ABSTRACT
Thermostable amylases are the most important enzymes in present with potential industrial applications. The main objective of this study was to isolate and characterize thermophilic amylases from Bacilli found in starch rich soil. Amylase producing bacilli were isolated and their enzymes were also characterized. Effect of temperature, pH, substrate and salt concentration on amylases activity were determined. All amylases produced by different isolates were hydrolyzed greater than 91% of starch after 60 h of fermentation. There was no significant (P ≥ 0.05) variation in enzyme productivity along with fermentation time. Amylase producing isolates were designated as Isolate-1, Isolate-2, Isolate-3, Isolate-4, Isolate-5 and Isolate-6. Amylases activities of all isolates were reached their optimum at 60°C. Amylases from all bacilli isolates were shown hydrolysis capacity of starch ranging from 91.4 to 95.7%. The optimum enzyme activity of amylase from Isolate-2 was extended from pH 7 to 8 with starch hydrolysis efficiency of 98% but other isolates enzyme activity reaches 99.5 to 100% at pH 8. The crude amylase extract has an activity with inversely proportional with substrate concentration. The bacterial dry weight increases as the course of incubation time increases and NaCl concentration greater than 5 molar significantly decreases the activity of the crude amylase extract. Amylases of this finding with thermophilic, alkalophilic and halophilic characteristics have wide range of huge potential for industrial applications. Besides, further purification of the crude extract could be conducted to meet thermophilic amylase enzyme requirements of pharmaceuticals and clinical sectors.
Key words: Alkalophilic, amylase, bacillus, industrial application, thermostable.
Amylases are among the most important enzymes widely studied in biotechnology. They constitute a class of industrial enzymes having approximately 25% of the enzyme in the market (Rajagopalan and Krishnan, 2008).
They have wide area of potential application in baking and bread industry, paper industry, textile desizing, detergent industry, starch liquefaction and saccharification, food and pharmaceutical industries and lastly analyses in medical and clinical chemistry. In addition they play an important role in the biogeochemical cycle of carbon (Gupta et al., 2003). One of the best characteristics of such enzymes is stability at high temperature. Therefore, thermostability is a desired characteristic of most of the industrial enzymes. Thermostable enzymes isolated from thermophilic organisms have a number of commercial and industrial applications because of their stability. Amylases working at high temperature are important for industrial application. This high temperature help to decrease viscosity of the medium, increase substrate solubility and reduce risk of microbial contamination (Kuchner and Arnold, 1997).
Enzymatic liquefaction and saccharification of starch are performed at high temperatures (100 to 110°C) by the help of thermostable amylolytic enzymes. To get such potential thermostable amylolytic enzymes, currently investigation of improved microbial strains from different ecological niches contaminated with starchy substances are being conducted. Amylases produced from such potential microorganism are significant for starch degradation to produce valuable products like crystalline dextrose, glucose, maltose, dextrose syrup and maltodextrins (Asgher et al., 2007).
Almost all microorganisms of the Bacillus genus synthesized thermostable amylase, thus this genus has the potential to dominate the enzyme industry: Bacillus amyloliquifaciens, Bacillus licheniformis, Bacillus stearothremophillus, Bacillus subtilis, Bacillus megaterium and Bacillus circulans (Riaz et al., 2003). The capacity of bacillus strains to produce large quantities of enzymes has placed them among the most important industrial enzyme producers. Indeed, they produce about 60% of commercial enzymes. They are known to be good producers of thermostable-amylase and have been widely used for commercial production of the enzyme for various applications (Prakash and Jaiswal, 2009). They have wide range of useful applications in the food, brewing, textile, detergent and pharmaceutical industries. In detergents production, they are applied to improve cleaning effect and are also used for starch de-sizing in textile industry (Chengyi et al., 1999).
Thermostable-amylases have been reported from several bacterial strains and have been produced through the use of submerged fermentation as well as solid state fermentation (Teodoro and Martins, 2000). Submerged fermentation utilizes free flowing liquid substrates or broths efficiently to produce desired bioactive compounds. Normally, such bioactive compounds are secreted into the fermentation broth. In general, this fermentation technique is appropriate for bacteria that normally require high moisture content. An additional advantage of this technique is that recovery of products is relatively simple (Subramaniyam and Vimala, 2012). Another best advantage of submerged culture is the technique for sterilization and process control is easier to engineer in these systems (Vidyalakshmi et al., 2009).
In the production of microbial amylase one of the factor is optimization of the culture conditions. A number of investigators have conducted researches to optimize culture condition for amylase production (Saxena et al., 2007). The chemical and physical parameters like pH, temperature, salt concentration and incubation time of microbial fermentation process play great role in enzyme production. Improving the yield of amylase and consequent cost reduction depends on the selection of strains, optimization of the factors affecting biosynthesis, genetic improvement, kinetic studies and biochemical characterization of enzyme (Andualem, 2014).
Isolation and identification of soil microorganisms with best amylase activity could contribute a lot for the discovery of novel potential amylases appropriate for different industrial and biotechnological applications (Mohapatra et al., 2003). The bacilli isolated from soil are considered as an ideal source for the production of bulk extracellular amylase for industrial application (Riaz et al., 2003). Therefore, isolation and screening of thermophilic bacteria from soil samples are significant to discover novel new industrial enzymes. There is a need to isolate and screen amylase producing bacilli bacteria from starch rich soil associated with high temperature environment. Most of the time, the temperature in environment around cereal grinding machine is high and the soil is normally rich in content of starch of cereal flour. The soil in such area may be rich in bacilli species of bacteria with huge potential to produce thermostable amylase (Andualem, 2014).
The purpose of the current investigation is to screen thermophilic Bacillus species from soil sample taken from grain mill houses rich in starchy flours and to study their suitability with regard to thermostable amylase production and characterization of the crude enzyme.
Isolation and screening of amylase producing thermophilic Bacillus strains
This study was conducted from January to May, 2011. Samples were collected from Azozo, Arada and Maraki (Gondar town) grain mill house soils rich in content of starchy flours. The sample site was located in Northwest part of Ethiopia. To isolate an aerobic, rod shaped, gram-positive, thermophilic and spore-forming Bacillus sp., from each soil samples 1 g was introduced into 9 ml of distilled sterilized water and heated at 80°C for 10 min and then 1 ml was first enriched on starch broth medium containing 1% soluble starch (w/v), 0.5% peptone (w/v) and 0.5% yeast extract (w/v) at pH 7 and incubated at 45°C for 24 h with constant shaking at 150 rpm. Two percent of the enriched liquid medium was then spread on starch agar medium (1% soluble starch, 0.5% peptone, 1.5% yeast extract, 1.5% agar (Andualem and Gessesse, 2013). The plates were incubated at 45°C for 48 to 72 h until bacteria typical colonies were obtained. The colonies were further sub-cultured on starch agar plates to get pure colonies. Iodine solution (1% iodine in 2% potassium iodide (w/v) was flooded over the surface of the plate in order to select amylase producing isolates. Those colonies having clear zone (more than 10 mm diameter) were selected for further investigation. Morphological characteristics of isolates were identified using gram staining techniques and colony morphology. The cultures were maintained on nutrient agar slants at 4°C.
Enzyme production
Amylase activity was assayed using starch as substrate. The selected bacterial isolates (designated as Isolate-1, Isolate-2, Isolate-3, Isolate-4, Isolate-5 and Isolate-6) were separately cultured at 45°C for 120 h in 100 ml of starch broth medium (1% starch, 0.5% peptone and 1.5% yeast extract) at pH 7 in 250 ml flask and constantly mixed using rotary shaker at 150 rpm. The broth from each culture was centrifuged at 6000 rpm for 20 min and the supernatant was collected as crude amylase enzyme extract. The crude extract was used for characterization of the enzyme activity and stability in different conditions (Bajpai and Bajpai, 1998).
Enzyme assay and characterization
The assay was carried out based on the reduction in blue color intensity due to amylase enzyme hydrolysis of starch (Bajpai and Bajpai, 1988; Oboh, 2005; Andualem and Gessesse, 2013). In this assay, 1 ml crude amylase enzyme (cell free supernatant) and 10 ml of 1% starch solution were mixed and incubated at 45°C for 10 min. The reaction in the test tube was stopped by adding 10 ml of 0.1 N HCl. One more dilution was made by mixing 1 ml of this acidified solution with additional 10 ml of 0.1 N HCl. From this, 1 ml was added to 10 ml iodine solution (0.05% iodine in 0.5% KI). Optical density (OD) of the solution was measured by spectrophotometer at 660 nm. A standard curve was prepared using starch (0 to 2.0 mg/ml) and a linear regression analysis was used to determine the total reducing sugar present as % starch equivalents. The same procedure was done using 1 ml distilled water instead of 1 ml enzyme sample (Yang and Liu, 2004). One unit of activity was defined as the amount of enzyme that reduces the intensity of blue color of starch-iodine solution by 1% at the assay conditions. Stock solution and solution for enzyme assay were prepared from 1% or 1000 mg/100 ml soluble starch.
Effect of temperature on amylase activity
The effect of temperature on amylase activity was determined at different temperatures (40, 45, 50, 55, 60, 65, 70, 75 and 80°C) at pH 7. One milliliter of crude culture extract enzyme was mixed with 10 ml of 1% soluble starch in sodium phosphate buffer (pH 7) and incubated in a water bath at different temperature for 10 min. The reaction was stopped by adding 10 ml of 0.1 N HCl. One more dilution was made by mixing 1 ml of this acidified solution with additional 10 ml of 0.1 N HCl. Then 1 ml from this solution was added to 10 ml iodine reagent containing 0.05% iodine and 0.5% KI. The OD-value was measured at 660 nm (Andualem and Gessesse, 2013).
Effect of pH on amylase activity
The effect of pH on amylase activity was determined on starch solutions (1%) at different acetate buffer and sodium phosphate buffer; acetate buffer of starch solution of pH 4 and 5 and sodium phosphate buffer starch solution of pH 6, 7, 8 and 9 at 60°C for 10 min. The amylase activity was similarly determined from reduction in blue color intensity (Andualem and Gessesse, 2013).
Effect of substrate concentration on amylase activity
Amylase activity of various crude amylase preparations was assayed at various substrate concentrations of 0.5, 1.0, 1.5, 2.0, 3.0 and 4.0% starch (w/v) solutions in sodium phosphate buffer at pH 8 and temperature of 60°C for 10 min of incubation. Finally the absorbance was measured at 660 nm using UV spectrophotometer (Oboh, 2005; Andualem and Gessesse, 2013).
Effect of NaCl concentration on amylase activity
The effect of different NaCl concentration on the amylase activity was done by subjecting the enzymes to different NaCl concentrations (0, 1, 2, 3, 4, 5 and 8 M). The enzymes were incubated in 1% starch solution containing different concentration of NaCl solution for 10 min at 60°C and pH 8 and the absorbance was measured at 660 nm (Andualem and Gessesse, 2013).
Growth rate and enzyme production dynamics
Growth rate, production and enzyme activity were determined by taking samples aseptically from submerged fermentation at the interval of 20 h (0, 20, 40, 60, 80, 100 and 120 h) (Andualem, 2014).
The time course of starch hydrolysis by crude amylase extract
The enzyme activity was done by measuring the residual activity of the enzyme after being incubated for specific period at specific pH and temperature (Yang and Liu, 2004). Determination of incubation time was carried out on reaction mixture to estimate the time required for maximum amylase activity. At specific time interval (2, 7, 17, 32 and 47 min) the activity of enzyme was determined (Andualem, 2014).
Data analysis
The data in this study were analyzed using SPSS version 16.0. Means and standard deviations were calculated using analysis of variance (ANOVA) to analyze the significant differences between the means using Duncan’s multiple range test (p < 0.05) when the F-test demonstrated significance. The triplicates mean values of tests were analyzed. Significant difference was defined as p < 0.05.
Many species of Bacillus are widely known to produce various kinds of extracellular enzymes having wide range of industrial application. Of those enzymes, amylases are the most significant for industrial application. Characteristics of all bacterial isolates and their amylase activity were determined after 120 h of fermentation (Figure 1). The production of amylase and rate of bacterial isolates growth (Figure 2) were analyzed in the medium containing 1% starch as source of carbon and 0.5% peptone as a source of nitrogen. This medium was supplemented with 1.5% yeast extract. The measurement of cell growth rate and enzyme activity of all bacterial isolates were carried out at every 20 h time intervals as shown in Figures 1 and 2.

All isolates which are designated as Isolate-1, Isolate-2, Isolate-3, Isolate-4, Isolate-5 and Isolate-6 were gram positive bacilli and they were screened based on size of clear zone diameter (> 10 mm) formed on starch agar. In all bacterial isolates, amylase production was increased together with cell mass increment after 40 h of fermentation. Moreover, that isolates showing high concentration of product (fermentable sugars), that is, above 90% after 120 h fermentation were selected for further investigation. With regard to enzyme production kinetic or dynamics of amylase producing spore former bacilli, all isolates were able to hydrolyze > 91% of starch after 60 h of submerged fermentation. High biomass (OD) of amylase producing thermopile Bacillus bacteria was seen after 120 h of incubation period. Isolate-5 was shown the highest (0.36 OD) biomass in comparison with other five isolates (range from 0.28 to 0.34 OD) at stationary phase after 120 h incubation. Among studied isolates, there was no significant (P ≥ 0.05) variation in enzyme productivity but there was significant (P≤0.05) difference in biomass. When the isolated bacteria were cultured in submerged fermenter, gradual increment of biomass up to 120 h of fermentation but the amylases activity were continued to remain up to 120 h of fermentation. In brief enzyme synthesis started after 40 h of fermentation and extended up to 120 h of fermentation process. Determination of period of bacterial growth and amylase productivity are significant to optimize time of product recovery.
Determination of effect of temperature and pH for amylases produced from thermophilic spore former bacilli are significant to optimize fermentation process during enzyme production. The optimum temperature of amylase reaction was analyzed by the incubation of crude amylase extract at temperature range of 40 to 80°C (Figure 3). Crude amylase activity of all isolates was relatively increased from 40°C up to 55°C and sharply reaches their optimum reaction activity at 60°C. After optimum temperature, as temperature increases, amylase activities of all isolates were reduced. This could be due to the breakage of secondary, tertiary and quaternary bonds that maintain the three dimensional structure of enzymes at high temperature and thereby would lead to conformational changes of the enzyme active sites. It is known that enzyme activity increases with increasing temperature up to the optimum temperature as the result of increasing kinetic energy, which can favor rate of collisions between substrate and enzyme during hydrolysis process. In this study, the optimum temperature for amylases produced from all bacteria isolates was 60°C. At this optimum temperature, amylase from Isolate-1, Isolate-2, Isolate-3, Isolate-4, Isolate-5 and Isolate-6 have shown starch hydrolysis capacity of 98.5, 99, 98.8, 98.9, 98.5 and 99% respectively.

High optimum temperature activity of the amylase offers some advantages for industrial processes such as reducing the cooling cost, lowering viscosity of the substrate, reducing the risk of microbial contamination and provides better solubility at high temperature (Burhan et al., 2003). The amylase activities of all enzymes produced from six bacterial isolates in this study were in line with some scientific reports (Burhan et al., 2003; Afiukwa et al., 2009; Andualem, 2014). However, the optimum temperature of amylases reported in this study was lower in comparison with amylases produced from the spices of Thermus (70°C) (Shaw et al., 1995) and Thermus filiformis (95°C) (Egas et al., 1998).Thus, amylases with optimum activity at 60°C, like in this study, have properties considered to be significant for industrial starch liquefaction. Generally, all amylases produced from all bacterial isolates have not shown significant (P ≥ 0.05) activity along with different temperature treatment.
The effect of pH on the enzyme activity of all isolates in this study is shown on Figure 4. The amylase activity of Isolate-1 was increased from 97.5% at pH 4 to 98.7% at pH 7 and the activity was decreases from 100% at pH 8 to 96% at pH 9. At the same time, amylase activity of isolate-2 was increased from 97.5% at pH 4 to 99.5% at pH 8 and then decreased to 95.5% at pH 9. Thus, its optimum amylase activity was at pH 8. On the other hand, amylase activity of Isolate-3, Isolate-4, Isolate-5 and Isolate-6 was increased from 97 to 100%, 97 to 99.5%, 97 to 100% and 97 to 98% from pH 4 to 8 respectively. The optimum amylase activity (98%) of Isolate-6 was significantly (P ≤ 0.05) reduced in comparison with other isolates at pH 8.

Amylase activity of all bacterial isolates were increased with increment of pH up to optimum and reduced after their optimum pH, that is, pH 8. This is a basic property of all enzymes and is probably due to concomitant alteration in the conformation of the enzyme protein caused by changes in pH of its environment (Afiukwa et al., 2009). The optimum pH of amylases activities found in this study was in line with that of optimum amylase activity reported by Fatoni and Zusfahair (2012). Changes in pH, like that of temperature, could change the three dimensional structure of active sites. And also, the enzymes and substrates binding speed to produce maximum product will be highly reduced due to pH change (Garrett and Grisham, 1999). The optimum pH of the amylase in this study was in line with the optimum pH produced by Thermus sp. (Fatoni and Zusfahair, 2012) and amylase from Bacillus KSM-K38 (Hagihara et al., 2001). However, this optimum pH was higher when compared with that of amylase produced by other Thermus filiformis of 5.5 to 6.0 (Egas et al., 1998) and Thermus sp. of 5.5 to 6.5 (Shaw et al., 1995). In other studies it was shown that optimum pH could range from 5.0 to 10.5. Amylase isolated from such bacteria has high enzymatic activity at alkaline pH which has huge potential for detergent industry. It is known that enzymes in detergents have potential abilities to remove tough stains without any environmental effects. Amylases are the second type of enzymes used for formulation of enzymatic detergents (Gupta et al., 2003; Hmidet et al., 2009). Currently, such type of enzyme formulations are widely used for laundry and automatic dish washing in order to remove starchy food substances derived from gravies, potatoes, chocolate, custard, and other smaller oligosaccharides (Mukherjee et al., 2009).
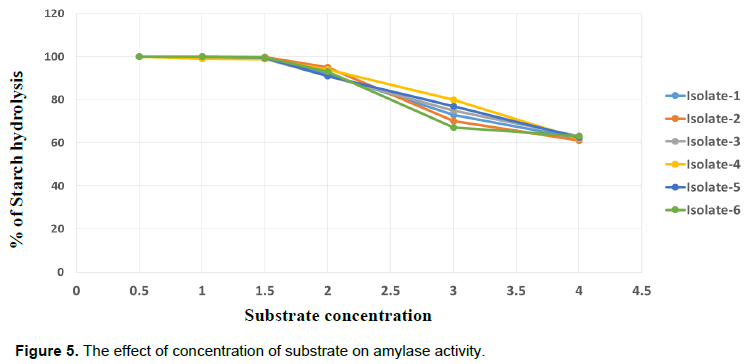
The starch hydrolysis activity of amylases from all bacterial isolates was measured at optimal pH and temperature (pH 8 and 60°C) (Figure 5). In all bacilli isolates, there was a high enzyme activity in the range between 0.5 and 2% of starch concentration. This result was in agreement with that of Alli et al. (1998) and Oboh (2005). In this result, the amylase activity of Isolate-1 was only reduced from 100 to 62% from the range of 0.5 to 4% starch substrate. The activity of Isolate-2, Isolate-3, Isolate-4, Isolate-5 and Isolate-6 were reduced from 100% to 61.1, 63, 61.8, 62.4 and 63% respectively in increasing the substrate 0.5 to 4% of starch. According to this finding optimal hydrolysis of starch substrate may not surpasses over 2%. This data is significant to optimize fermentation process within this range of substrate concentration.
Effect of salt on enzyme activity was presented on Figure 6. Salt tolerance of amylases in this study was evaluated in the presence of different NaCl concentration (0 to 8 M NaCl). The activity of the enzyme or starch hydrolysis capacity of all the isolates was 100% starting from 0 to 5 M NaCl solution. The amylase activity of all the isolates (Isolate-1, Isolate-2, Isolate-3, Isolate-4, Isolate-5 and Isolate-6) were sharply decreased after 5 M Nacl concentration that is 99.2, 99.3, 99.6, 99.7, 99.1 and 99.4% at 8 M NaCl concentration respectively. This result implies that the enzymes of these isolates are halotolerate and are comparable with some halophilic amylases reported (Carvalho et al., 2008). Different slats like that NH4C1, FeC13 and KC1 have an effect on the activation of amylase enzyme. But NaCl which had no effect up to 5 M concentration may probably be due to the presence of chloride ions in the soil sample. Previous studies has shown that metallic chlorides are usually potent activators of amylase (Oboh and Ajele, 1997;
Mohapatra et al., 1998).
The incubation time of amylases activity of bacterial isolates were determined and measured at various incubation times (Figure 7). The optimum enzyme activity of all the isolates, Isolate-1, Isolate-2, Isolate-3, Isolate-4, Isolate-5 and Isolate-6, were 99.6, 99.3, 99.5, 99.2, 99.4 and 99.1% respectively, at 32 min of incubation. Determination of time course of starch hydrolysis is significant to hydrolyze the substrate efficiently during industrial fermentation process.
In this study, six best amylase producing bacilli were isolated from soil rich in starch at grain milling house. Determination of period of bacterial growth and amylase productivity are significant to optimize time of product recovery. Amylases obtained with optimum activity at 60°C could be significant for industrial starch liquefaction. Amylase isolated from such bacteria in this study with high enzymatic activity at alkaline pH has huge potential for detergent industry. Thermostable amylases from six bacilli isolates may be used for starch hydrolysis up to 60°C, which is a basic property of an enzyme for industrial starch hydrolysis. A wide range of pH stability (pH 4 to pH 9) has significant advantage of preserving and handling the enzyme for commercial and industrial process. Generally, high temperature and pH stability of such enzymes have also potential application in industrial food biotechnology. The outcome of this study implies that the enzymes of these isolates are halotolerant. Determination of time course of starch hydrolysis is significant to hydrolyze the substrate efficiently during industrial fermentation process. Finally soil samples could be converted to wealth by developing a standard method of producing amylase and other useful enzymes. If further purification of the crude amylase was conducted it will have the ability to be applicable in pharmaceutical and clinical sectors.
The authors have not declared any conflict of interests.
REFERENCES
|
Afiukwa CA, Ibiam UA, Edeogu CO, Nweke FN, Chukwu UE (2009). Determination of amylase activity of crude extract from partially germinated mango seeds (Mangifera oraphila). Afr. J. Biotechnol. 8(14):3294-3296.
|
|
|
|
Alli AI, Ogbonna CIC, Rahman ATMF (1998). Hydrolysis of certain Nigerian Cereal starch using crude fungal amylase. Niger. J. Biotechnol. 9(1):24-36.
|
|
|
|
|
Andualem B, Gessesse A (2013). Isolation and identification of amylase producing yeasts in 'tella' (Ethiopian local beer) and their amylase contribution for 'tella' production. J. Microbiol. Biotechnol. Food Sci. 3(1):30-34.
|
|
|
|
|
Andualem B (2014). Isolation and screening of amylase producing thermophilic spore forming Bacilli from starch rich soil and characterization of their amylase activities using submerged fermentation. Int. Food Res. J. 21(2):831-837.
|
|
|
|
|
Asgher M, Asad MJ, Rahman SU, Legge RL (2007). A thermostable α-amylase from a moderately thermophilic Bacillus subtilis strain for starch processing. J. Food Eng. 79:950-955.
Crossref
|
|
|
|
|
Bajpai P, Bajpai P (1998). High-temperature alkaline α-amylase from Bacillus licheniformis TCRDC-B13. Biotechnol. Bioeng. 33:72-78.
Crossref
|
|
|
|
|
Burhan A, Unaldi N, Coral G, Colak O, Aygan A, Gulnaz O (2003). Enzymatic properties of a novel thermostable, thermophilic, alkaline and chelator resistant amylase from an alkaliphilic Bacillus sp. isolate ANT-6. Process Biochem. 38:1397-1403.
Crossref
|
|
|
|
|
Carvalho RVR, Correa TLM, Silva JC, Mansur LRCO, Martins MLL (2008). Properties of an amylase from tehrmophilic Bacillus sp. Braz. J. Microbiol. 39:102-107.
Crossref
|
|
|
|
|
Chengyi WH, Ming M, Jiang R (1999). Studies on the properties of alpha-amylase produced by Bacillus pumilus 289 (PBX96). Acta Microb. Sin. 32:400-404.
|
|
|
|
|
Egas MCV, da Costa MS, Cowan DA, Pires EMV (1998). Extracellular α-amylase from Thermus filiformis Ork A2: purification and biochemical characterization. Extremophiles 2:23-32.
Crossref
|
|
|
|
|
Fatoni A, Zusfahair (2012). Thermophilic amylase from Thermus sp. Isolation and its potential application for bioethanol production. Songklanakarin J. Sci. Technol. 34(5):525-531.
|
|
|
|
|
Garrett RH, Grisham CM (1999). Biochemistry. Saunder's College Publishing, Philadelphia, US.
|
|
|
|
|
Gupta R, Gigras P, Mohapatra H, Goswami VK, Chauhan B (2003). Microbial α-amylases: a biotechnological perspective. Process Biochem. 38:1599-1616.
Crossref
|
|
|
|
|
Hagihara H, Igarashi K, Hayashi Y, Endo K, Ikawa-Kitayama K, Ozaki K, Kawai S (2001). Ito S. Novel a-amylase that is highly resistant to chelating reagents and chemical oxidants from the Alkaliphilic Bacillus isolate KSM-K38. Appl. Environ. Microbiol. 4:1744-1750.
Crossref
|
|
|
|
|
Hmidet N, El-Hadj Ali N, Haddar A, Kanoun S, Alya S, Nasri M (2009). Alkaline proteases and thermostable α-amylase co-produced by Bacillus licheniformis NH1: Characterization and potential application as detergent additive. Biochem. Engl. J. 47:71-79.
Crossref
|
|
|
|
|
Kuchner O, Arnold FH (1997). Directed evolution of enzyme catalysts. Trends Biotechnol. 15(12):523-530.
Crossref
|
|
|
|
|
Mohapatra BR, Baerjee Vic, Bapuji M (1998). Characterization of a fungal amylase from mucor spp. Associated with marine sponge Spirastella sp. J. Biotechnol. 60:13-17.
Crossref
|
|
|
|
|
Mohapatra BR, Bapuji M, Sree A (2003). Production of industrial enzymes (Amylase, Carbo xymethylcellulase and Protease) by bacteria isolated from marine sedentary Organisms. Acta Biotechnol. 23(1):75-84.
Crossref
|
|
|
|
|
Mukherjee AK, Borah M, Raí SK (2008). To study the influence of different components of fermentable substrates on induction of extracellular α-amylase synthesis by Bacillus subtilis DM-03 in solid-state fermentation and exploration of feasibility for inclusion of α-amylase in laundry detergent formulations. Biochem. Engl. J. 43:149-156.
Crossref
|
|
|
|
|
Oboh G (2005). Nutrient enrichment of Cassava peels using a mixed culture of Saccharomyces cerevisae and Lactobacillus spp solid media fermentation techniques. Electrical J. Biotechnol. 9(1):46-49.
Crossref
|
|
|
|
|
Oboh G. Ajele JO (1997). Effect of some Metallic chlorides on the activity of β-amylase. Niger. J. Biochem. Mol. Biol.12:73-75.
|
|
|
|
|
Prakash O, Jaiswal N (2009). Alpha-Amylase: An Ideal Representative of Thermostable Enzymes. Appl. Biochem. Biotechnol. 160(8):2401-2414.
Crossref
|
|
|
|
|
Rajagopalan G, Krishnan C (2008). Alpha-amylase production from catabolite derepressed Bacillus subtilis KCC103 utilizing sugarcane bagasse hydrolysate. Bioresour. Technol. 99:3044-3050.
Crossref
|
|
|
|
|
Riaz N, Ul-Haq I, Qadeer MA (2003). Characterization of α-amylase by Bacillus subtilis. Int. J. Agric. Biol. 3:249-252.
|
|
|
|
|
Saxena KR, Dutt K, Agarwal L, Nayyar P (2007). A highly and thermostable alkaline amylase from a Bacillus sp. PN5. Bioresour. Technol. 98:260-265.
Crossref
|
|
|
|
|
Shaw JF, Lin FP, Chen SC, Chen HC (1995). Purification and properties of an extracellular α-amylase from Thermus sp. Bot. Bull. Acad. Sin. 36:195-200.
|
|
|
|
|
Subramaniyam R, Vimala R (2012). Solid state and submerged fermentation for the production of bioactive substances: a comparative study. Int. J. Sci. Nat. 3(3):480-486.
|
|
|
|
|
Teodoro CES, Martins MLL (2000). Culture conditions for the production of thermostable amylase by Bacillus sp. Braz. J. Microbiol. 31:298-302.
Crossref
|
|
|
|
|
Vidyalakshmi R, Paranthaman R, Indhumathi J (2009). Amylase Production on Submerged Fermentation by Bacillus spp. World J. Chem. 4(1):89-91.
|
|
|
|
|
Yang CH, Liu WH (2004). Purification and properties of a maltotriose-producing α-amylase from Thermobifida fusca. Enzyme Microb. Technol. 35(2-3):254-260.
Crossref
|
|





