeybees from different species of plants had values from 0.72 to 1.99 mg QE/g of pollen from Mimosa pudica and from 1.55 and 2.50 mg QE/g from Eucalyptus pollen. Moreira et al. (2008) described the values of antioxidant activity for propolis extracts of 33.00 mg/g for a concentration of 0.001 g/L and 94.00 mg/g for a concentration of 0.02 g/L.
Silva et al. (2013) reported the antioxidant activity of propolis extract dried by spray dryer with different proportions of arabic gum and modified starch, with concentrations from 2.5 to 5.00 mg/mL. Nori et al. (2011) applied the same technique to encapsulate propolis extracts using protein isolated from soya and pectin as encapsulating agents and obtained propolis in powder form. This was stable, and had its own antioxidant and antimicrobial activity against Staphylococcus aureus, and the property of controlled release in foods.
When analyzing the mean antioxidant activity values of the samples dried in a spray dryer with hydrolyzed collagen and arabic gum, it was possible to identify significant differences (p<0.05). Such reactions are the bases of the method used in this study for the evaluation of antioxidant activity. Moreira et al. (2008) attributed the differences observed to external factors such as handling, storage, and temperature.
The use of hydrolyzed collagen in the encapsulation of pollen allowed an increase in phenol concentration, which was accompanied by an increase in antioxidant activity. An antioxidant to be applied in industrial formulations should be used at low concentrations and present a low risk to the consumer (Bobbio and Bobbio, 2001). These attributes motivated this investigation, which attempts to make available to the market a concentrated option of possible compounds to be consumed as a supplement, industrial ingredient or gastronomic delicacy.
The authors recommended further research with this product such as: submitting the products to sensory testing to understand if pollen encapsulation can attenuate characteristic flavor and odor. Other encapsulation matrices such as starch, maltodextrins and other polysaccharides generate more economically accessible. Kinetic release assays and the testing of the involved mechanisms should be done; the spray dryer drying parameters need to be improved and the antimicrobial activity of the products obtained needs to be evaluated.
From a nutritional perspective, regardless of the type of drying used, the preparations of S. bipunctata pollen studied can be considered an excellent option as a nutritional supplement. This substance significant has phenolic content and capacity to eliminate free radicals, with promising nutritional and physiological implications with a positive effect on health promotion. However, lyophilized S. bipunctata pollen resulted in a product with considerably greater anti-radical activity than pollen dried in a spray dryer.
The use of hydrolyzed collagen considerably increased the final protein content, enhancing its nutritional value and resulting in an excellent option for supplement. Meanwhile, the arabic gum helped to obtain a product rich in fiber and with a low energy value, encouraging its use as an ingredient or additive.
The authors have not declared any conflict of interests.
The authors would like to thank the Conselho Nacional de Desenvolvimento Científico e Tecnológico (the National Council for Scientific and Technological Development) for financial support under process numbers 482947/2013-6 and 311663/2014-1, and the beekeeper Benedito Antonio Uczai of Mandirituba, Curitiba, in the state of Paraná, for supplying the samples of S. bipunctata pollen used in this study.
REFERENCES
|
Alothman M, Bhat R, Karim AA (2009). Antioxidant capacity and phenolic content of selected tropical fruits from Malaysia, extracted with different solvents. Food Chemistry 115(3):785-788.
Crossref
|
|
|
|
Andrade KCS, Carvalho CWP, Takeiti CY, Azeredo HMC, Corrêa JS, Caldas CM (2013). Goma de cajueiro (Anacardium occidentale): Avaliação das modificações químicas e físicas por extrusão termoplástica. Polímeros 23(5):667-671.
Crossref
|
|
|
|
|
Anvisa (2005). Agência Nacional de Vigilância Sanitária. Rotulagem nutricional obrigatória: manual de orientação às indústrias de alimentos – 2ª. Versão. Brasília 44 p.
|
|
|
|
|
Association of Official Analytical Chemists (AOAC) (2012). Association of Official Analytical Chemists. Official methods of analysis of the Association of Official Analytical Chemists. 19th ed., Arlington, USA. 1141 p.
|
|
|
|
|
Barajas J, Cortes-Rodriguez M, Rodriguez-Sandoval E (2012). Effect of temperature on the drying process of bee pollen from zones of Colombia. Journal of Food Process Engineering 35(1):134–148.
Crossref
|
|
|
|
|
Bobbio FO, Bobbio PA (2001). Química do processamento de alimentos. 3rd ed. São Paulo: Varela P 143.
|
|
|
|
|
Bogdanov S (2016). Pollen: Production, nutrition and health: A review. The Pollen Book pp.1-36.
|
|
|
|
|
Botrel DA, Borges SV, Fernandes RVB, Viana AD, Costa JMG, Marques GR (2012). Evaluation of spray drying conditions on properties of microencapsulated oregano essential oil. International Journal of Food Science and Technology47(11):2289-2296.
Crossref
|
|
|
|
|
Brasil (2001). Instrução Normativa n. 3, de 19 de janeiro de 2001. Regulamento técnico de identidade e qualidade de apitoxina, cera de abelha, geleia real, geleia real liofilizada, pólen apícola, própolis e extrato de própolis. Diário Oficial da República Federativa do Brasil, Brasília, DF, 23 de janeiro 2001, Seção 16-1, 18-23. Available at:
View
|
|
|
|
|
Campos MGR, Bogdanov S, Almeida-Muradian LB, Szczesna T, Mancebo Y, Frigerio C, Ferreira F (2008). Pollen composition and standardisation of analytical methods. Journal of Apicultural Research 47(2):154-161.
Crossref
|
|
|
|
|
Costa AJS, Imperatriz-Fonseca VL (2000). Intra and interspecific nestmate recognition in Scaptotrigona workers (Hymenoptera: Apidae: Meliponinae). Anais do IV Encontro sobre Abelhas. p. 283.
|
|
|
|
|
DerMarderosian A, Beuther JA (2005) Review of natural products: facts and comparisons. 4.ed. St Louis: Wolters Kluwer Health Inc. P 1343.
|
|
|
|
|
Dinkov D, Stratev D (2016). The content of two toxic heavy metals in Bulgarian bee pollen. International Food Research Journal 23(3):1343-1345.
|
|
|
|
|
Instituto Adolfo Lutz (2005). Métodos físico-químicos para análise de alimentos. 4 ed. Brasília: Ministério da Saúde.
|
|
|
|
|
Isengard HD, Kling R Reh CT (2006). Proposal of a new reference method to determine the water content of dried dairy products. Food Chemistry 96(3):418-422.
Crossref
|
|
|
|
|
KÄ™dzia B, HoÅ‚derna-KÄ™dzia E (2012). Nowe badania nad biologicznymi wÅ‚aÅ›ciwoÅ›ciami pyÅ‚ku kwiatowego. PostÄ™py Fitoterapii 13(1): 48–54.
|
|
|
|
|
Komosinska-Vassev K, Olczyk P, Kaźmierczak J, Mencner L, Olczyk K (2015). Bee pollen: Chemical composition and therapeutic application. Journal of Evidence-Based Complementary and Alternative Medicine pp. 1-6.
Crossref
|
|
|
|
|
Lima Neto JS, Lopes JAD, Moita Neto JM, Lima SG, Luz CFP, Citó AMGL (2017). Volatile compounds and palynological analysis from pollen pots of stingless bees from the mid-north region of Brazil. Brazilian Journal of Pharmaceutical Sciences 53(2):e14093.
Crossref
|
|
|
|
|
Melo ILP, Almeida-Muradian LB (2011). Comparison of methodologies for moisture determination on dried bee pollen samples. Ciência e Tecnologia de Alimentos 31(1): 194-197.
Crossref
|
|
|
|
|
Menezes JDS, Maciel LF, Miranda MS, Druzian JI (2010). Compostos bioativos e potencial antioxidante do pólen apícola produzido por abelhas africanizadas (Apis mellifera L.). Revista do Instituto Adolfo Lutz 69(2):233-242.
|
|
|
|
|
Mensor LL, Menezes FS, Leitão GG, Reis AS, dos Santos TC, Coube CS, Leitão SG (2001). Screening of Brazilian plant extracts for antioxidant activity by the use of DPPH free radical method. Phytotherapy Research 15(2):127-130.
Crossref
|
|
|
|
|
Moreira L, Dias LG, Pereira, JA, Estevinho L (2008). Antioxidant properties, total phenols and pollen analysis of propolis samples from Portugal. Food and Chemical Toxicology 46(11):3482-3485.
Crossref
|
|
|
|
|
Nori MP, Favaro-Trindade CS, Alencar SM, Thomazini M, Balieiro JCC, Castillo CJC (2011). Microencapsulation of propolis extract by complex coacervation. Journal of Food Science and Technology 44(2):429-435.
|
|
|
|
|
Oliveira PS, Vasconcelos MAM, Venturieri GC, Pontes MAM, Gonçalves ACS, Carvalho AV (2007). Composição físico-química de amostras de pólen de abelhas indígenas sem ferrão coletadas na região Amazônica. Anais do Simpósio Latino Americano de Ciência de Alimentos, P 1. Available at:
View
|
|
|
|
|
Prelipcean AA (2012). The dynamics of total polyphenols, flavonoids and antioxidant activity of beepollen collected from Moldavia area, Romania. Cercetări Agronomice în Moldova 45(1):81-92.
|
|
|
|
|
Rebelo KS, Ferreira AG, Carvalho-Zilse GA (2016). Physicochemical characteristics of pollen collected by Amazonian stingless bees. Ciência Rural 46(5):927-932.
Crossref
|
|
|
|
|
Rocha GA, Fávaro-Trindade CS, Grosso CRF (2012). Microencapsulation of lycopene by spray drying: Characterization, stability and application of microcapsules. Food and Bioproducts Processing 90(1):37-42.
Crossref
|
|
|
|
|
Roginsky V, Lissi EA (2005). Review of methods to determine chain-breaking antioxidant activity in food. Food Chemistry 92(2): 235-254.
Crossref
|
|
|
|
|
SAS Institute (2012). SAS/STATTM SAS user's guide for windows environment.9.3 ed. Cary: SAS Institute, USA.
|
|
|
|
|
Silva FC, Fonseca CR, Alencar SM, Thomazini M, Balieiro JCC, Pittia P, Favaro-Trindade CS (2013). Assessment of production efficiency, physicochemical properties and storage stability of spray-dried propolis, a natural food additive, using arabic gum and OSA starch-based carrier systems. Food and Bioproducts Processing 91(1):28-36.
Crossref
|
|
|
|
|
Silva GR, Natividade TB, Camara CA, Silva EMS, Santos FAR, Silva TMS (2014). Identification of sugar, amino acids and minerals from the pollen of Jandaíra stingless bees (Melipona subnitida). Food and Nutrition Sciences 5(11):1015-1021.
Crossref
|
|
|
|
|
Singleton VL, Orthofer R, Lamuela-Raventós RM (1999). Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Methods Enzymology 299C(1):152-178.
Crossref
|
|
|
|
|
Stoia M, Cotinghiu A, Budin F, Oancea S (2015). Total phenolics content of Romanian propolis and bee pollen. Acta Oecologica Carpatica 8:75-82.
|
|
|
|
|
Szczęsna T (2006). Long-chain fatty acids composition of honeybee-collected pollen. Journal of Apicultural Science 50(2):65-79.
|
|
|
|
|
Venturieri GC (2008). Criação de abelhas indígenas sem ferrão. 2nd. ed. Belém, PA: Embrapa Amazônia Oriental, 2ª impressão. Available at: View
|
|
|
|
|
Villas-Bôas J (2012). Manual tecnológico mel de abelhas sem ferrão. Brasília, DF–ISPN. Available at:
View
|
|