ABSTRACT
Globally, there is an increasing report of bacterial resistance to currently available antibiotics. The urgent need for newer therapeutic modalities was the incentive for the current evaluation of bactericidal and inhibitory effects of methanolic and aqueous extracts of Mitracarpus villosus on selected antibiotic resistant Gram-positive and Gram-negative bacteria. Ten-fold serial dilution was used to test the effects of the extracts. The percentage yield of extractable components was 22% for methanol and 8.3% for water. The minimum inhibitory concentration (MIC) of methanolic extract was 0.001 mg/mL against Streptococcus pyogenes, Enterococcus faecalis, Escherichia coli, Pseudomonas aeruginosa, and Salmonella Typhi showing resistance to antibiotics and 0.01 mg/mL for Staphylococcus aureus, Bacillus subtilis, and Klebsiella pneumoniae while the minimum bactericidal concentration (MBC) was 0.1 mg/mL for all the isolates except S. aureus and E. coli with MBC of 0.01 mg/mL. Similarly, the MIC of aqueous extract was 0.01 mg/mL for S. pyogenes, S. aureus, B. subtilis, and K. pneumoniae; 0.1 mg/mL against S. faecalis and E. coli; 0.001 mg/mL against P. aeruginosa and S. typhi. The MBC of the aqueous extract at 0.1 mg/mL was active against S. pyogenes, S. faecalis, P. aeruginosa, K. pneumonia, and S. typhi; 1.0 mg/mL was active against S. aureus, B. subtilis, and E. coli. Findings from the present study indicate that the extracts of M. villosus have the potential to be used for treatment and cure of infections caused by selected bacteria. The methanolic extracts show more potent activity. Further study on a larger scale at varied concentrations including a test for potential toxicity in cultured cells are needed to depict their safety profiles before recommendation for inclusion in the antibacterial armamentarium.
Key words: Minimum inhibitory concentration (MIC), minimum bactericidal concentration (MBC), M. villosus, bioactive compounds.
A large number of compounds of therapeutic values are traditionally produced from plants that possess antimicrobial activity (Khameneh et al., 2019). Successful defense mechanisms developed by these plants are responsible for the scarcity of infective diseases in those plants (Bolla et al., 2011). A report of Sivananthan (2013)
indicates the potential inhibitory effects of some bioactive compounds on the life processes of disease-causing bacteria, either alone or in combination with other agents. Several studies have explored the potential of medicinal plant administration for the treatment of bacterial related infections (Irobi and Daramola, 1994; Panda, 2014; Martins and Nunez, 2015). However, most of these published reports are from countries where traditional medicines for curative treatment of diverse diseases are frequently adopted owing to economic or practical reasons. Kokoska et al. (2019) and Shedoeva et al. (2019) mention the antimicrobial activity of Aloe vera that has been used for the local treatment of wounds, burns, and infections since ancient times from several in vitro, in vivo, and clinical studies. One major drawback is the concern of safety. A recent study highlighted the toxicity property of some of the diverse plants frequently used in traditional medicine (Mabona and Van Vuuren, 2013). While this needs to be addressed, it is not an exaggeration to say that the health benefits accrued to their use have been enormous.
The plant Mitracarpus villosus is abundantly distributed on farmlands as a weed across the Southern and Northern parts of Nigeria (Onyiloyi et al., 2005), and as also in Senegal, Mali, Liberia, and Gambia (Abere et al., 2007). Previous phytochemical studies and antimicrobial sensitivity tests of the leaf of M. villosus extract reported the potential antibacterial activity of the plants on a few organisms (Irobi and Daramola, 1994; Gbala and Anibijuwon, 2018). Most traditional medicines in West Africa use M. villosus as a remedy for toothaches, headaches (Dalziel, 1992), amenorrhea, leprosy, hepatic diseases, skin diseases (Kerharo and Adam, 1974), venereal disease (Medical Online News, 2019), neurological disorders (John-Africa et al., 2014), dyspepsia, and dysentery (Fabri et al., 2012). Antibacterial effects of the crude ethanolic extract have also been reported for Bacillus subtilis, Streptococcus pyogenes, Escherichia coli, and Staphylococcus aureus (Okoye and Okafor, 2013).
Information regarding the M. villosus (Swartz) DC plant commonly used for local treatment of wound infections is scarce. Traditional plants of known medicinal values are a rich source of active biological compounds with high antibacterial properties (Karygianni et al., 2014). The presence of compounds of low molecular weight, phytoalexins, in medicinal plants including the M. villosus plant has been shown to possess antibacterial activity (Hemaiswarya et al., 2008). Bioactive compounds including flavonoids, polyphenols, glycosteroids, and terpenoids have been reported in M. villosus (Hemaiswarya et al., 2008). Daiane and Cecilia (2015) and Ekpendu et al. (2001) reported a reasonable amount of triterpenes, terpenoids, and glycosides, in association with M. villosus known to exert various medicinal properties.
Nigeria has an abundant flora, and many species are believed to possess curative activities. Nevertheless, many of these claims have limited scientific validation. The current study may validate the antibacterial activities of M. villosus especially against multidrug-resistant bacteria including Pseudomonas aeruginosa, S. aureus, and E. coli, frequently implicated in delayed healing and infections.
Considering the increasing resistance to currently available antibiotics, the need to develop novel and more potent antibacterial agents to combat the global challenge of antimicrobial resistance is of high priority. Antibiotic resistance remains a serious threat to an effective prevention and treatment of an ever-increasing range of infections caused by bacteria (WHO, 2020). Antibiotic resistance contributes to increase in the cost of health care due to longer duration of illness, additional tests, use of more expensive drugs and more intensive care. Factors such as accumulation of mutations in bacterial genome due to drug selection pressure or natural mutations, misuse and overuse of antimicrobials are associated with increase prevalence of antibiotic resistance (WHO, 2020). The problem of multi-drug resistance is compounded by the widespread indiscriminate use of antibiotics that over time has enhanced bacterial pathogenic potential leading to default in treatment of infections caused by them. This study sought to determine the susceptibility of selected antibiotic resistant Gram-positive and Gram-negative bacteria commonly implicated in diseases in the study area to crude methanolic and aqueous extracts of M. villosus. Extract of M. villosus is frequently used in different communities in Africa for treating various diseases (Neuwinger, 2000) without knowing the active ingredients and pharmacological basis of their action. Identifying the bioactive compounds and its refinement as well as optimization may set the pace for future studies on the toxicity and safety profiles of the use of the plant extract.
Sample collection and preparation
The whole plant of M. villosus (Swartz) DC was collected from the Botanical Garden of Kogi State University, Anyigba, Nigeria (Figure 1). The plant was identified and authenticated in the Department of Plant Science and Biotechnology of the same university. The sample (whole plant of M. villosus) was washed and dried at room temperature in the laboratory for two weeks. Using mortar and pestle, the sample was pulverized to powdery form and placed in a polythene bag until ready for extraction. The maceration method with Soxhlet (solvent) and aqueous (water) were used to extract the bioactive agents.

Test organisms
Eight bacteria were used: S. pyogenes, S. aureus, Enterococcus faecalis, B. subtilis, E. coli, P. aeruginosa, Klebsiella pneumoniae, and Salmonella species. The bacteria are clinical isolates with resistance to cotrimoxazole, ampicillin, tetracycline, cefotaxime, amoxicillin clavulanic acid, ciprofloxacin, ceftazidime, cefoxitin, and gentamicin in the study area. They were obtained from the stock culture of the Microbiology Laboratory, Kogi State University, Anyigba.
Aqueous extraction was carried out as described by Irobi and Daramola (1994). Briefly, about 120 g of the powdered plant sample of M. villosus was weighed and dispensed in 250 mL of distilled water in a clean and sterile beaker. The resulting solution was boiled for 20 min on a Bunsen burner and then cooled. The extract was filtered and the filtrate was collected in a sterile and clean conical flask.
For Soxhlet extraction, about 120 g of pulverized plant sample was subjected to Soxhlet extraction as described by Akinyemi et al. (2000) using 800 mL of extraction solvent (methanol) at 80°C for 6 h for complete extraction. This was performed in the chemistry laboratory of Kogi State University, Anyigba. 120 g of the powdered plant sample was weighed, packaged in a serviette paper, tied with a thread, and placed inside the thimble. The latter was loaded into the main chamber of the Soxhlet extractor. The solvent for extraction was placed in a distillation flask which was then connected to the heating element. The Soxhlet extractor was placed atop the flask followed by a reflux condenser and the cycles of extraction began. After 6 h of several cycles, the compound was concentrated in the distillation flask and evaporated to dryness on a rotary evaporator at 78°C for 60 min. The soluble component was retained while the non-soluble portion of the extracted solid still in the thimble was discarded. The same procedure was repeated until the desired material was completely extracted from the plant.
Phytochemical screening of extracts
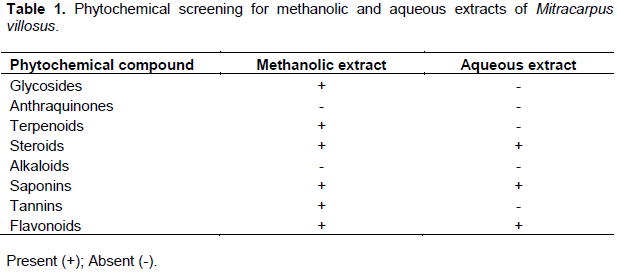
Both water and methanol extracts were screened for the presence of tannins, saponins, alkaloids, glycosides, flavonoids, carbohydrates, terpenes, and anthraquinones by adopting the methods described by Trease and Evans (2009) (Table 1).
Test for carbohydrates
One gram of the extract (water or methanol) was transferred to the test tube and 5 mL of an equal mixture of Fehling’s solution A and B was added and boiled in a water bath, the formation of a brick-red precipitate is suggestive of the presence of reducing sugar.
Test for cardiac glycosides
One gram of the extract was dissolved in 1 ml of glacial acetic acid containing traces of Ferric chloride solution and the resulting mixture was transferred into a dry test tube and 1 ml of concentrated sulphuric acid was added down the side of the test tube to form a lower layer at the bottom. The formation of a purple-brown ring is suggestive of cardiac glycoside's presence (Bhatt, 2019).
Test for saponins
To 1 g of the extract, 10 mL of distilled water was added, shaken vigorously for 30 s, and the tube allowed to stands in an upright position. The tube was observed for 30 min for honeycomb froth that persisted for 10-15 min, indicative of saponins.
Test for steroids and triterpenes
One gram of the extract and equal volume of acetic acid anhydride were mixed gently in a test tube after which 1 mL of concentrated sulphuric acid was added down the side of the test tube to form a layer. Color changes were observed immediately and over 1 h. Immediate formation of blue color at the upper layer and a reddish color indicated the presence of steroids and triterpenes, respectively.
Test for flavonoids
To 1 g of extract, a few drops of ferric chloride solution were added and the formation of a green precipitate indicated the presence of phenols.
Test for tannins
Three to 5 drops of ferric chloride solution were added to a portion of 1 g of the extract, and the resulting dark green precipitate indicated the presence of tannins.
Test for alkaloids
A few drops of Wagner's reagent were added to a portion of 1 g extract and the formation of a whitish precipitate is suggestive of alkaloids presence.
Test for free anthraquinones
Five milliliter of chloroform was added to a portion of 1 g extract in a dry test tube and then shaken to mix properly. The resulting solution was filtered to obtain filtrate, mixed with an equal volume of 10% ammonia solution. The formation of bright pink color in the aqueous (upper) layer is suggestive of free anthraquinones.
Determination of percentage yield of plant extract
The following is the equation for plant extract (Terblanche et al., 2017):
Preliminary screening of extract concentrations for antibacterial activity by agar well diffusion method
One milliliter of each test organism at 108 CFU/mL was used to flood Mueller-Hinton agar plates (prepared separately for methanolic and aqueous extracts) at aseptic conditions and allowed to solidify. On each of the solidified agar plates, six wells were done with a sterile cork-borer (diameter of 6 mm). The prepared plant extracts, 100% extract concentration, diluted concentrations (1, 0.1, 0.01 and 0.001 mg/mL) and distilled water as control, were loaded into each of the wells separately. Following the incubation at 37°C for 24 h, the presence of visible zones of inhibition around the wells shows the extract can inhibit the test organism at that concentration. The wells filled with distilled water show no visible zone of inhibition (NCCLS, 2006).
Evaluation of antibacterial activity by macrodilution technique
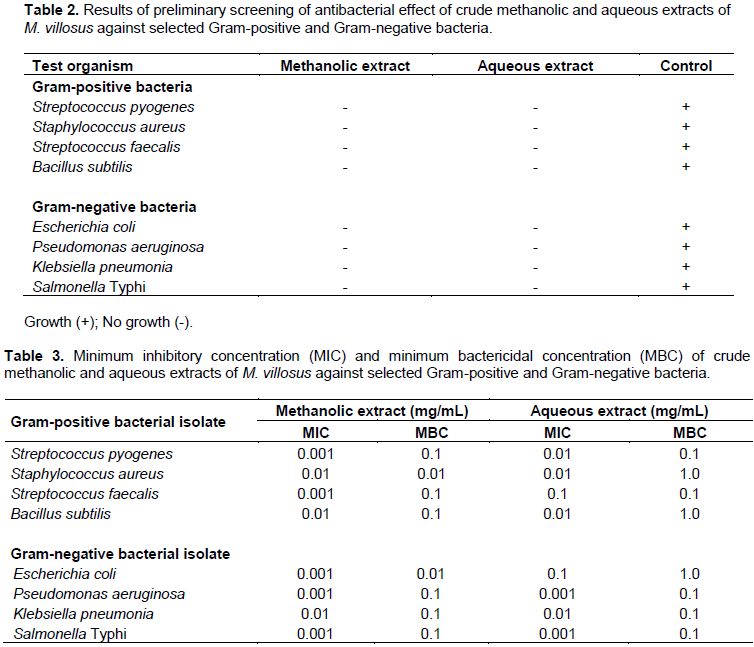
Following the National Committee for Clinical Laboratory Standards (2006), suspensions of micro-organisms was prepared in sterile normal saline and adjusted to 0.5 MacFarland standard (1.5 × 108 Cfu/mL), minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) were determined (Jones et al., 2002; Wikler, 2006). Mueller-Hinton broth was added (1 mL) to each tube. Then, 100% concentration of the whole plant extract was added to the first well and the other four concentrations of the extract (1, 0.1, 0.01 and 0.001 mg/mL, respectively) were prepared by serial macrodilution technique. Bacterial suspensions (1.5 × 108 CFU/mL), 1 mL each was added to each tube, and mixed. In each case of the extract (aqueous or methanolic), a medium in the tube containing bacterial suspension and another containing extracts were used as positive and negative controls, respectively. The tubes were incubated for 24 h at 37°C and the cloudiness was examined via unaided eyes. Tubes without turbidity were considered as MIC and subcultured on a Mueller-Hinton broth, incubated for 24 h at 37°C. The MBC was determined according to the methods described by Irobi and Daramola (1994) with little adjustments. The lowest concentration at which the extract did not permit any growth of a particular microorganism was considered MBC. This experiment was repeated three consecutive times for each methanolic and aqueous extracts.
The percentage yield of crude aqueous extract and methanol extract was 9.4 g (corresponding to 8.3%) and 26.4 g (corresponding to 22%), respectively. Table 1 depicts the results of the phytochemical screening. In the methanolic extract, anthraquinones and alkaloids tested negative while glycosides, terpenoids, steroids, saponin, tannin, and flavonoids tested positive. In the aqueous extract, glycosides, anthraquinones, terpenoids, alkaloids, and tannins tested negative while flavonoids, steroids, and saponins tested positive. The results of the antibacterial effects of the crude extract varied at different concentrations against both Gram-positive and Gram-negative bacteria are shown in Table 2. Results showed that none of the selected organism could grow in the presence of either the methanolic or water crude extract preparations. The Minimum Inhibitory Concentrations (MICs) and the Minimum Bactericidal Concentrations (MBCs) of the crude extracts against the selected bacteria are depicted in Table 3. The MIC of methanolic extract at 0.001 mg/mL was active against S. pyogenes, S. faecalis, E. coli, P. aeruginosa, and S. Typhi except three isolates. The MBC of the methanolic extract was 0.1 mg/mL for all the isolates except S. aureus and E. coli which was at 0.01 mg/mL. Similarly, the MIC of aqueous extract at 0.01 mg/mL was active against S. pyogenes, S. aureus, B. subtilis, and K. pneumonia while the MBC was at 0.1 mg/mL against all the isolates except S. aureus, B. subtilis, and E. coli.
Our findings showed that both extracts (methanolic and aqueous) have strong inhibitory effects on the growth of the test organisms. The results of the phytochemical study in extracts of M. villosus revealed the presence of bioactive agents such as tannins, flavonoids, saponins, glycosides, terpenoids, and steroids, all of which exhibited antibacterial activity. Our finding supports previous reports by Ubani et al. (2012) and Gbala and Anibijuwon (2018) which indicated that tannins, flavonoids, saponins, glycosides, terpenoids, and steroids are capable of bacterial growth inhibition. However, variations in active substance detection have been documented. Ouadja et al. (2018) identified phenolic and alkaloids in a different species (Mitracarpus scaber) of the same plant which were not identified in the current study, suggesting that variability in bioactive compounds extraction or bio-responsiveness in plant estrogen levels may affect their detection. Ubani et al. (2012), also attributed differences in bioactive substance detection to variation in geographical location as a result of soil mineral concentration.
Results of MIC and MBCs fractions of the methanolic and aqueous extracts shown in Tables 2 and 3 revealed the promising antibacterial activity of the extracts against species of Gram-positive and Gram-negative bacteria such as S. pyogenes, S. aureus, S. faecalis, B. subtilis, E. coli, P. aeruginosa, K. pneumoniae, S. typhi, etc. This finding suggests that, isolation of the active compounds is needed to enable further development of this plant as a source for anti-bacterial substances and the potential pharmacognostic properties of the extract when developed, could surmounts the global treatment challenges associated with antibiotics resistant pathogens frequently encountered in clinical practice.
Findings from the present study showed that the methanolic extract of M. villosus inhibited the selected test organisms better than the aqueous extract, as can be seen from the lower MIC and MBC. This is supported by the higher amounts of bioactive compounds obtained in Table 1 that are known to inhibit and/or kill the bacteria at lower concentrations compared to aqueous (water) extract. Previous reports of Abdelrahim et al. (2017) also showed that the methanol extract of Mitracarpus has a higher inhibitory effect on the growth of B. subtilis, S. aureus, E. coli, and P. aeruginosa compared to the aqueous extract.
The fact that crude extracts of this medicinal herb inhibit some Gram-positive and Gram-negative bacteria indicates the presence of potent antibacterial activities which can be developed (Zahra et al., 2020). Although both the aqueous and methanolic extracts of M. villosus produced inhibitory action against the bacteria, the latter was more active against the bacteria. The need for lower concentrations of the methanol extracts for antibacterial activity compared to the aqueous extracts could be attributed to the efficacy of solvents for extraction of active compounds and also suggests that methanol is a better solvent for isolation of antibacterial principles.
In conclusion, methanolic and aqueous extracts of M. villosus have been shown to possess antibacterial activity on all selected test organisms in this study. The inhibitory effect demonstrated by this extract against test isolates even at their lower concentrations depicts the ethnopharmacological benefit. This observation supports other scientific articles that reported that extract of the plant has potential therapeutic values against S. pyogenes, S. faecalis, E. coli, P. aeruginosa, S. Typhi, S. aureus, B. subtilis, and K. pneumonia resistant isolates commonly associated with various ailments as claimed by traditional medicine healers. Despite the inhibitory effects of extracts of M. villosus on selected clinical isolates that have shown relative resistance to the conventional antibiotics including cotrimoxazole, ampicillin, tetracycline, cefotaxime, amoxicillin clavulanic acid, and ciprofloxacin in the study area (Mofolorunso et al., 2020), further study on individual isolated compounds and their potential for toxicity and safety profiles are needed.
The authors have not declared any conflict of interests.
The authors thank the laboratory staff of the Microbiology and Chemistry Departments for providing the enabling environments during the study.
REFERENCES
|
Abdelrahim A, Oibiokpa IF, Musa D, Inobenme A (2017). Phytochemical constituents and antimicrobial activity of some medicinal plants used for treating skin diseases in Bosso Local Government, Niger State, Nigeria. Journal of Complementary and Alternative Medical Research 3(3):1-9.
Crossref
|
|
|
|
Abere TA, Onwukaeme DN, Eboka CJ (2007). Phamacognostic evaluation of the leaves of Mitracarpus villosus. Topical Journal of Pharmacologial Research 6(4):849-851.
Crossref
|
|
|
|
|
Akinyemi KO, Coker AO, Bayagbon C, Oyefolu AOB, Akinsinde K, Omonigbehin EO (2000). Antimicrobial Screening of Five Nigerian Medicinal plants against S. typhi and S. paratyphi. Nigerian Journal of Infection Control 3:1-4.
Crossref
|
|
|
|
|
Bhatt R (2019). Chemical tests for glycosides: General and specific: GPAT-India.
View
|
|
|
|
|
Bolla JM, Alibert-Franco S, Handzlik J, Chevalier J, Mahamoud A, Boyer G (2011). Strategies for bypassing the membrane barrier in multi-drug-resistant Gram-negative bacteria. FEBS Letter 585(11):1682-1690
Crossref
|
|
|
|
|
Daiane M, Cecilia VN (2015). Secondary Metabolites from Rubiaceae Species. Molecules 20(7):13422-13495.
Crossref
|
|
|
|
|
Dalziel JM (1992). Useful Plants of West Tropical Africa. London: The Crown Agents for Over areas Colonican 32:661-663.
|
|
|
|
|
Ekpendu TOE, Adesomoju AA, Okogun JI (2001). Chemical Studies of Mitracarpus villosus (Sw.) Dc-A Medicinal Rubiaceous Weed. Journal of Chemical Society of Nigeria 26:69-71.
|
|
|
|
|
Fabri RL, Braga FG, Bouzada MLM, Matos MO, Moreira FO, Scio E, Coimbra ES (2012). Antileishmanial and antifungal activity of plants used in traditional medicine in Brazili. Journal of Ethnopharmacology 111:396-402.
Crossref
|
|
|
|
|
Gbala ID, Anibijuwon II (2018). Antibacterial activity of Terminalia glaucescens, Mangifera indica and Mitracarpus villosus on Carbapenem-resistant Enterobacteriaceae. African Journal of Clinical and Experimental Microbiology 19(4):251-259.
Crossref
|
|
|
|
|
Hemaiswarya S, Kruthiventi AK, Doble M (2008). Synergism between natural products and antibiotics against infectious diseases. Journal of Phytomedicine 15:639-652.
Crossref
|
|
|
|
|
Irobi ON, Daramola SO (1994). Bactericidal properties of crude extracts of Mitracarpus villosus. Journal of Ethnopharmacology 42(1):39-43.
Crossref
|
|
|
|
|
John-Africa LB, Danjuma NM, Anuka JA, Chindo BA (2014). Sedative properties of Mitracarpus villosus leave in mice. International Journal of Biology and Chemical Society 8(5):2132-2142.
Crossref
|
|
|
|
|
Jones RN, Pfaller MA, Rhomberg PR, Walter DH (2002). Tiamulin activity against fastidious and non-fastidious veterinary and human bacterial isolates: Initial development of in vitro susceptibility test methods. Journal of Clinical Microbiology 40:461-465.
Crossref
|
|
|
|
|
Karygianni L, Cecere M, Skaltsounis AL, Argyropoulou A, Hellwig E, Aligiannis N, Wittmer A, Al-Ahmad A (2014). High-level antimicrobial efficacy of representative Mediterranean natural plant extracts against oral microorganisms. BioMed Research International 2014:839019.
Crossref
|
|
|
|
|
Kerharo J, Adam JG (1974). In Phariacopeesene galasae traditional medicinal and toxic plants Editions, Vigot Freres. Paris. France pp. 692-693.
|
|
|
|
|
Khameneh B, Iranshahy M, Soheili V, Bazzaz BSF (2019). Review on plant antimicrobials: a mechanistic viewpoint. Antimicrobial Resistance and Infection Control 8:118.
Crossref
|
|
|
|
|
Kokoska L, Kloucek P, Leuner O, Novy P (2019). Plant-derived products as antibacterial and antifungal agents in human health care. Current Medicinal Chemistry 26(29):5501-5541.
Crossref
|
|
|
|
|
Mabona U, Van-Vuuren SF (2013). Southern African medicinal plants are used to treat skin diseases. South African Journal of Botany 87:175-193.
Crossref
|
|
|
|
|
Martins D, Nunez CV (2015). Secondary Metabolites from Rubiaceae Species. Molecules 20:13422-13495.
Crossref
|
|
|
|
|
Medical Online News (2019).
View
|
|
|
|
|
Mofolorunso KC, Ocheni HO, Aminu RF, Omatola CA, Olowonibi OO (2020). Prevalence and antimicrobial susceptibility of extended-spectrum beta lactamases-producing Escherichia coli and Klebsiella pneumoniae isolated in selected hospitals of Anyigba, Nigeria. African Health Sciences, WKRO-2020-01-0111-R1. eISSN:1680-6905. In Press.
|
|
|
|
|
National Committee for Clinical Laboratory Standards (NCCLS) (2006). Methods for dilution, antimicrobial susceptibility tests for bacteria that grow aerobically. 17th ed.
|
|
|
|
|
Neuwinger HD (2000). African Traditional Medicine. A dictionary of plant use and applications. Medpharm Scientific Publishers, Stuttgart pp. 63-72.
|
|
|
|
|
Okoye EI, Okafor KO (2013). Phytochemical screening of the leaves of Mitracarpus villosus and the production of phytodrug for the treatment of skin infections. International Journal of Science and Research 4:87-92.
|
|
|
|
|
Onyiloyi AG, Abdulkarim A, Sadiq Y, Umar ZA, Mukhtar AE (2005). Evaluation of five medicinal plants used in diarrhea treatment in Nigeria. Journal of Ethnopharmacology 101:27-30.
Crossref
|
|
|
|
|
Ouadja B, Anani K, Djeri B, Ameyapoh YO, Karou DS (2018). Evaluation of the phytochemical composition, antimicrobial and anti-radical activities of Mitracarpus scaber (Rubiaceae). Journal of Medicinal Plants Research 12(28):493-499.
Crossref
|
|
|
|
|
Panda SK (2014). Ethno-medicinal uses and screening of plants for antibacterial activity from Similipal Biosphere Reserve, Odisha. Indian Journal of Ethnopharmacology 151(1):158-175.
Crossref
|
|
|
|
|
Shedoeva A, Leavesley D, Upton Z, Fan C (2019). Wound healing and the use of medicinal plants. Evidence-Based Complementary Alternative Medicine. 2684108.
Crossref
|
|
|
|
|
Sivananthan M (2013). Antibacterial activity of 50 medicinal plants used in folk medicine. International Journal of Bioscience 3(4):104-121.
Crossref
|
|
|
|
|
Trease GE, Evans WC (2009). Trease and Evans pharmacognosy. 16th Edition, BalliereTindau W. B Sauders Company Ltd. London pp. 224-575.
|
|
|
|
|
Terblanche U, Semakalu CC, Mtunzi F, Pillay M (2017). Screening of Variables Influencing Extraction Yield of Cotyledon orbiculata: 2 3 Full Factorial Design. International Journal of Pharmacognosy and Phytochemical Research 9(3):303-312.
|
|
|
|
|
Ubani CS, Oje OA, Ihekogwo FNP, Eze EA, Okafor CL (2012). Effect Of Varying Soil Minerals And Phytochemical Parameters on Antibacterial Susceptibility Of Mitracarpus Villosus Ethanol Extracts; Using Samples From South East And South-Southern Regions Of Nigeria. Global Advanced Research Journal of Microbiology 1(7):120-125.
|
|
|
|
|
Wikler MA (2006). Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically: Approved standard. CLSI (NCCLS) 26:M7 A.
|
|
|
|
|
World Health Organization (WHO) (2020). Fact sheet on antimicrobial resistance.
|
|
|
|
|
Zahra G, Faezeh K, Reihaneh A, Zahra Z, Leila S, Rastin S (2020). Inhibitory effects of ethanolic, methanolic, and hydroalcoholic extracts of olive (Olea europaea) leaf on growth, acid production, and adhesion of Streptococcus mutants. Dental Research Journal 2020 | Published by Wolters Kluwer - Medknow.
|
|