ABSTRACT
This paper studied the safety and physiological effect of acute intake of Bifidobacterium adolescentis CH2 in albino rats using yogurt as a carrier. Thirty-six female albino rats were divided into three groups: control group received standard diet and water daily; yogurt group received standard diet, water, and 0.5 ml yogurt; B. adolenscentis-yogurt (probiotic yogurt) group received standard diet, water, and 0.5 ml B. adolenscentis CH2-yogurt, at a dose of 3.6 × 106 cfu B. adolescentis CH2/animal. Yogurt and B. adolenscentis CH2-yogurt administration was done daily by oral gavage for four weeks. Rats were monitored daily for feed intake and weight, and weekly for organ conditions and functions. Histology of liver and kidney were performed at week 3 and 4. There was no significant statistical difference in feed intake for the three groups. Results established no significant difference in average organ/body weight ratios of liver, lung, heart, and spleen in groups at week 4. Concentration of clinical parameters- albumin, bilirubin, aspartate aminotransferase, glucose, total protein, and urea- indicated no significant difference among groups. This study recorded lower triglycerides and total cholesterol levels in B. adolescentis-yogurt group in week 4. Kidney and liver histopathology confirmed that the studied B. adolescentis had no negative effects on rat liver and kidney. Significant reduction (P<0.05) in body weight gain for B. adolescentis-yogurt group was observed from week three. Our findings showed that B. adolescentis CH2 is safe for acute intake. Results suggested a hypolipidemic and weight reduction effect on prolong usage, indicating a potential for application in weight management and cardiovascular disease control.
Key words: Bifidobacterium adolescentis, probiotics, in vivo, safety, hypolipidemic.
Probiotics as key gut microbiome contribute functional genes that affect host physiology and health (Greenhalgh et al., 2016). Certain species of Lactic Acid Bacteria (LAB) and bifidobacteria are commonly used as probiotics because of their well-known beneficial effects to host health (Goldin and Gorbach, 1992; Oakey et al., 1995; O’Bryan et al., 2013). Studies have shown that probiotics can stimulate the immune system, decrease serum cholesterol, alleviate lactose intolerance, control infections, act as antibiotics, suppress tumors and protect against colon/bladder cancer (Bordoni et al., 2013; Di Gioia et al., 2014; Ejtahed et al., 2011; Isolauri et al., 2012; Scheinbach, 1998). The antiviral activity of
Bifidobacterium adolescentis was also reported (Cha et al., 2012;
Kim et al., 2014).
Biï¬dobacterium species
are among the most widely used and studied probiotic microorganisms.
Bifidobacterium species predominance in the gut is reported to be due to their possession of large number of genes required for the degradation and use of a wide range of carbohydrates, outnumbering what is found in other gut microbes (Milani et al
., 2014, 2016; O’Callaghan and van Sinderen, 2016). Species of bifidobacteria considered as probiotics include:
Bifidobacterium infantis,
B. adolescentis,
Bifidobacterium animalis subsp
animalis,
Bifidobacterium animalis subsp
lactis,
Bifidobacterium bifidum,
Bifidobacterium longum, and
Bifidobacterium breve (Fijan, 2014).
In vivo animal toxicity studies of probiotics at a dose relative to the anticipated intake provide suitable evidence of safety. Absence of observable harmful effect in acute toxicity test for a probiotic strain is considered a good indication of the possible safety of the strain for human consumption, although human studies are necessary before final application in human. In toxicological studies, abnormalities in animal weights, concentrations of blood biochemical parameters, organ/body weight ratios, and histopathology of relevant tissues are some biomarkers that underline evidence of negative impact of a consumed substance. For instance, a high level of total cholesterol and triglycerides indicate hyperlipidemia, a metabolic syndrome and risk factor in coronary heart disease (
Dhingra et al., 2014; Kolovou et al., 2005).
Live microorganisms, such as probiotics, are often dosed through a carrier. Dairy products are recognized as ideal vehicle for delivering probiotic bacteria to the human gastrointestinal tract (Homayouni et al., 2012). Yogurt is one of the most popular fermented dairy products known for centuries to have universal acceptance in terms of nutritional and health benefits. It is considered as a healthy food due to its high digestibility and bioavailability of nutrients (Yadav et al., 2015). Though researchers previously reported that ingestion of some Bifidobacterium strains caused no observable harmful effects in animals (Chenoll et al., 2011; Mohan et al., 2006; Odamaki et al., 2007; Salazar et al., 2011; Xiao et al., 2006) and European Food Safety Authority (2017) recognized B. adolescentis as presumptively safe, it is still needful to ensure the safety of any probiotic microorganism prior to its application in product development. Continuous surveillance is also necessary given the fact that most probiotic in vivo effects are strain specific and that microbial genetic change is always a possibility. The objective of this study was to evaluate the in vivo physiological effects of an isolated strain of B. adolescentis proposed to be used as a probiotic agent, in experimental rats, using yogurt as the delivery vehicle.
Bacterial strain and growth condition
B. adolescentis strain, designated B. adolescentis CH2, was isolated from chicken (Gallus gallus domesticus). Its identification and probiotic properties investigations were previously reported (Onyibe et al., 2013a, b). B. adolescentis CH2 was cultivated by growing in De Man Rogosa and Sharpe (MRS) medium (Oxoid) adjusted to pH 5.8 – 6.4 and supplemented with 0.05% L-cysteine hydrochloride. Culture was incubated anaerobically at 37°C for 48 h in Oxoid anaerobic jar system with Oxoid AnaeroGen: O2 below 1%, and CO2 between 9 - 13%.
Yoghurt and fortified (probiotic) yoghurt production
Production of yogurt and B. adolescentis CH2-yogurt (fortified yogurt/probiotic yogurt) was by the modified method of Heller (2001). Two hundred and ten grams (210 g) of milk powder and 40 g of granulated sugar were dissolved in 1-L sterile distilled water, pasteurized at 95°C for 10 min, cooled to 44°C and inoculated with yogurt starter culture - S. thermophilus, L. bulgaricus and L. acidophilus (Yogomet, Germany). Cooled pasteurized inoculated milk was distributed into 500-mL flasks after thorough mixing with magnetic stirrer. Flasks and contents were incubated in a water bath at 42-43°C. The pH was monitored until pH dropped to 4.5. Produced yogurt was pasteurized at 85°C for 15 min and refrigerated overnight. Pasteurized yogurt was subsequently divided into two and one portion was inoculated with B. adolescentis CH2 culture as an adjunct to produce the B. adolescentis CH2-yogurt (fortified yogurt/probiotic yogurt). Samples were stored refrigerated prior to use.
In vivo safety and physiological effect study in rats
Thirty-six two weeks old female albino rats were obtained from the Animal Facility of the College of Medicine, University of Lagos Teaching Hospital, Lagos, Nigeria. Animal handling procedure was in accordance with the U.S government principles for the utilization and care of vertebrate animals in testing, research, and training (National Academy of Sciences, 2011). The animals were maintained and housed in cages at room temperature (28 ± 3°C). They were fed with standard rodent diet and water ad libitum, and allowed to acclimatize for 2 weeks. After acclimatization, rats were randomly divided into three groups of 12 rats: group one (control) were given standard diet and water daily; group two (yogurt group) were given standard diet, water and daily oral dose of 0.5 ml yogurt; while group three (B. adolenscentis-yogurt/probiotic yogurt group) were given standard diet, water, and daily oral dose of 0.5 ml yogurt containing approximately 7.2 x 106 cfu/ml B. adolenscentis, representing an intake of about 3.6 x 106 cfu B. adolescentis per animal per day. This administered intake was based on the recommended viability of ≥ 106 cfu probiotic culture. Applying the uncertainty factor of 100-fold (10-fold corrections for species differences and 10-fold corrections for human variability), this amounted to an intake of 2.48 x 105/kg body weight/day which is equivalent to 1.74 x 107 per day for an average 70 kg human. The B. adolenscentis dosage was not adjusted for body weight throughout the study period. Yogurt and B. adolenscentis fortified yogurt were supplied biweekly to ensure freshness and culture viability. All animals in each cage were administered the same dose daily by the orogastric feeding tube for four weeks.
Rats were monitored daily for weight and physical appearance throughout the study. Body weight and feed intake were recorded daily. B. adolescentis CH2 acute toxicity was assessed by evaluating the rat development and organs, and by analyzing blood concentrations of some relevant biochemical compounds. The results obtained for the treated (B. adolescentis-yogurt) group were compared with results for the control and yogurt groups. For evaluation of organs conditions and functions, three rats from each group were sacrificed weekly by cervical dislocation under anesthesia. Their blood samples were collected, transferred into lithium heparin bottle, and centrifuged for 15 min to obtain plasma. Plasma concentrations of glucose, albumin, bilirubin, total protein, total cholesterol, high density lipoprotein-cholesterol, triglycerides, urea, and creatinine were determined using BIOLABO reagent kits, while Randox reagent kit was used for aspartate aminotransferase determination. Assays were carried out following the commercial kits’ instructions. The weight of organ was recorded weekly.
Histopathology
Histopathological evaluation of dissected rat’s kidney and liver were performed at week 3 and 4. The liver and kidney tissues were processed and embedded in paraffin. Sections were cut, stained using hematoxylin and eosin method, and subsequently viewed under the microscope.
Statistical analysis
Data obtained were statistically analyzed by one-way ANOVA with multiple comparisons using graph pad prism 6.0. A P value less than 0.05 (P<0.05) was considered significant.
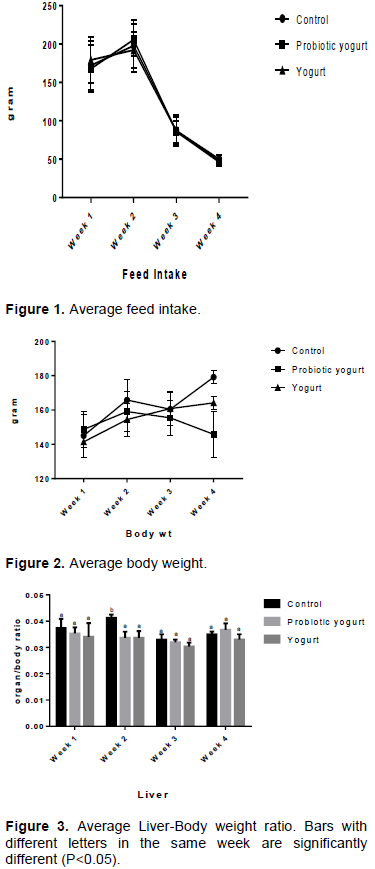
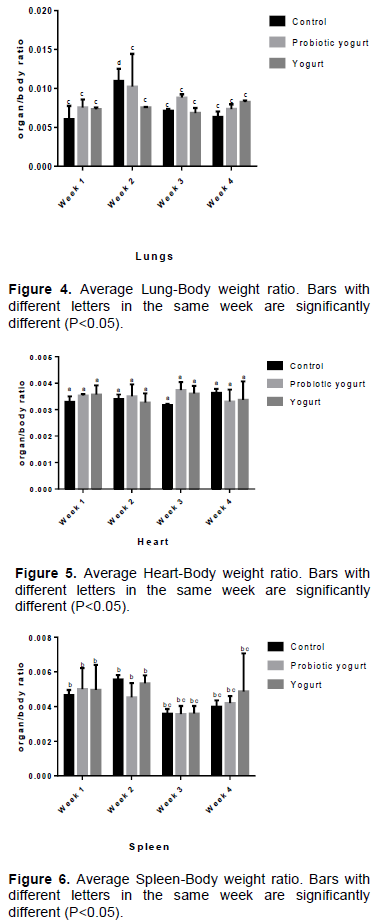
No incidence of animal mortality was recorded in any group. There was no significant difference in feed intake among the groups throughout the period of investigation (Figure 1). Results indicate no statistical significant difference in rats development and body weight in all groups up to day 15 of treatment. There was a reduction (P<0.05) in body weight gain of the B. adolescentis-yogurt (probiotic yogurt) group from week 3 of administration, while the body weights of the control and yogurt groups were increased (Figure 2).
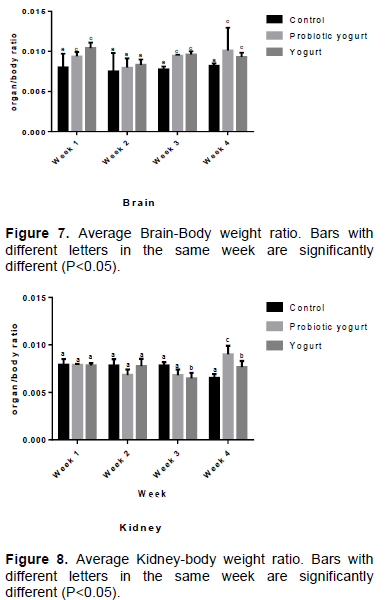
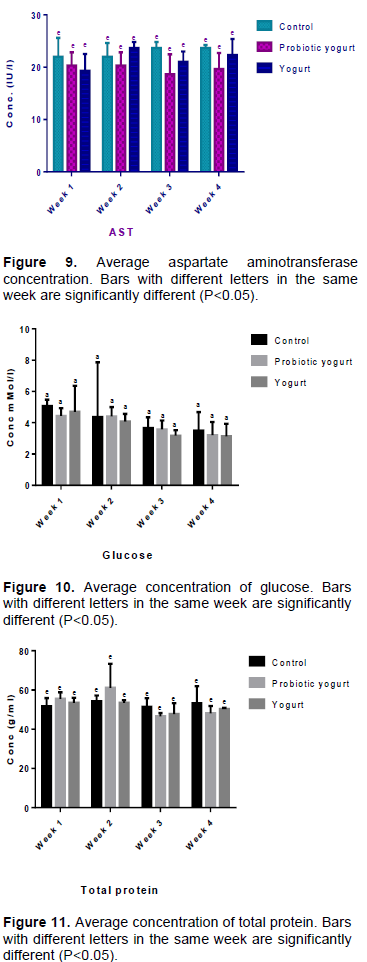
There was no significant difference (P>0.05) in the average organ/body ratios of the liver, heart, lung, and spleen between the B. adolescentis-yogurt and yogurt groups, and with each group compared with the control at week 4 (Figures 3 to 6). Evidence obtained from results inferred that there was no significant difference (P>0.05) in the average organ/body weight ratio of the lung and liver of the B. adolescentis-yogurt and yogurt groups compared with the control group at week 1, 3, and 4 of administration. The average lung/body weight ratio and liver/body weight ratio for B. adolescentis-yogurt and yogurt groups indicated no significant difference over the four weeks including week 2 of intake. However, results of the average liver/body and lung/body weight ratios of the control group at week 2 compared to the yogurt and B. adolescentis-yogurt groups showed significant difference.
Figure 7 represent the average brain/body weight ration. When compared with the control group, a significant difference was detected in the average brain/body weight ratio (P<0.05) of the B. adolescentis- yogurt and the yogurt group throughout the four weeks of administration except at week 2. We observed no significant difference in the brain/body weight ratio between the B. adolescentis-yogurt and the yogurt group from weeks 1 to 4. However, the organ/body weight ratio of the brain for the B. adolescentis-yogurt and the yogurt group were significantly higher (P<0.05) compared to the control group in weeks 1, 3, and 4 of investigation. The brain/body weight ratio at week 4 was 0.0082, 0.0101, and 0.0093 for the control, B. adolescentis-yogurt, and yogurt group respectively. There was no significant difference (P≥0.05) in the organ/body weight ratio values of the kidney in the three groups from week 1-2. However, values were significantly higher (P=0.001) in the yogurt and probiotic yogurt group in week 4 (Figure 8).
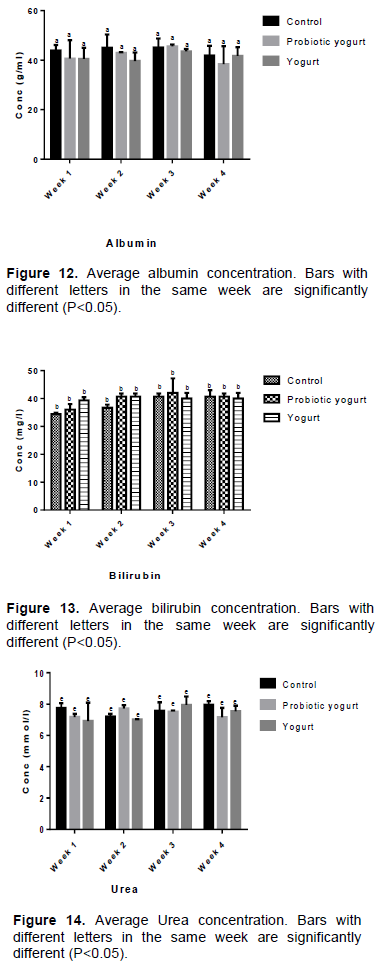
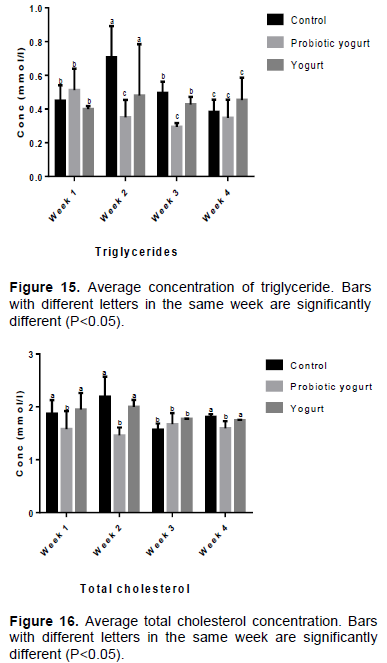
As illustrated in Figures 9 to 14, there were no significant statistical differences (P>0.05) in the average blood concentrations of Aspartate aminotransferase (AST), Glucose, Total protein, Albumin, Bilirubin, and Urea between the B. adolescentis-yogurt and yogurt groups as well as with each group compared to the control group throughout the study period. The blood plasma level of triglycerides was lower for the B. adolescentis-yogurt group than for the control and yogurt groups at week 2, 3, and 4 (Figure 15). The value for the average concentration of total cholesterol in B. adolescentis-yogurt group was lower compared to the control group and yogurt group by week 4 of administration (Figure 16).
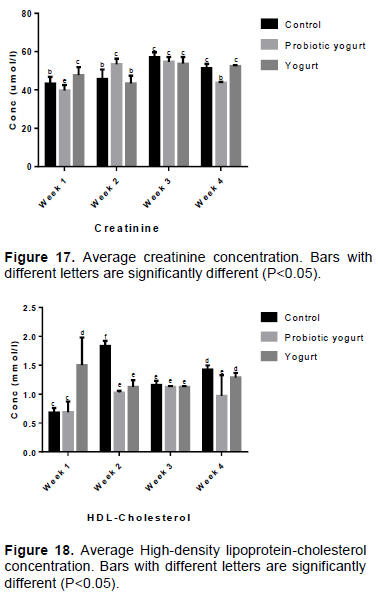
Following the four weeks intake of B. adolescentis CH2-yogurt, the blood concentration of creatinine for the B. adolescentis-yogurt group was significantly lower (P=0.0001) compared to the control group and yogurt group by week 4 as shown in Figure 17. The average high-density lipoprotein-cholesterol concentration in the B. adolescentis-yogurt animals did not reveal a significant difference when compared against its values in the control group at week 1 and 3, and with yogurt group at week 2 and 3. By week 4 of the investigation, the concentration was reduced in B. adolescentis-yogurt animals when compared with the control and yogurt animals (Figure 18).
As presented in Figures 19 and 20, the administered B. adolescentis CH2-yogurt did not result in any abnormalities in animal kidney or liver. The histopathological examination of kidney for all groups did not detect any abnormalities (Figures 19). The liver histological section for the control and B. adolescentis-yogurt did not reveal any difference between the tested group (B. adolescentis-yogurt) and the control group, while the liver histopathological examination for yogurt group showed sinusoidal congestion (Figure 20).
B. adolescentis enjoy the GRAS (Generally Regarded As Safe) and presumptively safe species for human and animal consumption status (FAO/WHO, 2006; European Food Safety Authority, 2017). Past researches have also documented the safety of B. adolescentis and other Bifidobacteriun species (Munoz et al., 2011; Patole et al., 2016; Salazar et al., 2011). Despite the B. adolescentis safe for consumption status, it is important that new isolates be confirmed safe before application as probiotic agents in animals and human. Some microbial characteristics or effects are strain specific. Moreover, continuous surveillance of probiotic microorganisms is necessary to continuously guarantee consumer safety. In this in vivo study we evaluated the effect of B. adolescentis CH2 dose on female albino rats by comparing the results obtained for B. adolescentis-yogurt group with that obtained for the yoghurt and control groups.
Effect of Bifidobacterium adolescentis CH2 on feed intake and body weight
There was no statistical significant difference in all groups for feed intake rate throughout the study period. Results up to week 2 (day 15) of investigation showed comparable growth for all groups. At week 3 and 4, despite the evidence of no significant difference in feed intake for all groups during the investigation period of four weeks, there was a significant reduction in average body weight for the B. adolescentis-yogurt group when compared with the average body weight of the control group. While the control and yoghurt group average weights increased from 145 g (week 1) to 179.167 g (week 4) and 141.458 g (week 1) to 164.167 g (week 4) respectively, the B. adolescentis-yogurt group average weight increased from 148.958 g (week 1) to 159.167 g (week 2) and subsequently decreased to145.833 g at week 4. This is an indication that while acute intake of B. adolescentis CH2 at the applied dose of 2.48 x 105/kg body weight/day did not affect animal weight, chronic (prolonged) intake may lead to weight reduction. An et al. (2011), in a study with male Sprague Dawley rats, also reported lower body weight in group fed high fat diet (HFD) and bifidobactia than the group fed HFD without bifidobacteria, even though the two groups had no significant difference in caloric consumption values. They concluded that the Bifidobacterium species (B. pseudocatenulatum SPM 1204, B. longum SPM 1205, and B. longum SPM 1207) possessed antiobesity potentials. B. adolescentis ability to reduce body weight was previously documented (Chen et al., 2012; Lim and Kim, 2017).
Effect of Bifidobacterium adolescentis CH2 on organ-body weight ratio
An important requirement in toxicological experiments is the evaluation of the effects of a substance on specific organs. Effect on organ weight and the ratio of the organ weight to body weight in toxicology studies is an important indicator for identification of potentially harmful effects of a substance. This investigation did not establish any important significant difference in the organ-body ratio of the liver, lung, heart, and spleen of the B. adolescentis-yogurt and yogurt groups, as well as the B. adolescentis-yogurt group compared with the control group. This is an indication that the B. adolescentis CH2 fortified yogurt exerted no toxicology effect on these organs of the albino rats.
There was no significant difference in the average brain-body ratio between the B. adolescentis-yogurt and the yogurt group throughout the four weeks of study. However, the average organ/body ratio of the brain of the B. adolescentis-yogurt and the yogurt groups were higher compared to the control group over the 4 weeks of administration (Figure 7), indicating that this is not attributable to the administered B. adolescentis CH2 strain, since the recorded difference also occurred in the yogurt group. The differences in the brain-body weight ratios of the B. adolescentis-yogurt and yogurt groups when compared with the control are likely results of the lower body weights (Figure 2) of the B. adolescentis-yogurt and yogurt group animals. Differences in body weights occur and affect organ-body ratios. Long et al. (1998) reported that brain weight has low variability compared with other organs which may impact on brain-body ratio.
There was no significant difference (P≥0.05) in the organ/body ratio value of the kidney in the three groups from week 1-2, but the value increased in the
B. adolescentis CH
2-yogurt and yogurt groups in week 4. At the end of four weeks administration of yogurt and the
B. adolescentis CH
2-yogurt, the average kidney/body weight rations differed among the groups. The average kidney/body weight ration for the
B. adolescentis CH
2-yogurt group increased from 0.0079 at week 1 to 0.0090 at week 4, while the control and yogurt groups decreased from 0.0079 to 0.0065 and 0.0078 to 0.0077 respectively. However, the kidney histology of the
B. adolescentis CH
2-yogurt group at week 3 and 4 revealed no abnormality. The significant increase in
B. adolescentis-yogurt rats’ kidney/body weight ratio in week 4 is likely due to the low body weight of the animals in the
B. adolescentis-yogurt group during the period. Effect of variations in results of organ weights and organ-body weight ratios have previously been reported (Bailey et al., 2004; Long et al., 1998;
Piao et al
., 2013).
Effect of Bifidobacterium adolescentis CH2 on rat kidney and liver histology
The histopathological analysis of kidney for all groups at week 3 (Figure 19) and week 4 (result not shown) were normal, indicating absence of any negative effect of treatment. There was no necrosis, inflammation or haemorrhage in the control, B. adolescentis-yogurt, and yogurt groups.
Histological section of liver for the control and B. adolescentis-yogurt groups at week 3 (Figure 20) and week 4 (result not shown) revealed preservation of hepatic architecture. There were no intracellular inclusions, sinusoids congestion, haemorrhage or inflammation, and no areas of necrosis. However, the analyzed histological sections for animals in the yogurt group showed mild sinusoidal congestion at week 3 (Figure 20) and steatosis, revealing hepatocytes with intracellular accumulations of large clear fat vacuoles at week 4 (result not shown). This observed difference in the liver of the yogurt group compared to the control group may have resulted from the daily consumption of yogurt by the animals. The absence of this same effect in the B. adolescentis-yogurt group, even though animals in this group received the same daily intake of yogurt as those in the yogurt group, may be due to a hypolipidemic effect of the B. adolescentis CH2 strain, indicating that the probiotic strain repressed this probable yogurt effect and ameliorated the fatty liver symptom. B. adolescentis CGMCC 15058 was reported to relieve increased serum and liver alanine aminotransferase and lipopolysaccharide-binding protein in rat in an induced acute liver injury condition (Li et al., 2019). According to Li et al. (2019), their studied B. adolescentis CGMCC 15058 protected the induced rats against liver damage and failure.
The results of kidney and liver histopathology confirmed that the four weeks B. adolescentis CH2-yogurt consumption had no harmful effects on the albino female rats liver and kidney.
Effect of Bifidobacterium adolescentis CH2 on rat blood biochemistry
Exposure of an animal to toxicity reflects in the blood biochemistry which usually results from effect of the substance on the organ and its functions. Authors agree that high level of some blood biochemical biomarkers can indicate metabolic organ failure and inefficient performance due to inflammation, infection, damage, or injury (Lee, 2011; Kumar et al., 2013). The results of clinical parameters for liver function tested- Albumin, Bilirubin, Aspartate aminotransferase (AST), Total protein- showed no significance difference in concentrations between the experimental groups. This showed that the administered B. adolescentis CH2-yogurt did not alter the functionality of the liver of the experimental animals. The average blood concentrations of glucose, total protein, and urea of rats in the B. adolescentis-yogurt and yogurt groups compared favorably with the control group.
Creatinine and urea levels in the blood of animals are kidney functions tests. Creatinine, a waste product from normal muscle metabolism is easily filtered out of the blood by well-functioning kidneys which stabilize its concentration in the bloodstream.
High blood levels of urea or creatinine is an indication of malfunctioning kidney and/or kidney failure or damage in animals. The results of creatinine and urea analysis indicated that the functionality of the kidney in the experimental rats was not affected negatively by the B. adolescentis CH2-yogurt. While the urea values did not vary significantly in all groups over the four weeks of evaluation, the creatinine level in the B. adolescentis-yogurt group was significantly lower (P=0.0001) compared to the control group and yogurt group by week 4 of administration (Figure 17).
Authors previously documented triglyceride, cholesterol, and lipid lowering activities of some
Bifidobacterium species and strains (An et al., 2011; Tsai et al., 2014). Lim and Kim (2017) noted that oral administration of B. adolescentis IM38 lowered high fat diet-induced lipopolysaccharide levels in blood and colonic fluid of mice, with subsequent inhibition of body and epididymal fat weight gain. In an attempt to correct the lipoprotein imbalance found in the blood of children with dyslipidemia,
Guardamagna et al. (2014) examined the effects of a probiotic which contained three
Bifidobacterium strains. Their results established a decrease in total cholesterol and low-density lipoprotein cholesterol. Experiments using mice also confirmed the ability of
Bifidobacterium breve B-3 in administered skim milk to reduce the accumulation of epididymal fat and improve total cholesterol level (Kondo et al., 2010).
This study established lower average concentrations of triglycerides and total cholesterol in rats fed with B. adolescentis CH2-yogurt compared with the control group at week 4 of the study.
The blood concentration of triglycerides decreased from 0.51 mmol/L in week 1 to 0.35 mmol/L by week 4 in B. adolescentis-yogurt group, slightly decreased from 0.45 to 0.38 mmol/L in control group, while it slightly increased in yogurt group from 0.40 to 0.45 mmol/L. At week 4 the average blood content of total cholesterol (mmol/L) in control group was 1.81, B. adolescentis-yogurt group 1.60, and yogurt group 1.75. The hypolipidemic effect potential of B. adolescentis CH2 will be investigated further to explore its possible use as a biotherapeutic.
Furthermore, animals in the B. adolescentis CH2-yogurt treatment group recorded lower average weight compared to the other groups (Figure 2), showing that B. adolescentis CH2 or B. adolescentis CH2-yogurt could be an agent for weight management.
The study findings suggest that B. adolescentis CH2 is a toxicologically safe probiotic for acute intake. Short-term consumption of B. adolescentis CH2 supplemented yogurt revealed no undesirable effect. A hypolipidemic activity and weight reduction effect was observed at week four of daily consumption of B. adolescentis CH2, suggesting that B. adolescentis CH2 may have a potential for application in weight management and cardiovascular disease control.
The authors have not declared any conflict of interests.
The authors thank Prof. O. A. Magbagbeola and Mr. F. Akinrodoye of Biochemistry Department, College of Medicine, University of Lagos, Nigeria, for their technical assistance with the animal study.
REFERENCES
|
An HM, Park SY, Lee DK, Kim JR, Cha MK, Lee SW, Lim HT, Kim KJ, Ha NJ (2011). Antiobesity and lipid-lowering effects of Bifidobacterium spp. in high fat diet-induced obese rats. Lipids in Health and Disease 2011(10):116.
Crossref
|
|
|
|
Bailey SA, Zidell RH, Perry RW (2004). Relationships between organ weight and body/brain weight in the rat: what is the best analytical endpoint? Journal of Toxicologic Pathology 32:448-466.
Crossref
|
|
|
|
|
Bordoni A, Amaretti A, Leonardi A, Boschetti E, Danesi F, Matteuzzi D, Roncaglia L, Raimondi S, Rossi M (2013). Cholesterol-lowering probiotics: In vitro selection and in vivo testing of bifidobacteria. Applied Microbiology and Biotechnology 9:8273-8281.
Crossref
|
|
|
|
|
Cha MK, Lee DO, An HM, Lee SW, Shin SH, Kwon JH, Kim KJ, Ha NJ (2012). Antiviral activity of Bifidobacterium adolescentis SPM1005-A on human papillomavirus type 16. BMC Medicine 10:72.
Crossref
|
|
|
|
|
Chen J, Wang R, Li X-F, Wang R-L (2012). Bifidobacterium adolescentis supplementation ameliorates visceral fat accumulation and insulin sensitivity in an experimental model of the metabolic syndrome. British Journal of Nutrition 107:1429-1434.
Crossref
|
|
|
|
|
Chenoll E, Casinos B, Bataller E, Astals P, Echevarria J, Iglesias JR, Balbarie P, Ramon D, Genoves S (2011). Novel probiotic Bifidobacterium bifidum CECT 7366 strain active against the pathogenic bacterium Helicobacter pylori. Applied and Environmental Microbiology 77:1335-1343.
Crossref
|
|
|
|
|
Dhingra D, Lamba D, Kumar R, Gauttam S (2014). Antihyperlipidemic activity of aloe succotrina in rats: possibly mediated by inhibition of HMG-CoA reductase. ISRN Pharmacology 2014.
Crossref
|
|
|
|
|
Di Gioia D, Aloisio I, Mazzola G, Biavati B (2014). Bifidobacteria: their impact on gut microbiota composition and their applications as probiotics in infants. Applied Microbiology and Biotechnology 98:563-577.
Crossref
|
|
|
|
|
Ejtahed HS, Mohtadi-Nia J, Homayouni-Rad A, Niafar M, Asghari-Jafarabadi M, Mofid V, Akbarian-Moghari A (2011). Effect of probiotic yogurt containing Lactobacillus acidophilus and Bifidobacterium lactis on lipid profile in individuals with type 2 diabetes mellitus. Journal of Dairy Science 94:3288-3294.
Crossref
|
|
|
|
|
European Food Safety Authority (2017). EFSA Panel on Biological Hazards 2017. Scientific Opinion on the update of the list of QPS-recommended biological agents intentionally added to food or feed as notified to EFSA. EFSA Journal 15(3):4664.
|
|
|
|
|
FAO/WHO (2006). Probiotics in food: Health and nutritional properties and guidelines for evaluation. Report of a Joint FAO/WHO Expert Consultation. Published by FAO and WHO. pp. 1-56.
|
|
|
|
|
Fijan S (2014). Microorganisms with Claimed probiotic properties: an overview of recent literature. International Journal of Environmental Research and Public Health 11:4745-4767.
Crossref
|
|
|
|
|
Goldin BR, Gorbach SL (1992). Probiotics for humans. In Probiotics the scientific basis, Edited by Fuller R., London: Chapman and Hall. Chapter 13:355-376.
Crossref
|
|
|
|
|
Greenhalgh K, Meyer KM, Aagaard KM, Wilmes P (2016). The human gut microbiome in health: establishment and resilience of microbiota over a lifetime. Environmental Microbiology 18:2103-2116.
Crossref
|
|
|
|
|
Guardamagna O, Amaretti A, Puddu PE, Raimondi S, Abello F, Cagliero P (2014). Bifidobacteria supplementation: effects on plasma lipid profiles in dyslipidemic children. Nutrition 30:831-836.
Crossref
|
|
|
|
|
Heller KJ (2001). Probiotic bacteria in fermented foods: Product characteristics and starter organisms. The American Journal of Clinical Nutrition 73:374S-379S.
Crossref
|
|
|
|
|
Homayouni H, Payahoo L, Azizi A (2012). Effects of Probiotics on lipid profile: A review. American Journal of Food Technology 7:251-265.
Crossref
|
|
|
|
|
Isolauri E, Rautava, S, Salminen S (2012). Probiotics in the development and treatment of allergic disease. Gastroenterology Clinics of North America 41:747-762.
Crossref
|
|
|
|
|
Kim MJ, Lee DK, Park JE, Park IH, Seo JG, Ha NJ (2014). Antiviral activity of Bifidobacterium adolescentis SPM1605 against Coxsackievirus B3. Biotechnology and Biotechnological Equipment 28:681-688.
Crossref
|
|
|
|
|
Kolovou G, Anagnostopoulou K, Cokkinos D (2005). Pathophysiology of dyslipidaemia in the metabolic syndrome. Postgraduate Medical Journal 81:358-366.
Crossref
|
|
|
|
|
Kondo S, Xiao J Z, Satoh T, Odamaki T, Takahashi S, Sugahara H, Yaeshima T, Iwatsuki K, Kamei A, Abe K (2010). Antiobesity effects of Bifidobacterium breve strain B-3 supplementation in a mouse model with high-fat diet-induced obesity. Bioscience Biotechnology and Biochemistry 74:1656-1661.
Crossref
|
|
|
|
|
Kumar V, Ahmed D, Anwar F, Ali M, Mujeeb M (2013). Enhanced glycemic control, pancreas protective, antioxidant and hepatoprotective effects by umbellif eron-a-D-glucopyranosyl-(21 – 111)-a-D-glucopyranoside in streptozocin induced diabetic rats. SpringerPlus 2:639.
Crossref
|
|
|
|
|
Lee HC (2011). Liver function tests as indicators of metabolic syndrome. The Korean Journal of Hepathology 17:9-11.
Crossref
|
|
|
|
|
Li Y, Lv L, Ye J, Fang D, Shi D, Wu W, Wang Q, Wu J, Yang L, Bian X, Jiang X, Jiang H, Yan R, Peng C, Li L (2019). Bifidobacterium adolescentis CGMCC 15058 alleviates liver injury, enhances the intestinal barrier and modifies the gut microbiota in D-galactosamine-treated rats. Applied Microbiology and Biotechnology 103:375-393.
Crossref
|
|
|
|
|
Lim SM, Kim DH (2017). Bifidobacterium adolescentis IM38 ameliorates high-fat diet-induced colitis in mice by inhibiting NF-KB activation and lipopolysacharride production by gut microbiota. Nutrition Research 41:86-96.
Crossref
|
|
|
|
|
Long GG, Symanowski JT, Roback K (1998). Precision in data acquisition and reporting of organ weights in rats and mice. Journal of Toxicologic Pathology 26:316-318.
Crossref
|
|
|
|
|
Milani C, Lugli GA, Duranti S, Turroni F, Bottacini F, Mangifesta M (2014). Genomic encyclopedia of type strains of the genus bifidobacterium. Applied and Environmental Microbiology 80:6290-6302.
Crossref
|
|
|
|
|
Milani C, Turroni F, Duranti S, Lugli GA, Mancabelli L, Ferrario C, van Sinderen D, Ventura M (2016). Genomics of the genus Biï¬dobacterium reveals species-speciï¬c adaptation to the glycan-rich gut environment. Applied and Environmental Microbiology 82:980–991.
Crossref
|
|
|
|
|
Mohan R, Koebnick C, Schildt J, Schmidt S, Mueller M, Possner M, Radke M, Blant M (2006). Effects of Bifidobacterium lactis Bb 12 supplementation on intestinal microbiota of preterm infants: a double-blind, placebo-controlled, randomized study. Journal of Clinical Microbiology 44:4025-4031.
Crossref
|
|
|
|
|
Munoz JAM, Chenell E, Casinos B, Bataller E, Ramon D, Genoves S, Montava R, Ribes J M, Buesa J, Fabrega J, Rivero M (2011). Novel probiotic Bifidobacterium longum subsp. Infantis CECT 7210 strain active against rotavirus infections. Applied and Environmental Microbiology 77:8775-8783.
Crossref
|
|
|
|
|
National Academy of Sciences (2011). Guide for the care and use of laboratory animals. Eighth edition. National Academies Press. USA.
|
|
|
|
|
O'Bryan CA, Pak D, Crandall PG, Lee SO, Ricke SC (2013). The role of prebiotics and probiotics in human health. Journal of Probiotics and Health 1(2):108.
|
|
|
|
|
O'Callaghan A, van Sinderen D (2016). Biï¬dobacteria and their role as members of the human gut microbiota. Frontiers in Microbiology 7:925.
Crossref
|
|
|
|
|
Oakey HJ, Harty DW, Knox KW (1995). Enzyme production by Lactobacilli and the potential link with infective endocarditis. Journal of Applied Bacteriology 78:142-148.
Crossref
|
|
|
|
|
Odamaki T, Xiao JZ, Iwabuchi N, Sakamoto M, Takahashi H, Enomoto T, Benno Y (2007). Influence of Bifidobacterium longum BB 536 intake on faecal microbiota in individuals with Japanese cedar pollinosis during pollen season. Journal of Medical Microbiology 56:1301-1308.
Crossref
|
|
|
|
|
Onyibe JE, Asagbra AO, Bankole AO, Elemo GN, Sanni AI (2013b). Hydrophobicity and autoaggregation of Bifidobacterium species of human and avian origin. Analytical Science Journal 1:5-16.
|
|
|
|
|
Onyibe JE, Oluwole OB, Ogunbanwo ST, Sanni AI (2013a). Antibiotic susceptibility profile and survival of Bifidobacterium adolescentis and Bifidobacterium catenulatum of human and avian origin in stored yoghurt. Nigerian Food Journal 31:73-83.
Crossref
|
|
|
|
|
Patole SK, Rao SC, Keil AD, Nathan EA, Doherty DA, Simmer KN (2016). Benefits of Bifidobacterium breve M-16V Supplementation in preterm neonates-A retrospective cohort study. PLoSONE 11(3):e0150775. doi:10.1371/journal. pone.0150775.
|
|
|
|
|
Piao Y, Liu Y, Xie X (2013). Change trends of organ weight background data in Sprague Dawley rats at different ages. Journal of Toxicologic Pathology 26:29-34.
Crossref
|
|
|
|
|
Salazar N, Binetti A, Gueimonde M, Alonso A, Garrido P, Gonzalez del Rey C, Gonzalez C, Ruas Madiedo P, de los Reyes-Gavilan, CG (2011). Safety and intestinal microbiota modulation by the exopolysaccharide-producing strains Bifidobacterium animalis IPLA R1 and Bifidobacterium longum IPLA E44 orally administered to Wistar rats. International Journal of Food Microbiology 144:342-351.
Crossref
|
|
|
|
|
Scheinbach S (1998). Probiotics: functionality and commercial status. Biotechnology Advances 16:581-608.
Crossref
|
|
|
|
|
Tsai CC, Lin PP, Hsieh YM, Zhang ZY, Wu HC, Huang CC (2014). Cholesterol-lowering potentials of lactic acid bacteria based on bile-salt hydrolase activity and effect of potent strains on cholesterol metabolism in vitro and in vivo. The Scientific World Journal 2014.
Crossref
|
|
|
|
|
Xiao JZ, Kondo S, Yanagisawa N, Takahashi N, Odamaki T, Iwabuchi N, Miya K, Iwatsuki K, Togashi H (2006). Probiotics in the treatment of Japanese cedar pollinosis: a double-blind placebo controlled trial. Clinical Experimental Allergy 36:1425-1435.
Crossref
|
|
|
|
|
Yadav A, Jawal P, Jaiswal M, Kumar N, Sharma R, Shailendra R, Prasad GBKS, Bisen P (2015). Concise Review: Importance of probiotics yogurt for human health improvement. IOSR Journal of Environmental Science, Toxicology and Food Technology 9:25-30.
|
|