ABSTRACT
Studies were conducted in the Department of Plant Pathology, College of Agriculture, Orissa University of Agriculture and Technology, Odisha, India in 2010-2011 on serological detection methods for identification of bacterial pathogens associated with rotted potato tubers. One hundred and two rotten potato tubers of KufriJyoti variety were collected from freshly harvested lot of All India Co-ordinated Potato Improvement Project, Orissa University of Agriculture and Technology. Tubers were cut into two equal halves and categorized into 6 groups, on the basis of internal symptoms exhibited, that is, (A) cut tubers showing brownish discolouration along the vascular region, (B) cut tubers showing brownish discolouration along the vascular region with soft rotten cavities filled with whitish ooze, (C) cut tubers showing brownish blackish discolouration along the vascular region and soft rotten tissues extending towards centre, (D) cut tubers showing soft rotten tissues extending towards centre without brownish discolouration, (E) cut tubers showing soft rotten tissues extending towards centre with brownish black discolouration, (F) cut tubers showing dry tissues with cavities surrounded by soft tissues. The association of two bacterial species was assayed following tube agglutination test using the known antiserum for each bacterium, Ralstonia solanacearum and Pectobacterium carotovorum. It was revealed that R. solanacearum could be associated exclusively with 54.10% of diseased tubers with symptom Category–A. No other bacteria could be detected from the rest of the samples belonging to the said category. Similarly, exclusive association of P. carotovorum could be detected in 87.50% of the rotten tubers with symptom Category–D. In the symptom categories B, C, E and F, both test bacteria were found to be associated either singly or as mixture. Least bacterial infection due to P. carotovorum (12.5%) was observed in symptom Category–F. It is a very quick detection method which can reveal the percentage of bacterial pathogen association after two hours of testing.
Key words: Rotten potato tubers, bacteria, bacterial pathogen, serological detection, Ralstonia solanacearum, Pectobacterium carotovorum.
Rotting of potato tubers is commonly noticed at the time of harvest, in storage at country stores and also at cold stores. The rotten tubers exhibit brown rot, soft rot and mixed symptoms. Accurate identification of the causal pathogen is necessary because management strategy is almost different for different organisms. The usual method of identification of the causal bacterial plant pathogen involves isolation, pathogenicity test followed by Gram staining, microscopic studies, growing them on selective medium, a series of biochemical tests and carbohydrate tests and studies at molecular levels followed in different countries like Japan and Mauritius (Rodney et al., 2010; Harita et al., 2010; Suga et al., 2013). Molecular level of isolation and characterization of the phytopathogenic bacteria has recently gained wide acceptance (Tamura et al., 2011; Zhou et al., 2012). Rahman et al. (2012) characterized soft rot bacterial strain of potato following physiological and biochemical tests such as (i) potato soft rot test; (ii) Gram reaction test; (iii) glucose fermentation, oxidase reaction; (iv) catalase test and (v) gelatine liquefaction test, nitrite test, indole test and lecithinase test. Ravari et al. (2011) isolated forty strains from macerated potato tubers and water soaked lesions of some ornamental plants in north parts of Iran, proved pathogenicity in their respective hosts. The causal organisms were identified as Pectobacterium spp. based on their physiological and biochemical assays and confirmed by species and subspecies specific PCR and RFLP analysis of 16S-23S intergenic transcribed spacer region. Dhital et al. (2001) characterized the strains of Ralstonia solanacearum (the causal agent of bacterial wilt disease from Nepal and Thailand) on the basis of pathogenicity, biochemical/physiological and serological tests. Among different recent techniques, one is detection of quorum sensing molecules in R. solanacearum which may be responsible for virulence (Kumar et al., 2016). Similarly, molecular methods of characterization in Potato virus Y was studied in different countries by different workers (Chikh-Ali et al., 2016). Isolation and characterization of R. solanacearum was studied by several workers (Lemessa and Zeller, 2007; Alvarez et al., 2010; Tamura et al., 2011).
Both bacteria (R. solanacearum and P. carotovorum) caused rotting at harvest. P. carotovorum continues to enhance rotting in transit and storage and spread easily by contact. Quick detection will help in adopting adequate management practices at earliest possible time and the loss can be reduced subsequently. Among different methods, the serological detection method is also the most accurate, simple and quick technique. By using specific antiserum, the occurrence of particular race or strain of a particular plant pathogenic bacteria and virus can be studied easily and also at earliest time and further studies can be conducted. In the present study, serological detection methods were followed for identification of bacteria associated with rotted potato tubers.
One hundred and two apparently rotten potato tubers were collected from the harvested lot of All India Co-ordinated Potato Research Project, Orissa University of Agriculture and Technology, Bhubaneswar and cut into two equal halves. On the basis of internal rotting symptom, the tubers were categorized into six different rotting groups. The association of two bacterial species were assayed following tube agglutination test using the known antiserum for each bacteria: R. solanacearum and P. carotovorum.
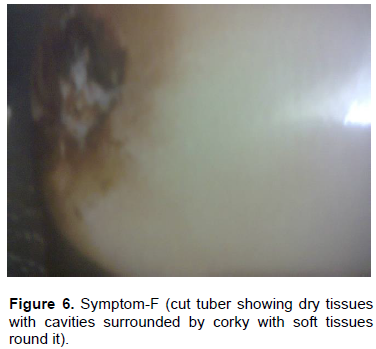
The rotting symptoms observed were categorized into 6 groups namely; (A) cut tubers showing brownish discoloration along the vascular region (24), (B) cut tubers showing brownish discolorations along the vascular region with soft rotten cavities filled with whitish ooze(16), (C) cut tubers showing brownish blackish discolorations along the vascular region and soft rotten tissues extending towards centre (24), (D) cut tubers showing soft rotten tissues extending towards the center without brownish discoloration (8), (E) cut tubers showing soft rotten tissues extending towards the centre with brownish black discoloration (22), (F) cut tubers showing dry tissues with cavities surrounded by soft tissue (8) as presented in Table 1. Each tuber collected from experimental field was carefully washed in tap water using a soft brush. Care was taken not to damage the skin of tuber or disturb the disease symptom. Then it was blot dried and examined for characteristic symptom developed. Based on the symptom, the tubers were separated. Individual tubers in each symptom group were tested for association of test bacteria following modified tube agglutination test. Completely rotten portion of individual tuber in a group was separated and the apparently healthy tissues adjacent to disease portion were cut into small pieces of about 1.0 cm size. Five cut pieces were transferred into 0.5 ml of sterile water taken in a culture tube. It was kept under laboratory condition for 30 min for bacterial oozing.
For detection of test bacterial species present in the bacterial suspension of each diseased tuber, sample was carried out following tube agglutination test. For each sample, two agglutination tubes were taken for the two known antisera. 0.5 ml of 10.0% diluted solution of each known antiserum was transferred into one tube and the other known anti serum to the second tube and leveled properly. To each of the two tubes, 0.5 ml of bacterial suspension collected from one diseased sample was transferred and mixed thoroughly using Pasteur pipettes. The process was repeated for subsequent disease samples. The tubes were incubated in a hot water bath maintained at 37±1°C for 2 h. Formation and deposition of precipitate at the base of the tube indicated positive reaction on the basis of positive reaction against the known antiserum.
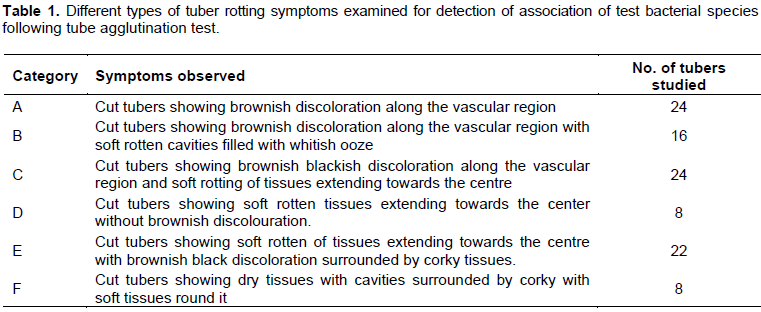
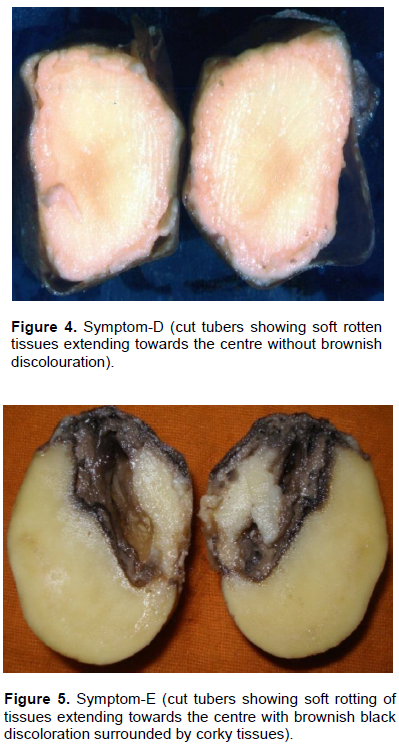
Results revealed that out of total diseased tubers, 23.52% of the tubers exhibited symptom categorized with brownish discoloration along the vascular region without any soft rotting of tissues (Category–A) (Table 2 and Figure 1). About 15.68% of tubers exhibited symptom with brownish discoloration along the vascular region with soft rotten cavities filled with whitish ooze (Category–B) (Figure 2). It was closely followed by the Category–C (23.52%) (Figure 3) with brownish black discoloration along the vascular region with soft rotting of tissues extending towards the centre. About 7.84% tubers exhibited symptom both in categories– D and F each (Figure 4). In Category–D (Figure 4), the tubers showed soft rotten tissues extending towards the center without brownish discoloration and in Category–F (Figure 6), the tuber exhibited dry cavities surrounded by corky layer with soft tissues round it. In Category–E (Figure 5), 21.56% of the tubers showed soft rotten tissues extending towards the centre with brownish black discoloration.
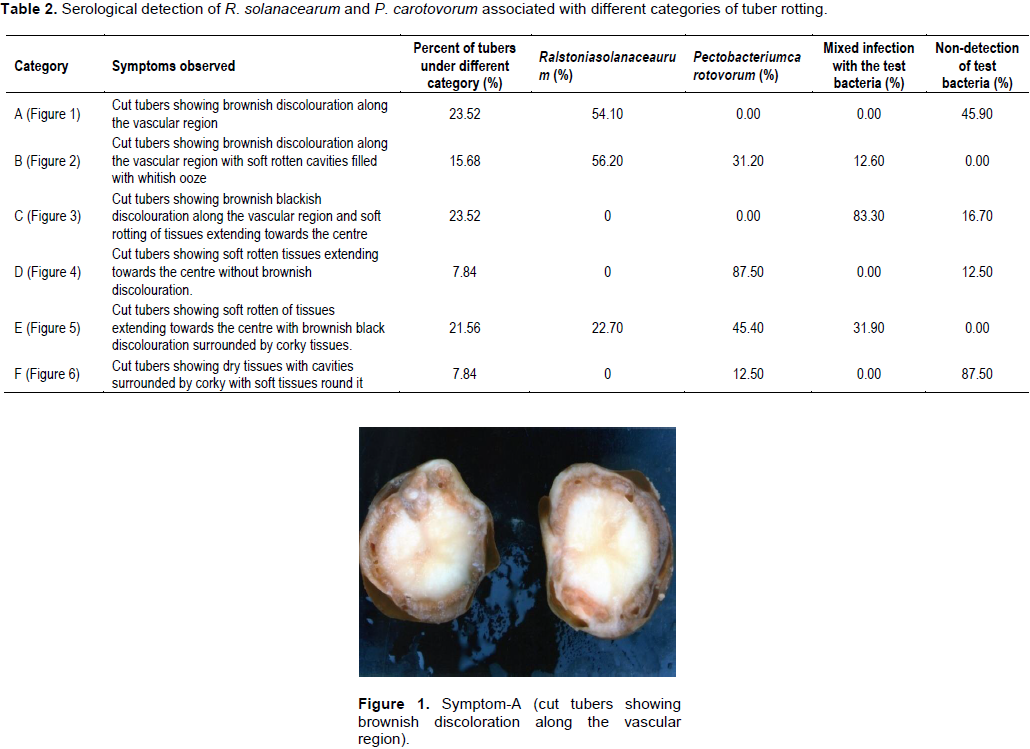
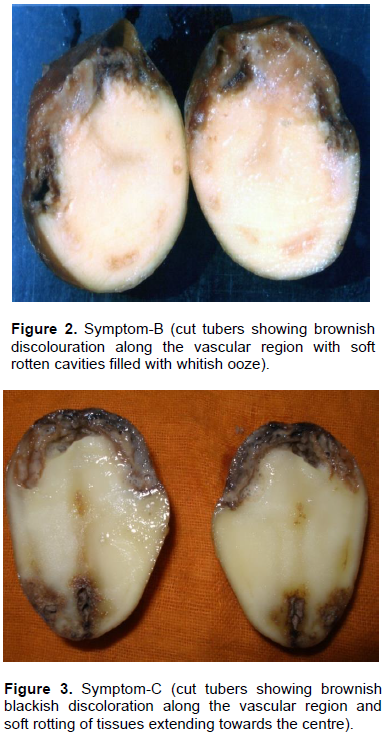
R. solanacearum could be found associated exclusively in 54.10% of the diseased tubers of symptom Category–A. No other bacteria could be detected from the rest of the samples belonging to the said category. Similarly, exclusive association of P. carotovorum could be detected in 87.50% of the rotten tubers of symptom category–D. In the symptom category B and E, both test bacteria were found to be associated either singly or as mixture. However, maximum infection of 56.20% was caused by R. solanacearum in symptom Category–B followed by P. carotovorum.
Similarly P. carotovorum was found to be associated with maximum 45.40% of tuber infection as given in Category–E. Mixed infection of both bacteria could be detected in 31.9% of diseased tubers as per Category-E. Only 12.50% of tubers found were associated with P. carotovorum in symptom Category-F and no bacterial infection was found in the rest 87.50% of tubers.
Brown rot (R. solanacearum) and soft rot (P. carotovorum) are the two major devastating diseases of potato at harvest in Odisha India. Present studies followed sero-diagnosis methods and revealed that most tubers rotted due to P. carotovorum followed by R. solanacearum. Mixed infection by both bacteria was also recorded.
Serological detection methods were also used by different workers around the globe in different crops in addition to strains on potato. Khder et al. (2014) studied in detail, the serological characteristics of Erwinia carotovora isolated from fields in Egypt. Silveria et al. (2002) worked on production of antisera of soft rotting Pectobacteria. Improved monospecific serum technology was also used for diagnosis of E. Amylovora (Bokszan and Sadlak, 2001). Serological methods was successfully quantified for the potato seed contamination by E. carotovora sub sp. atroseptica (Perombelon and Hyman, 1995). Serological studies were done with 11 isolates of Erwinia carotovora sub sp. atroseptica (Mierzwa et al., 1979). Tanii and Akaii (1975) demonstrated black leg was caused by serologically specific strain of E. carotovora. Dobias (1973) detected the serological relationship of strains of E. carotovora. Prez (1962) worked serologically on identification of Pseudomonas solanacearum. Several workers also adopted serological methods in studies of viral and fungal diseases. Galvino-Costa et al. (2012) worked together on molecular and serological typing of Potato virus Y isolates from Brazil. Karasev et al. (2011) studied serologically, the genetic diversity of the ordinary strain of Potato virus Y (PVY) and origin of recombinant PVY strains. Serological properties of ordinary and necrotic isolates of PVY were taken into account to survey the occurrence for both isolates in the U.S seed crop (Karasev et al., 2010). Baldauf et al. (2006) worked on biological and serological properties of Potato Virus Y isolates in Northeastern United States potato in New York. Mahmoud et al. (2010) followed serological method for detection of Phytophthora infestans in infected symptomatic and asymptomatic potato tissues (leaves and tubers) and could provide important interaction and disease development. Llave et al. (1999) followed serological analysis for coat protein sequence determination of potato virus Y pepper pathotypes. Sukla et al. (1988) compared four strains of potato viruses Y, that is, PVY-D, PVY-10, PVY-18 and PVYPVY-43 on the basis of their biological, serological and coat protein structural properties. McDonald and Singh (1996) established the serology of the isolates of PVY that share properties with both PVYN and PVY0 strain groups. De Boer et al. (1979) categorized sero groups of E. carotovora potato strains. Dobias (1973) detected the serological relationship of strains of E. carotovora. Serological detection methods has already been followed in other crops, that is, poplar (Zhang et al., 2001), wheat (Shneider et al., 1978) and also in hyacinth and calla (Kabashna, 1977). Hence, by using specific antiserum, the occurrence of particular race or strain of a particular plant pathogenic bacteria and virus in potato can be studied easily. Quick detection technique is very much essential for effective management of the disease in stored condition and also in field during growing and harvesting stage.
The authors have not declared any conflict of interests.
REFERENCES
|
Baldauf PM, Gray SM, Perry KL (2006). Biological and serological properties of Potato virus Y isolates in northeastern United States potato. Plant Dis. 90(5):559-566. .
Crossref
|
|
|
|
De Boer SH, Copeman RJ, Vruggink H (1979). Serogroups of Erwiniacarotovora potato strains determined with diffusible somatic antigens. Phytopathology 69(4):316-319.
Crossref
|
|
|
|
Bokszan O,'Sadlak A (2001). Improved monospecific serum technology based on haemoagglutination and its use in diagnosis of E. amylovora. Ochro. Ros. 45(9/10):39-40.
|
|
|
|
Dhital SP, Thaveechai N, Shrestha SK (2001). Characteristics of Ralstonia solanacearum strains of potato wilt disease from Nepal and Thailand. Nepal Agric . Res. J. 4&5:42-47
|
|
|
|
Dobias K (1973). The serological relationship of strains of Erwinia carotovora (Jones) Holland isolates from potato. Rostl Vyroba 19(3):277-284.
|
|
|
|
Galvino-Costa SBF, Figueira AR, Rabelo-Filho FAC, Moraes FHR, Nikolaeva OV, Karasev AV (2012). Molecular and serological typing of Potato virus Y isolates from Brazil reveals a diverse set of recombinant strains. Plant Dis. 96(10):1451-1458.
Crossref
|
|
|
|
Harita M, Suga Y, Ooshiro A, Tsuchiya K (2010). Analysis of genetic and biological characters of Japanese potato strains. J. Gen. Plant Pathol. 76(3):196-207.
Crossref
|
|
|
|
Kabashna LV (1977). Serological study on Erwinia aroideae (Townsend) Holland and E. carotovora (Jones) Holland isolated from hycianth and calla. Mikrobiol. Zhul. 39(4):452-457.
|
|
|
|
Karasev AV, Nikolaeva OV, Hu X, Sielaff Z, Whittworth J, Lorenzen JH, Gray SM (2010).Serological properties of ordinary and necrotic isolates of Potato virus Y:A Case Study of PVYN Misidentification. Am. J. Potato Res. 87(1):1-9.
Crossref
|
|
|
|
Karasev J, Kerlan C, Nikolaeva OV, Crosslin JM, Grey SM (2011). Genetic diversity of the ordinary of the ordinary strain of potato virus Y (PVY) and origin of recombinant PVY strain. Phytopathology 101(7):778-785.
Crossref
|
|
|
|
Llave C, Martinez B, Diaz-Ruiz JR, Lopez-Abella D (1999). Serological analysis and coat protein sequence determination of Potato Virus Y (PVY) pepper patho types and differentiation from other PVY strains. Euro. J. Plant Pathol. 105(9):847-857.
Crossref
|
|
|
|
Mahmoud HEK, Eid MAT, Sayed MA, Ebtsam MES (2010). Serological and molecular detection of late blight pathogen and disease development in potato. Int. J. Agric. Biol. 12(2):161-170.
|
|
|
|
McDonald JG, Singh RP (1996). Host range, symptomatology and serology of potato virus Y (PVY) that share properties with both the PVYN and PVYO strain groups. Am. Potato J. 73(7):309-315.
Crossref
|
|
|
|
Mierzwa Z, Komorowska, Jedrys J (1979). Serological characteristics of Erwinia carotovora sub. sp atroseptica. Biuletynin stytutu Ziemniaka 31:125-128.
|
|
|
|
Perombelon MCM, Hyman LJAD (1995). Serological methods to quantify potato seed contamination by Erwiniacarotovora subsp. atroseptica. EPPO Bulletin 25(1â€2):195-202.
Crossref
|
|
|
|
Khedr AA, Mehiar F, EL-Kad MA, Gabr MA, Elsharkaairus WY MM, Shimizu M (2014). Serological characteristics of Erwinia carotovora isolated from potato fields in Egypt. Plant Pathol. J .13:246-256.
Crossref
|
|
|
|
Prez JE (1962). The use of Foller's formaide method in the serological identification of P. solanacearum. J. Agric. Univ. Puerto Rico 46:144-153.
|
|
|
|
Rahman MM, Equab Ali M, Khan AA, Hasim U, Akanda AM, Hakim MA (2012). Serological and molecular detection of late blight pathogen and disease development in potato. Int. J. Agric. Biol. 12(2):161-170.
|
|
|
|
Ravari BS, Rahimiam H, Shoms B, Lopez-solanila E, Antanez-Lamas M, Rodriguez-Palenzuela P (2011). Characteriation of Pectobacterium species from Irn using biochemical and molecular methods. Euro. J. Plant Pathol. 10:3050-3059.
|
|
|
|
Rodney KH, Ganoo MH, Saumtally ES (2010). Molecular characterization and epidemiology of Ralstonia solanacearum Race 3 biovar 2 causing brown rot of potato in Mauritius. J. Phytopathol. 158(7-8):503-512.
Crossref
|
|
|
|
Kumar JS, Umesha S, Prasad KS, Niranjana P (2016). Detection of Quorum Sensing Molecules and Biofilm Formation in Ralstonia solanacearum. Curr. Microbiol. 72(3):297-305.
|
|
|
|
Shneider Y, Ilyukhin MK, Gerrrasimova KF (1978). Serological diagnosis of bacterial diseases of winter wheat. Zasch. Rest.11:40-41.
|
|
|
|
Silveria JRP, Castro LAS de, Courto MEO, Martins OM, Barni V (2002). Production of antisera for diagnosis of soft-rotting Pectobacteria in potato. Pesq. Agro. Gau. 8(1/2):7-14.
|
|
|
|
Suga Y, Horita M, Umekita M, Furya N, Tsuchiya K (2013). Pathogenic characters of Japanese potato strains of Ralstonia solanacearum. J. Gen. Plant Pathol. 79(2):110-114.
Crossref
|
|
|
|
Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S (2011). MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance and maximum parsimony methods. Mol. Biol. Evol. 28:2731-2739.
Crossref
|
|
|
|
Tanii A, Akai J (1975). Blackleg of potato plant caused by a serologically specific strain of Erwinia carotovora var. carotovora (Jones) Dye. Ann. Phytopathol. Soc. Jpn. 41:513-517.
Crossref
|
|
|
|
Zhang JH, Liu JH, Dong AR, He BZ, Xiang CZ, Zhu H, Wang CW, Lin HB, Jiang GY, Pan SY, Su GL (2001).Serology detection techniques for poplar INA bacterial canker disease. J. North East Forest. Univ. 29(3):128-133.
|
|
|
|
Zhou T, Chen D, Li C, Sun Q, Li L., Liu F, Shen Q, Shen B (2012). Isolation and characterization of Pseudomonas brassicacearum J12 as antagonist against Ralstonia solanacearum and identification of its antimicrobial components. Microbiol. Res.167:388-394.
Crossref
|




