ABSTRACT
Diclofenac is an analgesic and anti-inflammatory drug, used to relief the secondary complications of diabetes. Wound infections are much more serious particularly in diabetic patients. Proteus mirabilis are Gram negative rods, show a wide range of pathogenesis based on arsenal of diverse virulence factors. Recently, P. mirabilis and other Gram negative rods were isolated from diabetic foot ulcers; moreover, these isolates showed an increase resistance and more aggressive virulence behavior. The promising approaches to overcome such kind of infections include the improvement of patient’s immunity and/or challenging the bacterial virulence. Diclofenac is used frequently by diabetic patients, and it showed antimicrobial activity. This study was conducted to screen the effect of diclofenac on the virulence of P. mirabilis isolated from diabetic foot. Interestingly, diclofenac significantly inhibited or decreased the P. mirabilis virulence which indicates its additional beneficial use in diabetic foot patients.
Key words: Proteus mirabilis, diabetic foot, diclofenac.
Proteus, Homer’s Odyssey, was known by his ability to foretell the future to anyone capable of capturing him; he changed shape to evade his followers. Hauser (1885), first used the name Proteus in bacterial nomenclature to describe a shapeshifting bacterium isolated from putrefied meat. Proteus mirabilis, family Enterobacteriaceae, is a Gram-negative, motile, non-lactose fermenter and produce hydrogen sulphide by incubation in triple sugar iron media. P. mirabilis is dimorphic and has the ability to differentiate from short rods into elongated, multinucleate swarm cells that express thousands of flagella. Members of the genus Proteus are widely distributed in nature and can be isolated from stagnant water, sewage, soil, and the intestinal tract (Armbruster and Mobley, 2012). P. mirabilis can cause a wide range of pathogenesis to the infected host that varied from pyelonephritis, urolithiasis to prostatitis. Moreover, it is one of the major causes of catheter-associated urinary tract infections (CAUTIs) as it causes approximately 3% of all nosocomial infections and up to 44% of CAUTIs in the United States (O’Hara et al., 2000; Jacobsen et al., 2008).
Diabetic foot ulcers are known to be a complicated serious problem of diabetes as it increases the risk of amputation (Grayson, 1995). P. mirabilis is considered one of the most common infectious agents of diabetic foot ulcers (Sekhar et al., 2014; Perim et al., 2015). The most critical that it is showing a vast resistance to several antimicrobial agents which worsen the diabetic foot ulcers and delay the treatment (Tansarli et al., 2013; Perim et al., 2015). The understanding of bacterial behavior, life style and virulence factors is a crucial determinant in developing vaccines and introducing more efficient antimicrobial treatments (Hegazy and Hensel, 2012). The virulence of P. mirabilis is described on genetic basis which are chromosomally integrated or extra-chromosomally imported. For instance, 94 Kb ICE PM1 mobile pathogenicity island of P. mirabilis is present with up to 100% sequence identity for some genes in several Proteus species, which indicate DNA transfer between these species (Flannery et al., 2009). The virulence factors of P. mirabilis are widely diverse, adhesive fimbria, flagella swarming motility and production of toxins as haemolysin and extracellular enzymes as protease and urease all are working cooperatively on pathogenesis enhancement (Jacobsen et al., 2008; Morgenstein et al., 2010; Armbruster and Mobley, 2012). The biofilm formation by P. mirabilis constitutes an additive obstacle in antibiotic treatment of infections and its prevention is considered as an aim (Jacobsen et al., 2008; Zhao and Hu, 2013).
In serious bacterial infections as in diabetic foot ulcers, in order to control the aggressive bacterial invasion, it is a mandatory not only to prevent microbial infections by antibiotics, but also to inhibit microbial virulence to guarantee effectiveness. Controlling of diabetic foot ulcers, the surgical intervention and treatments are both applied. Diabetes traditional treatment regimens include basically analgesics and anti-inflammatory which are used to mask the other complications and relief the pain (Park and Anand, 2015; Santema et al., 2016).
Diclofenac inhibits synthesis of prostaglandin by inhibition of cyclooxygenase; it is widely used as sodium salt or potassium salt as anti-inflammatory (Chakraborti et al., 2010). Moreover, it is available indifferent dosage forms which ease its use and its safety is approved. Several drugs which are available in the market and commonly used by diabetic patients for their activity against bacterial virulence were screened. In this study, the effect of direct effect of diclofenac was studied on the virulence factors of a highly resistant P. mirabilis isolated from ulcerated diabetic foot. The effect of diclofenac in combination with antibiotics was studied.
Bacterial strain
Clinical isolate of P. mirabilis was obtained from diabetic foot ulcers from patients admitted to the Surgery Department in Zagazig University Hospitals. The isolate was identified by morphology, Gram staining and biochemical reactions (Koneman et al., 1997).
Determination of minimum inhibitory concentration (MIC)
MIC of antibiotics or diclofenac sodium salt (Novartis, Egypt) was determined by the broth microdilution method according to Clinical Laboratory and Standards Institute Guidelines (CLSI) (Wayne, 2006). Briefly, bacterial inoculum were prepared and standardized to have a turbidity matching that of ½ McFarland standards. Sterile saline was used to dilute the bacterial suspensions to achieve a cell density approximating 106 CFU/ml. Equal volumes of antibiotics or diclofenac sodium salt and aliquots of the bacterial suspensions in Mueller-Hinton broth were added. After incubation of the plates at 37°C overnight, the MIC was calculated as the lowest concentration that showed no visible growth in the tubes. The test was repeated triplicate.
Swarming and swimming motilities assay
The effect of diclofenac on swimming and swarming was examined (Liaw et al., 2001, 2004). For swarming assay, overnight culture of P. mirabilis was prepared and 5 μl from this culture was inoculated on the center of the surface of dried LB swarming agar (1.5%) plates containing different sub-inhibitory concentrations of diclofenac (½ MIC or ¼ MIC). The plates were incubated overnight at 37°C, the swarming zones diameters were measured in mm. Control plates were also prepared and inoculated in the same way. The experiment was repeated in triplicates and the mean and standard deviation were calculated. In order to differentiate swarmer cells, sections of agar from swarming assay plates with and without inhibitor were cut under aseptic conditions. The sections were cut from the center of the colony which contains vegetative cells and from the edge of the colony with swarmer cells. After removal of the bacteria from the cut agar pieces with phosphate buffered saline, they were simple stained with crystal violet and examined under the oil immersions lens.
For swimming assay, the overnight P. mirabilis culture was stabbed into the center of the dried LB swimming agar (0.4%) with diclofenac (½ MIC or ¼ MIC). After overnight incubation of the plates at 37°C, the swimming zones diameters were measured in mm. Control plates were also prepared and inoculated in the same way. The experiment was repeated in triplicate and the mean and standard deviation were calculated.
Protease assay
The tested strain was grown overnight in LB broth with and without sub-MIC (½ MIC or ¼ MIC) of diclofenac at 37°C. The bacterial suspension was centrifuged and the supernatant was collected for Protease assay (Keay et al., 1970). Protease activity was measured in the presence and absence of diclofenac using a casein substrate; 1 ml of the culture supernatant was mixed with 1 ml 0.05 M phosphate buffer-0.1 M NaOH (pH 7.0) containing 2% casein, and incubated for 10 min at 37°C. The reaction was stopped by adding 2 ml 0.4 M trichloroacetic acid. After 30 min stand at room temperature, the precipitate was removed by centrifugation and the optical density of the assays was measured at 660 nm. The positive and negative controls were prepared in the same way. The assay was repeated in triplicate and the mean and standard deviation were calculated.
Hemolysis assay
The tested strain was grown overnight in LB broth with and without sub-MIC (½ MIC or ¼ MIC) of diclofenac at 37°C. The bacterial suspension was centrifuged and supernatant was collected for Haemolysin assay (Liaw et al., 2004). Briefly, bacterial suspension (50 μl) was mixed with a 2% erythrocyte suspension (450 μl) in 0.85% NaCl and 20 mM CaCl2 and incubated at 40°C for 15 min. Hemolytic activity was determined by the haemoglobin release using a 100% positive haemolysin-positive reference and the optical density of the assays was measured at 540 nm. The assay was repeated triplicate and the mean and standard deviation were calculated.
Urease activity assay
To examine the effect of diclofenac on urease production, a modification of Koneman et al. (1997) method was applied. An overnight culture of P. mirabilis was prepared and 5 μl from this culture was inoculated on the center of the surface of dried Christensen’s urea agar plates containing different sub-inhibitory concentrations of diclofenac (½ MIC or ¼ MIC). The plates were incubated overnight at 37°C, the activity of urease was indicated by the pH indicator color change from yellow to pink and pink zones diameters were measured in mm. Control plates were also prepared and inoculated in the same way. The experiment was repeated in triplicate and the mean and standard deviation were calculated.
Biofilm formation
Assessment of biofilm production
Overnight cultures of P. mirabilis tested isolate was prepared, diluted with fresh tryptone soya broth and adjusted to a cell density of 1 × 106 CFU/ml for assessment of biofilm production (Stepanovic et al., 2000). Aliquots of 200 μl of the adjusted bacterial suspension were inoculated in sterile 96-well polystyrene microplates, incubated for 24 h at 37°C. The wells were gently aspirated and washed three times with sterile phosphate buffered saline (pH 7.2). The adherent cells were fixed with 200 μl of 99% methanol for 20 min and stained with 200 μl crystal violet (1%) for 20 min. The excess dye was washed out under running distilled water, and then the plates were air dried. The crystal violet bound dye was extracted by 95% ethanol and the optical densities were measured at a wavelength of 590 nm. The test was repeated three times, and the mean optical densities were calculated. The cut-off OD (ODc) was defined as three times standard deviations above the mean OD of the negative control. The tested isolate was categorized into one of four groups; non-biofilm forming (OD ≤ ODc), weak biofilm forming (OD > ODc, but ≤ 2x ODc), moderate biofilm forming (OD>2x ODc, but ≤ 4x ODc), or strong biofilm forming (OD> 4x ODc).
Inhibition of biofilm formation
For evaluation of the inhibitory effect of diclofenac on biofilm formation, the same procedure described for assessment of biofilm production was followed. Aliquots of 100 μl of the prepared bacterial suspension were added to the wells of sterile 96-well polystyrene microplate containing 100 μl of ½ MIC or ¼ MIC of diclofenac. The optical densities of the stained adherent biofilms were measured in the presence and absence of diclofenac at a wavelength of 590 nm. The assay was repeated in triplicate and the means and standard deviations were calculated.
Determination of minimum biofilm inhibitory concentration (MBIC)
The MBICs of the antibiotics or diclofenac, the minimum concentrations which inhibit regrowth of the bacterial biofilm cells, were determined by broth dilution method in polystyrene microtiter plates (Cernohorská and Votava, 2008). Briefly, an overnight culture adjusted with TSB to achieve a turbidity equivalent to that of a ½ McFarland standard, 75 μl aliquots of the inoculated media were added to the wells of microtiter plates. The plates were incubated for 24 h at 37°C. The wells were washed three times with PBS under aseptic conditions. Volumes of 100 μl of appropriate two-fold dilutions of the respective antimicrobial agents or diclofenac in Mueller–Hinton broth were transferred into the dried wells with established biofilms. The microtiter plates were incubated for 18 to 20 h at 37°C and MBIC was determined, as the lowest concentration of antibiotic showed no visible growth in the wells. A positive control and a negative control were included in all experiments. The experiment was repeated in triplicate.
Adhesion assay
Overnight cultures of P. mirabilis tested isolate was prepared, diluted with fresh tryptone soya broth and adjusted to a cell density of 1 × 106 CFU/ml for adhesion assay (Vesterlund et al., 2005).
Adhesion to epithelial cells
Epithelial cells were collected from pregnant urine, washed and resuspended in phosphate buffer saline (PBS). Epithelial cells were counted by methylene blue method and distributed eventually in microtitre-plate and co-cultured with bacterial strain in total volume 150 µl in absence and presence of diclofenac in concentration ½ MIC or ¼ MIC. Cells were incubated at 37°C for 1 h, washed 3 times with PBS, fixed at 60°C for 20 min, stained with equal volume of crystal violet (0.1%) for 45 min and washed 5 times with PBS. Finally, 150 µl 20 mole/L citrate buffer (PH 4.3) was used to lysis cells for 45 min and optical density was measured at 570 nm. The experiment was repeated in triplicate and the means and slandered deviations were calculated.
Adhesion to abiotic surface
Bacterial strain was cultured with diclofenac in concentration ½ MIC or ¼ MIC in micro-titer plate, incubated at 37°C for 1 h, washed 3 times with PBS, fixed at 60°C for 20 min, stained with equal volume of crystal violet (0.1%) for 15 min and washed 5 times with PBS.
Then, ethanol was added and optical densities were measured at 590 nm. The assay was repeated triplicate and the means and slandered deviations were calculated.
Combination of antibiotics and diclofenac
To determine the effect of combining diclofenac with antimicrobial agents, the MICs of these antimicrobial agents were determined in the presence of ¼ MIC of diclofenac. The wells of microtiter plates with 50 μl of 4 fold the final concentration of each diclofenac and antibiotics were inoculated with standardized bacterial suspensions to have a final inoculum of 5×105 CFU/ml and incubated at 37°C overnight. The MIC was calculated as the lowest concentration of antimicrobial agent that can completely inhibit visible growth in the wells. Fractional inhibitory concentration (FIC) of antibiotic was determined according to Mackay et al. (2000). FIC of drug A= MIC drug A in combination/MIC drug A alone. The result of the combination may be synergistic (FIC ≤ 0.5), indifferent (FIC > 0.5 to 4), or antagonistic (FIC > 4).
Statistical analysis
The assays were repeated in triplicates and the data are presented as median and range unless specified. The differences between the control and diclofenac were analyzed by t-test using the Graphpad Prism 5 software. The relationship between variables was evaluated using the Pearson rank correlation test. A two-tailed P value <0.05 was considered statistically significant. The percentage of inhibition of diclofenac was calculated.
Identification of P. mirabilis isolates
P. mirabilis isolate was identified as Gram-negative rods. They produced lactose non-fermenting colonies on MacConkey’s agar and showed swarming on nutrient agar. They produced hydrogen sulphide from triple sugar iron agar and were urease positive and indole fermentation negative.
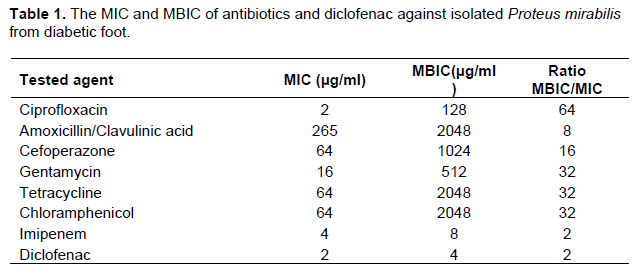
Determination of MIC
The MIC of antibiotics or diclofenac sodium salt was determined by the broth microdilution method according to Clinical Laboratory and Standards Institute Guidelines (CLSI). The results were summarized in Table 1. The MIC for tested antibiotics was determined in the presence of diclofenac (¼ MIC) and FIC was calculated for combinations. It was shown that diclofenac synergistically decrease the MIC of tested antibiotics, and FIC ranged from 0.25 to 0.5, except combination with imipenem was indifferent (FIC = 1). The results of diclofenac combination with antibiotics were summarized in Table 3.
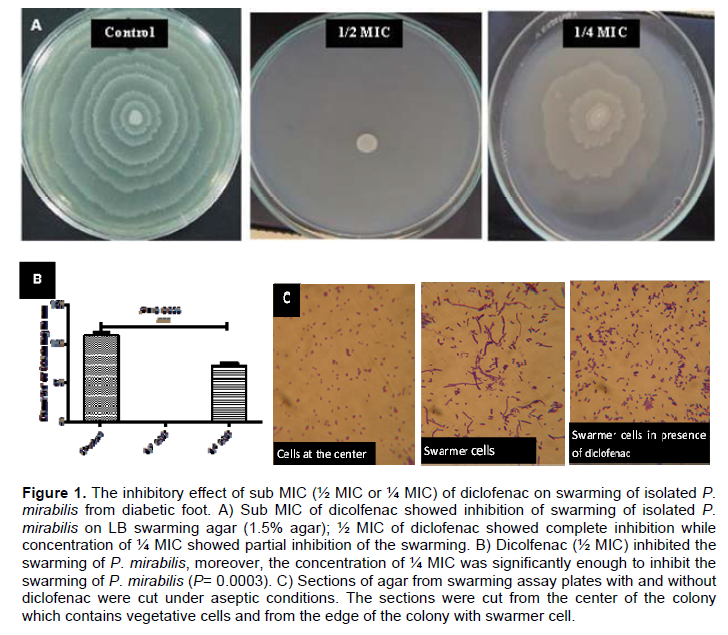
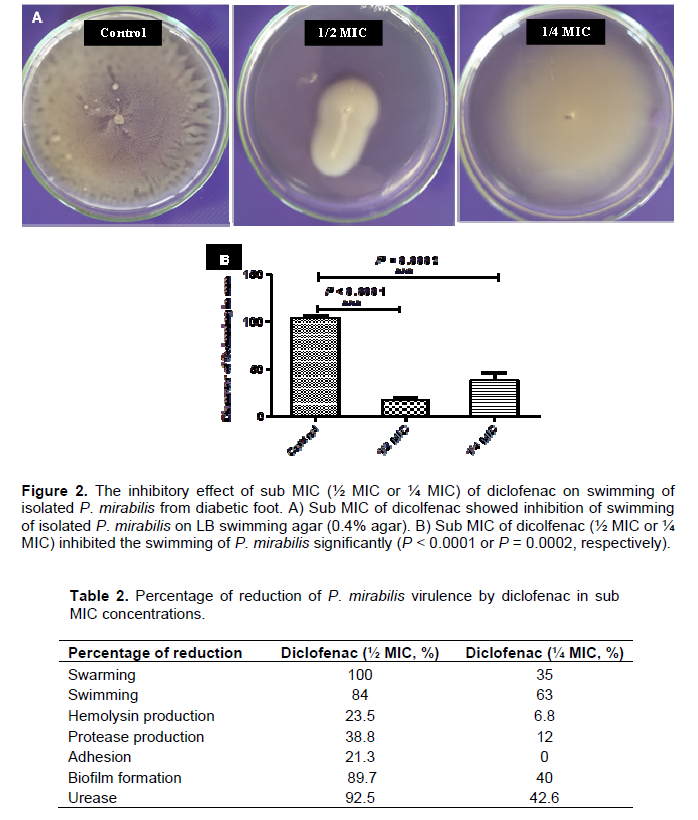
Inhibition of swarming and swimming motilities
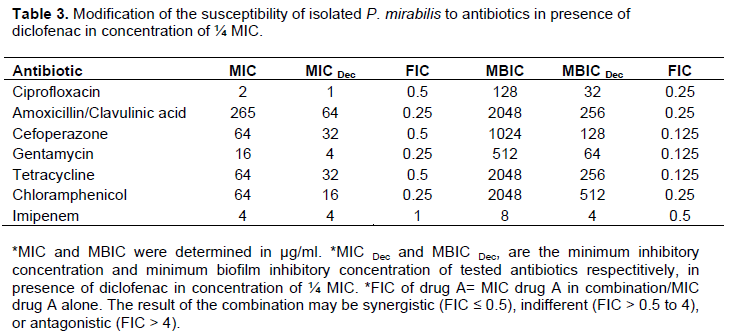
An overnight culture of P. mirabilis was prepared and 5 μl from this culture was inoculated on the center of the surface of dried LB swarming agar (1.5%) or LB swimming agar (0.4%) plates containing different sub-inhibitory concentrations of diclofenac (½ MIC or ¼ MIC). The plates were incubated overnight at 37°C, the diameters of swarming zones or swimming zones were measured in mm. Control plates were also prepared and inoculated in the same way (Figures 1A and 2A). In order to differentiate swarmer cells, sections of agar from swarming assay plates with and without diclofenac were cut under aseptic conditions. The sections were cut from the center of the colony which contains vegetative cells and from the edge of the colony with swarmer cells. The swarmer cells in the presence of diclofenac were shorter and more or less similar to vegetative cells (Figure 1C). The experiment was repeated in triplicate and the mean, standard deviation and significance of inhibition were calculated (Figures 1B and 2B). Sub-inhibitory concentrations of diclofenac inhibited the swarming and swimming motilities significantly. Diclofenac (½ MIC) inhabited swarming completely and swimming significantly (P < 0.0001), moreover diclofenac in ¼ MIC showed significant inhibition for swarming and swimming (P = 0.0003 and P = 0.0002, repetitively).
The percentage of the inhibition of swarming was 100% as in case of ½ MIC concentrations, while decreased to 35% in concentration of ¼ MIC (Table 2). Diclofenac inhibited swimming activity of isolated P. mirabilis 84 and 63% in concentrations ½ MIC and ¼ MIC, repetitively (Table 2).
Protease assay
Protease activity was measured in the presence and absence of diclofenac using a casein substrate. Sub MIC of dicolfenac (½ MIC or ¼ MIC) decreased the protease activity of P. mirabilis significantly (P = 0.0001 or P = 0.0021, respectively) (Figure 3). The percentage of inhibition of protease activity varied from 12 to about 39% in ¼ MIC and ½MIC concentrations of dicolfenac (Table 2).
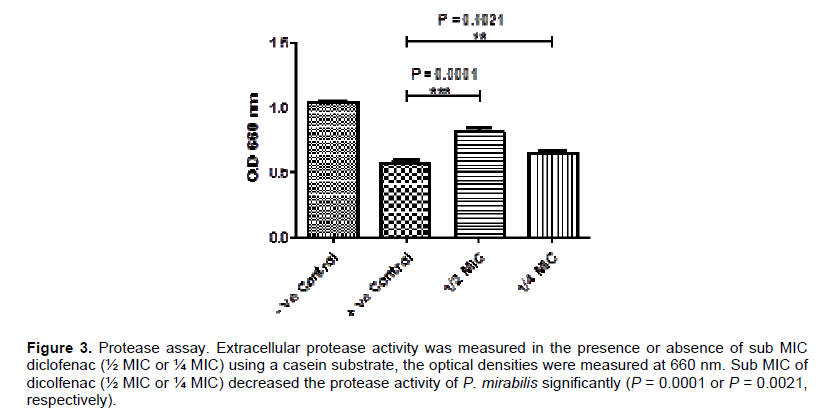
Hemolysis assay
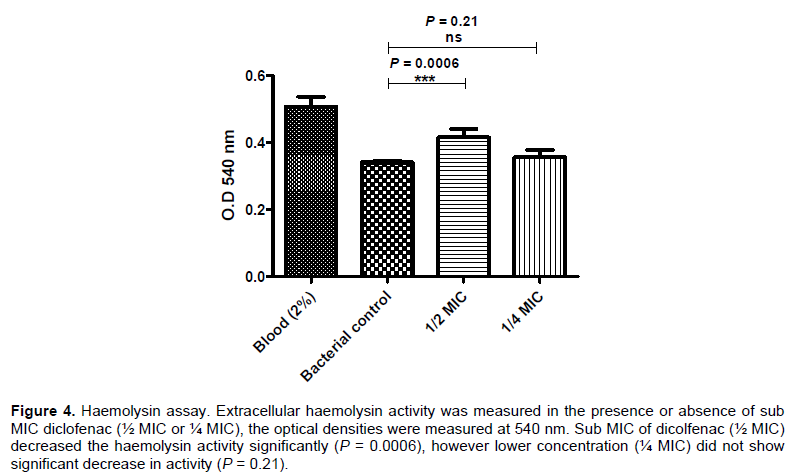
The tested strain was grown with and without sub-MIC (½ MIC or ¼ MIC) of diclofenac at 37°C. The bacterial suspensions were centrifuged and the supernatant was collected for Haemolysin assay. Dicolfenac (½ MIC) showed a significant inhibition in haemolysin activity (P = 0.0006) and the reduction percentage was about 24%, however, it did not show a significant inhibition in ¼ MIC concentration (P = 0.21) (Figure 4 and Table 2).
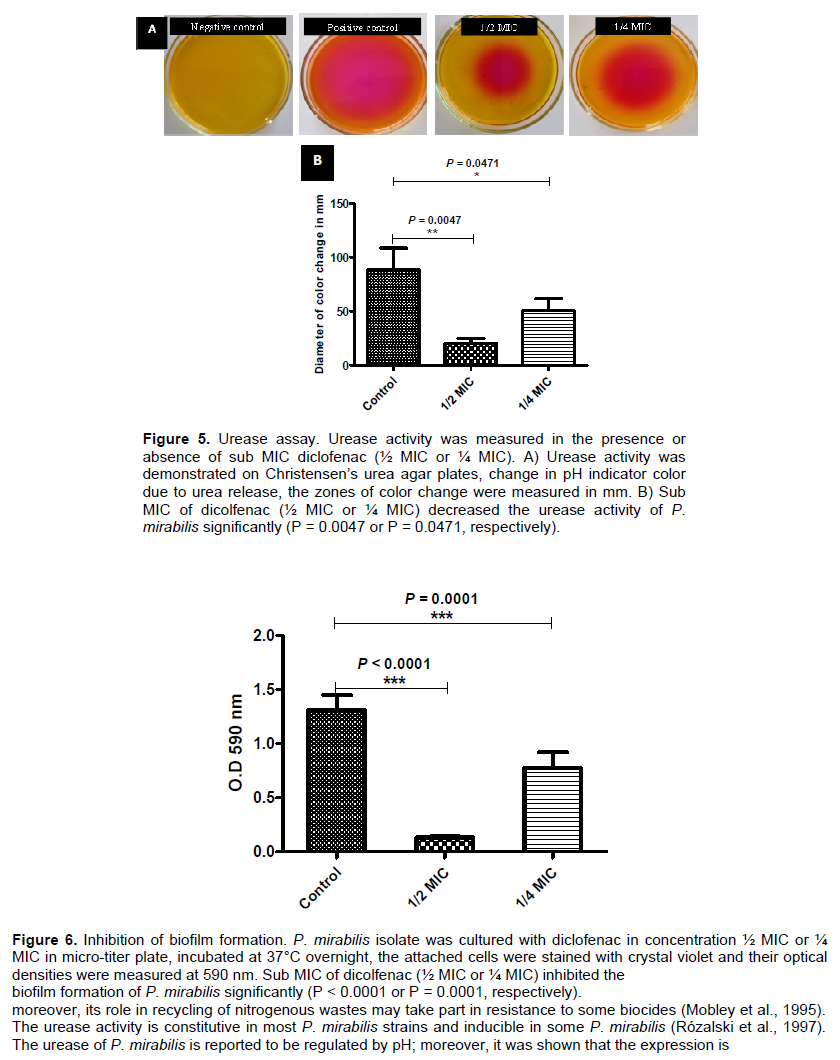
Urease activity assay
An overnight culture of P. mirabilis was prepared and 5 μl from this culture was inoculated on the center of the surface of dried Christensen’s urea agar plates containing different sub-inhibitory concentrations of diclofenac (½ MIC or ¼ MIC) (Figure 5A). The diameter of color change were measured in mm and statistically calculated. Sub MIC of dicolfenac (½ MIC or ¼ MIC) showed a decrease in the urease activity of P. mirabilis significantly (P = 0.0047 or P = 0.0471, respectively) (Figure 5B). The percentages of reduction of urease activity were about 93 and 43% for ½ MIC and ¼ MIC of diclofenac (Table 2).
Biofilm formation
For assessment of biofilm production, the ODc and OD of tested P. mirabilis were determined. ODc was 0.064 and OD was 0.344 (OD > 4x ODc), P. mirabilis isolate was considered strong biofilm forming according to Stepanovic et al. (2000). The minimum biofilm inhibitory concentration was performed using sterile 96-well polystyrene microplate plates against tested antibiotics and diclofenac (Table 1).
For evaluation of the inhibitory effect of diclofenac on biofilm formation, the same procedure described forassessment of biofilm production was followed in sterile 96-well polystyrene microplate containing ½ MIC or ¼ MIC of diclofenac. Diclofenac in sub MIC concentrations showed a significance inhibition in biofilm formation (P < 0.0001 and P = 0.0001 in ½ MIC and ¼ MIC) (Figure 6). The percentages of inhibition were calculated about 90 and 40% in concentration of ½ MIC or ¼ MIC of diclofenac repetitively (Table 2). Moreover, diclofenac (¼ MIC) decreased the MBIC of tested antibiotics, the FIC ranged from 0.125 to 0.5 (synergism). The summary of synergistic effect of diclofenac and antibiotics is summarized in Table 3.
Adhesion assay
For evaluation the effect of diclofenac on adhesion of isolated P. mirabilis, epithelial cells were collected from pregnant urine and distributed eventually in microtitre-plate and co-cultured with bacterial strain in absence and presence of diclofenac in concentration ½ MIC or ¼ MIC (Figure 7A). The cells were incubated at 37°C for 1 h, fixed at 60°C for 20 min, stained with equal volume of crystal violet and the adherent cells were lysed with citrate buffer (pH 4.3) and optical density was measured at 570 nm. No significant inhibition was observed to be considered.
Furthermore, the inhibitory effect of diclofenac on adhesion of P. mirabilis to abiotic surface was examined. P. mirabilis was cultured with diclofenac in concentration ½ MIC or ¼ MIC in micro-titer plate, incubated at 37°C for 1 h. The adherent cells were stained with crystal violet, ethanol was added and optical densities were measured at 590 nm. Compatible to findings with adhesion to epithelial cells, dicolfenac did not show a much significant inhibition of adhesion as shown in biofilm inhibition. Diclofenc did not show a significant inhibition of adhesion in concentration of ¼ MIC (P = 0.56), while it showed inhibition in concentration of ½ MIC (P = 0.01) (Figure 7.B).
Proteus is a causative agent of wide range of infections; the potential of its virulence is not only due to production of several extracellular enzymes, but also due to inherent capability of pretrichrous flagellar translocation and its biofilm formation capability (Morgenstein et al., 2010; Armbruster and Mobley, 2012). In the last decades, resistance development is one of the serious emerging problems and it is owed to genotypic and/or phenotypic modifications. In general, bacterial virulence is thought to be a factor of resistance and overcoming the virulence is hypothesized to enhance the antimicrobial eradication process. Overcoming the bacterial virulence is more crucial in immunocompromised patients, as in diabetes. Inspite, the vast advances in controlling diabetes, complications usually happen. The most serious complications are those due to bacterial infections, particularly, in immunocompromised patients (Assmann et al., 2015). In this study, P. mirabilis was isolated from diabetic foot ulcer and its virulence behavior and resistance to common prescribed antibiotics were evaluated. Anti-inflammatory drugs and analgesics are widely prescribed for diabetic patients for several medical reasons. Diclofenac is one of these anti-inflammatory and analgesic drugs which showed some antibacterial activity. This work was to evaluate the effect of diclofenac sodium salt on inhibition or reduction of important virulence factors of P. mirabilis.
Swarming and swimming describe flagellum-dependent movement across a surface or through liquid or soft agar. This form of motility allows P. mirabilis to migrate across the infected ulcer, spreading the infection (Rather, 2005; Jacobsen et al., 2008). Moreover, P. mirabilis biofilms contain protruding swarm cells which enhance the microbial resistance (Jones et al., 2007). It showed that sub-inhibitory concentrations of diclofenac inhibited the swarming and swimming motilities significantly which may affect the infection spread which may influence the pathogenesis as a consequence. Several factors participated in the regulation of swarming motility including the up-regulator of flagellar master operon (Umo) proteins and other factors reviewed elsewhere (Morgenstein et al., 2010). Moreover, all flagellum-related genes are arranged within a single 53.3-kb locus (Pearson et al., 2008), which may indicate the significance of inhibition of the transcriptional regulator on motility. There are several approaches which described the mode of diclofenac action; one of them is its capability to down regulate Umo proteins (work in progress).
Biofilms are closely associated microbial cells embedded in dynamic communities within a hydrated extracellular polymeric substance on an air-liquid interface, or adherent to inert (abiotic) or living surfaces constituting the major proportion of bacterial biomass in nature (De Kievit et al., 2001). Biofilms are formed in a sequential manner, started by reversible attachment of free-floating cells to surfaces. A variety of direct interactions, generally associated with the onset of the production extracellular polymeric substances, are responsible for the transition to irreversible attachment which entraps bacteria and results in aggregation of cells (Gómez-Suárez et al., 2002). Diclofenac in sub-inhibitory concentration did not affect the bacterial adhesion significantly, while the formation of biofilms are significantly inhibited in the same concentrations, which indicate its action on prevention of late stages of biofilm formation. Many species of bacteria use a system of stimuli and response correlated to population density called quorum sensing (QS). Intercellular signaling regulates functions contributing to virulence of many bacterial pathogens. Thus, interference with signaling is a promising approach to improve the outcome of bacterial, and in particular, biofilm infections (Zhang and Li, 2016). It was assumed that effect of diclofenac on biofilm formation may be due to interference with QS signaling (under investigation) which may explain its significant inhibition of biofilm versus non considerable inhibition of bacterial adhesion. Diclofenac in sub- inhibitory concentrations do not only lowered the inhibitory concentration of antibiotics in combination, but also lowered their inhibitory concentration in the presence of biofilm.
The effect of diclofenac is extended to inhibit extracellular enzymes which may contribute significantly in lowering the pathogenesis. Urease enzyme is a significant virulence factor in human and animal infections of the urinary and gastrointestinal tracts; developmentally regulated as swarm cells have higher levels of urease and of urease transcript (Mobley et al., 1995). Protease is an extracellular proteolytic enzyme which cleaves two classes of antibodies, IgA and IgG, as well as non-Ig proteins such as gelatin, secretory component, casein, and bovine serum albumin (Loomes et al., 1990). It had been demonstrated that the differentiation of P. mirabilis short vegetative rods into hyper-flagellate swarmer cells is accompanied by substantial increases in the activities of virulence factors including proteases (Allison et al., 1994). The ability of P. mirabilis to invade human epithelial cells is basically characteristic of swarmer cells but not vegetative cells; the protease activity is hypothesized to be relevant to P. mirabilis pathogenesis (Rózalski et al., 1997). Moreover, P. mirabilis strains synthesize urease, which degrades urea, providing alkaline optimal conditions for the action of proteases (Senior et al., 1993). Diclofenac showed a significant inhibition on both protease and urease which may reduce bacterial virulence potentially.
Haemolysin is another virulence factor of P. mirabilis because of its cytotoxicity on epithelial cells (Armbruster and Mobley, 2012). It is most interesting that mutation in the gene encoding Haemolysin hpmA did not affect the colonization or tissue damage during infection (Alamuri et al., 2009), which may indicate that its activity is either diminished in-vivo or masked by contribution of other virulence factors (Armbruster and Mobley, 2012). P. mirabilis genome is transcribed in several distinguished rRNA operons, surprisingly the two-partner secretion system containing the hemolysin genes hpmBA are transcribed in separate operons than those in which protease and urease are transcribed (Pearson et al., 2008). Diclofenac showed a significant inhibition of haemolysin production in higher concentrations in comparison to that needed to inhibit protease or urease, that may be due to an expected inhibitory effect of diclofenac on transcription of urease or protease (under investigation). Conclusively, diclofenac showed a significant inhibition of P. mirabilis virulence, which may be helpful to diabetic patients who need anti-inflammatory and analgesic treatments. This study showed the effect of diclofenac phenotypically while it will be more interesting to show the molecular basis of this effect (work in progress). Diclofenac is widely used drug in different medical conditions and in different pharmaceutical formulations; inspite of the fact that our study lacks the in-vivo evidence of diclofenac inhibitory effect on virulence, it may be more helpful to prescribe diclofenac for diabetic patients when needed.
The authors have not declared any conflict of interests.
REFERENCES
|
Alamuri P, Eaton KA, Himpsl SD, Smith SN, Mobley HL (2009). Vaccination with proteus toxic agglutinin, a hemolysin-independent cytotoxin in vivo, protects against Proteus mirabilis urinary tract infection. Infect. Immun. 77(2):632-641.
Crossref
|
|
|
|
Allison C, Emödy L, Coleman N, Hughes C (1994). The role of swarm cell differentiation and multicellular migration in the uropathogenicity of Proteus mirabilis. J. Infect. Dis. 169(5):1155-1158.
Crossref
|
|
|
|
Armbruster CE, Mobley HLT (2012). Merging mythology and morphology: the multifaceted lifestyle of Proteus mirabilis. Nat. Rev. Microbiol. 10(11):743-754.
Crossref
|
|
|
|
Assmann TS, Brondani Lde A, Bouças AP, Canani LH, Crispim D (2015). Toll-like receptor 3 (TLR3) and the development of type 1 diabetes mellitus. Arch. Endocrinol. Metab. 59(1):4-12.
Crossref
|
|
|
|
Cernohorská L, Votava M (2008). Antibiotic synergy against biofilm-forming Pseudomonas aeruginosa. Folia Microbiol. 53(1):57-60.
Crossref
|
|
|
|
Chakraborti AK, Garg SK, Kumar R, Motiwala HF, Jadhavar PS (2010). Progress in COX-2 inhibitors: a journey so far. Curr. Med. Chem. 17(15):1563-1593.
Crossref
|
|
|
|
De Kievit TR, Gillis R, Marx S, Brown C, Iglewski BH (2001). Quorum-sensing genes in Pseudomonas aeruginosa biofilms: their role and expression patterns. Appl. Environ. Microbiol. 67(4):1865-1873.
Crossref
|
|
|
|
Flannery EL, Mody L, Mobley HLT (2009). Identification of a modular pathogenicity island that is widespread among urease-producing uropathogens and shares features with a diverse group of mobile elements. Infect Immun. 77:4887-4894.
Crossref
|
|
|
|
Gómez-Suárez C, Pasma J, van der Borden AJ, Wingender J, Flemming HC, Busscher HJ, van der Mei HC (2002). Influence of extracellular polymeric substances on deposition and redeposition of Pseudomonas aeruginosa to surfaces. Microbiology 148(4):1161-1169.
Crossref
|
|
|
|
Grayson ML (1995). Diabetic foot infections, Antimicrobial therapy. Infect Dis Clin North Am. 9:143-161.
|
|
|
|
Hegazy WA, Hensel M (2012). Salmonella enterica as a vaccine carrier. Future Microbiol. 7(1):111-127.
Crossref
|
|
|
|
Jacobsen SM, Stickler DJ, Mobley HLT, Shirtliff ME (2008). Complicated catheter-associated urinary tract infections due to Escherichia coli and Proteus mirabilis. Clin. Microbiol. Rev. 21:26-59.
Crossref
|
|
|
|
Jones SM, Yerly J, Hu Y, Ceri H, Martinuzzi R (2007). Structure of Proteus mirabilis biofilms grown in artificial urine and standard laboratory media. FEMS Microbiol. Lett. 268:16-21.
Crossref
|
|
|
|
Keay L, Moser PW, Wildi BS (1970). Proteases of the genus Bacillus. II. Alkaline proteases. Biotechnol. Bioeng. 12(2):213-249.
Crossref
|
|
|
|
Koneman EW, Allen SD, Janda WM, Schreckenberger PC, Winn WC (1997). Color atlas and textbook of diagnostic microbiology. Lippincott, Philadelphia 5th ed: pp. 253-320.
|
|
|
|
Liaw SJ, Lai HC, Ho SW, Luh KT, Wang WB (2001). Characterization of p-nitrophenylglycerol-resistant Proteus mirabilis super-swarming mutants. J. Med. Microbiol. 50(12):1039-1048.
Crossref
|
|
|
|
Liaw SJ, Lai HC, Wang WB (2004). Modulation of swarming and virulence by fatty acids through the RsbA protein in Proteus mirabilis. Infect. Immun. 72(12):6836-6845.
Crossref
|
|
|
|
Loomes LM, Senior BW, Kerr M (1990). A proteolytic enzyme secreted by Proteus mirabilis degrades immunoglobulins of the immunoglobulin A1 (IgA1), IgA2, and IgG isotypes. Infect. Immun. 58(6):1979-1985.
|
|
|
|
Mackay ML, Milne K, Gould IM (2000). Comparison of methods for assessing synergic antibiotic interactions. Int J Antimicrob Agents. 15(2):125-129.
Crossref
|
|
|
|
Mobley HL, Island MD, Hausinger RP (1995). Molecular biology of microbial ureases. Microbiol. Rev. 59(3):451-480.
|
|
|
|
Morgenstein RM, Szostek B, Rather PN (2010). Regulation of gene expression during swarmer cell differentiation in Proteus mirabilis. FEMS Microbiol. Rev. 34(5):753-763.
Crossref
|
|
|
|
O'Hara CM, Brenner FW, Miller JM (2000) Classification, identification, and clinical significance of Proteus, Providencia, and Morganella. Clin Microbiol. Rev. 13:534-546.
Crossref
|
|
|
|
Park TH, Anand A (2015). Management of diabetic foot: Brief synopsis for busy orthopedist. J. Clin. Orthop. Trauma 6(1):24-29.
Crossref
|
|
|
|
Pearson MM, Sebaihia M, Churcher C, Quail MA, Seshasayee AS, Luscombe NM, Abdellah Z, Arrosmith C, Atkin B, Chillingworth T, Hauser H, Jagels K, Moule S, Mungall K, Norbertczak H, Rabbinowitsch E, Walker D, Whithead S, Thomson NR, Rather PN, Parkhill J, Mobley HL (2008). Complete genome sequence of uropathogenic Proteus mirabilis, a master of both adherence and motility. J. Bacteriol. 190(11):4027-4037.
Crossref
|
|
|
|
Perim MC, Borges JC, Celeste SR, Orsolin EF, Mendes RR, Mendes GO, Ferreira RL, Carreiro SC, Pranchevicius MC (2015). Aerobic bacterial profile and antibiotic resistance in patients with diabetic foot infections. Rev. Soc Bras. Med. Trop. 48(5):546-554.
Crossref
|
|
|
|
Rather PN (2005). Swarmer cell differentiation in Proteus mirabilis. Environ. Microbiol. 7:1065-1073.
Crossref
|
|
|
|
Rózalski A, Sidorczyk Z, KoteÅ‚ko K (1997). Potential virulence factors of Proteus bacilli. Microbiol. Mol. Biol. Rev. 61(1):65-89.
|
|
|
|
Santema TB, Poyck PP, Ubbink DT (2016). Skin grafting and tissue replacement for treating foot ulcers in people with diabetes. Cochrane Database Syst Rev. 11;2:CD011255.
|
|
|
|
Sekhar S, Vyas N, Unnikrishnan M, Rodrigues G, Mukhopadhyay C (2014). Antimicrobial susceptibility pattern in diabetic foot ulcer: a pilot study. Ann. Med. Health Sci. Res. 4(5):742-745.
Crossref
|
|
|
|
Senior BW (1993). The production of HlyA toxin by Proteus penneri strains. J. Med. Microbiol. 39(4):282-289.
Crossref
|
|
|
|
Stepanovic S, Vukovic D, Dakic I, Savic B, Svabic-Vlahovic M (2000). A modified microtiter-plate test for quantification of Staphylococcal biofilm formation. J. Microbio.l Methods. 40(2):175-9.
Crossref
|
|
|
|
Tansarli GS, Athanasiou S, Falagas ME (2013). Evaluation of Antimicrobial Susceptibility of Enterobacteriaceae Causing Urinary Tract Infections in Africa. Antimicrob. Agents Chemother. 57(8):3628-2639.
Crossref
|
|
|
|
Vesterlund S, Paltta J, Karp M, Ouwehand AC (2005). Measurement of bacterial adhesion-in vitro evaluation of different methods. J. Microbiol. Methods. 60(2):225-233.
Crossref
|
|
|
|
Wayne PA (2006). Clinical and Laboratory Standards Institute Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; approved standard. 7th ed. CLSI Document M7-A7.
|
|
|
|
Zhang W, Li C (2016). Exploiting Quorum Sensing Interfering Strategies in Gram-Negative Bacteria for the Enhancement of Environmental Applications. Front. Microbiol. 8(6):1535.
Crossref
|
|
|
|
Zhao WH, Hu ZQ (2013). Epidemiology and genetics of CTX-M extended-spectrum β-lactamases in Gram-negative bacteria. Crit. Rev. Microbiol. 39(1):79-101.
Crossref
|