ABSTRACT
The potato (Solanum tuberosum L.) is a tuberous herbaceous plant that belongs to the Solanaceae family. Several biotic and abiotic factors affecting its production include fungi such as mildew. In this present work, we proposed to evaluate the antagonistic power of Trichoderma viride against parasite Phytophthora infestans of potato tubers. We conducted two tests; for the first, in confrontation in-vitro, the direct confrontation was performed on an agar PDA medium to demonstrate the inhibitory capacity of T. viride on P. infestans. For the second test, in- vivo confrontation, we realized two methods; the first was carried out using a mycelia disk on the antagonist and the second was done by injecting the spore solution of T. viride into the potato tubers after this pathogen was applied. The results of the in- vitro test revealed that the confrontation between T. viride and the P. infestans showed the inhibitory capacity of T. viride, be it directly (68%) or remotely (58%) on the growth medium. Interesting results were also obtained in- vivo. The injection of the tubercles with a T. viride spore solution reduced the development of P. infestans with an average penetration of 3.28 mm for the T. viride and of 1.65 mm for the P. infestans. The findings of the mycelia discs method were similar to the injection method with a penetration average of 2.62 mm for T. viride and 1.81 mm for P. infestans. The test results in -vitro showed the efficiency of T. viride against P. infestans; while for in-vivo, it was proved that this antagonist possesses a very significant inhibitory effect that suppresses the spread of the pathogen.
Keywords: Biological control, pathogen, confrontation, symptoms, in vivo, in vitro.
The potato (Solanum tuberosum L.) is a herbaceous tuber plant, originally from Latin America, that belongs to the Solanaceae family (Sonnewald and Sonnewald, 2014). It is a species that is vegetatively propagated and cultivated for its tubers, storage and multiplication organs rich in nutrients, mostly carbohydrate (starch). Three types of production are distinguished: potato for consumption, potato starch, and potato seeds or plants (Bohl and Johnson, 2010). Several biotic and abiotic factors affect its production and these include fungi such as mildew. Mildew is a dreadful disease caused by Oomycete Phythophtora infestans and a heterothallic species with two mating mates A1 and A2. It can affect all the plant organs and cause considerable yield losses (Singh and Islam, 2010). To face these losses, it requires fighting this disease such as the use of living micro-organisms (biological control) as Trichoderma viride. This fungus has an inhibiting activity on the plant Phyto-pathogenic, which typically occurs either by competition, hyperparasitism or by antibiosis (Struik, 2007). The aim of this research was to study in–vitro and in-vivo antagonist power of T. viride against Phytophthora infestans pest of potato tubers.
Biological materials
Pathogenic agent
In this work, we have used as target micro-organisms P. infestans isolated from the potato tubers and identifies in Mohammed Sadik Ben Yahiya Laboratory of Applied Microbiology in Jijel (Figure 1A).
Antagonist agent
The antagonist agent used to fight against the P. infestans is the T. viride, which comes from the applied mycology laboratory at Mentouri University 1 Constantine (Figure 1B).
Vegetative materials
The potato cultivar: the potato variety tested in the present study was gotten from pesticide sellers in Jijel (Taher).
Antagonist activity in-vitro
Direct confrontation of Trichoderma on an agar medium was studied by Chet (1990).This technique consists of placing in the same Petri dishes PDA medium (20 g of agar, 20 g of glucose, 200 g of potato, 1000 g) ml of distilled water; and two agar pellets (6 mm in diameter), one carrying the T. viride and the other carrying the P. infestans. The two pellets were positioned along a diametrical axis of 3 cm and equidistantly from the center of the box. Transplanting is performed simultaneously (Benhamou and Chet, 1996). The incubation was carried out at 30°C for 7 days.
A notation regarding the diametric growth inhibition of the P. infestans and invasion by the mycelium of T. viride was conducted every two days. Also, microscopic observations on the direct effect of the antagonist agent on the P. infestans mycelia were made. The witness sample consists of subculturing of the pathogen at the box center.
Evaluation of the inhibition exerted by T. viride is estimated by calculating the percent inhibition of mycelial growth using the following formula:
I (%) = (1- Cn / Co) x 100
Where: Cn is the mean diameter of the colonies in the presence of the antagonist and Co the average diameter of control colonies.
Antagonistic activity in-vivo of T. viride
An in-vivo antagonism test for T. viride and P. infestans was performed by applying both methods to demonstrate the inhibitory capacity of T. viride.
Method of injection
The tubers were superficially disinfected with sodium hypochlorite solution (10%) for 5 min, and then rinsed thoroughly with distilled sterile water. Inoculation sites on the tubercles with the dimension of 6 mm width and depth was prepared. T. viride was applied by the injection of 100 µL on the sites of inoculation for 24 h before the application of the pathogen. The witness tubercles were treated similarly with distilled sterile water. The vaccination involves deposing an agar plate (6 mm diameter) colonized by the pathogen in the injuries that were sustained. Incubation of tubers was carried out at 25°C for 15 days.
After the incubation period, the potato tubers were longitudinally cut through for inoculation spots. The induced penetration parameters of the maximum width (w) and depth (d)) were noted. Penetration of the pathogenic and antagonist agents in tubers was calculated according to the formula of Elad and et al. (1994).
Method of mycelial disks
Potato tubers (spunta) were washed in running water and then disinfected by soaking them for 5 min in a hypochlorite sodium solution (10%). Thereafter, they were rinsed three times successively in distilled sterile water.
Injuries of 6 mm in width and depth in the tubers using a sterile punch were made. Furthermore, a disc of 6mm in diameter carrying the phytophthora infestans pathogenic agents to be dropped at each injury was prepared. The witness tubers were inoculated by 6mm in diameter of agar explants. Incubation was performed at 25°C for 24 h. As a result, we made the treatment by the use of discs of 6 mm containing T. viride and incubation for 15 days under the same conditions.
After the incubation period, the potato tubers were cut longitudinally over the infection spots. The induced penetration parameters of maximum width (w) and depth (d) were noted. Penetration of the agent and the antagonist in the tubers was calculated according to the formula of Elad et al. (1994).
Antagonist activity in- vitro
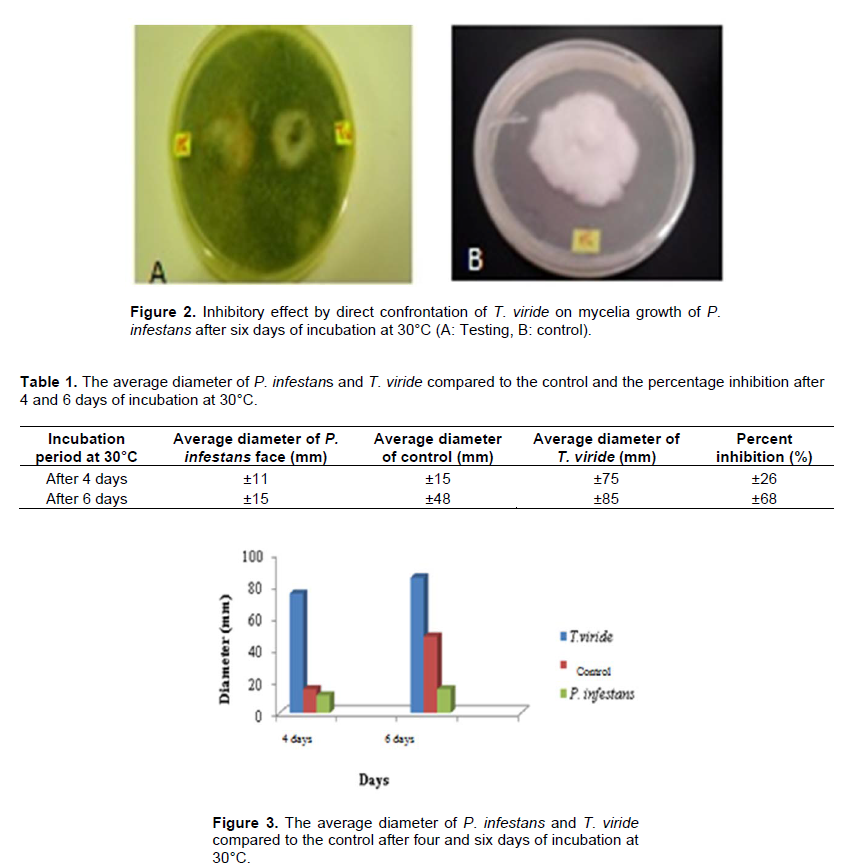
There was a direct confrontation on a culture medium between T. viride and P. infestans. Before the implementation on a culture medium between T. viride and Phytopathogenic fungi using organic products, it is necessary to know the antagonist behavior and interactions with the pathogen, which is why an antagonistic activity test was directed by T. viride through the confrontation between P. infestans (Figure 2). Simultaneous planting of T. viride and P. infestans showed faster growth of T. viride than the isolate of P. infestans. After four days of incubation, the box was almost completely invaded by the antagonist agent, while the isolate of P. infestans occupies only a surface of 11mm in diameter, which corresponds to an inhibition of mycelial growth of more than 26% (Table 1).
The P. infestans witness grown alone occupies an area of about 15 mm in diameter. Beyond this period, and after six days, T. viride invaded the P. infestans colony and even sporulates on them revealing its highly microparasitic power which corresponds to an inhibition of mycelial growth equal to 68% (Figure 3).
In the same sense, Benhamou and Chet (1996) reported an alteration of mycelium Sclerotium rolfsii caused by T. harzianum, resulting in aggregation, a retraction and a vacuolization of the cytoplasm which illustrates the highly micro-parasitic power that the T. harzianum possesses.
Similar results were obtained with T.lignorum which is capable of warping about the mycelium of Rhizoctonia solani causing the pathogenic cytoplasm to dissolve (Howell, 2003; Timothy and Widmer, 2014).
The microscopic observations realized at the contact area between T. viride and P. infestans showed that there was a profound change at the mycelia pathogen level. It was marked by recognition of the hyphae in strips and a winding start of the mycelium of the T. viride on that of P. infestans.
Antagonist activity in -vivo
Injection method
The results obtained showed a colonization of the incubation site by the antagonist agent after 15 days of incubation, with the appearance of small whitish spots representing the pathogenic agent on the superficial parts of the injuries (Figure 4).
This explains that T. viride exerts a competition mechanism, taking place before the arrival of the pathogen, and therefore hinders the development of mildew. The average antagonist penetration is estimated to be 3.28mm, while the average pathogen penetration is 1.65 mm (Table 2).
This phenomenon (competition) was not observed in the witness sample treated with distilled sterile water. It has been seen that the metabolites produced by the antagonist agent have a direct impact on the development of P. infestans penetration. This result explains that the antagonist agent has a great inhibitory capacity of the P. infestans as it is installed before the pathogen without damaging the plant tissues (Figure 5). Moayedi et al. (2010) opined that Trichoderma sp. demonstrated an antagonistic effect against Phytophthora root of potato rot, particularly in vitro and in vivo.
Discs method
The results obtained displayed appearances of different colors on the injuries. The white color represents P.infestans and the green color represents T. viride (Figure 6).
The average pathogen penetration is 1.81mm and the average antagonist penetration was estimated to be 2.62 mm (Table 3). This explains that T. viride exerts different antagonistic mechanisms to inhibit the development of P. infestans (Figure 7).
The results agree with the results of Yang et al. (2013). The studies of Kerroum et al. (2015) demonstrated the antagonist activity of Trichoderma sp. on P. infestans on potato tubers and tomato variety, and the antagonistic activities of Trichoderma species, including the competition and colonization against P. infestans.
This study has clearly demonstrated the antagonist effect of T. viride in relation to P. infestans which is the responsible agent of mildew of potato tubercles (Spunta). In effect, the confrontation attempts between P. infestans and T. viride showed that T. viride invaded the P.
infestans colony with an inhibition percentage equal to 68% in six days.
In the case of the remote confrontation and despite the lack of direct contact between the two fungi, a reduction of the P. infestans colony diameter has been observed compared to the untreated witness sample with an inhibition percentage equal to 68% in six days. This demonstrated that in addition to the microparasitic power of the antagonist agent, T.viride may act by the secretion of volatile substances which are able to distantly stop the development of the pathogenic agent.
Consequently, the in-vivo test showed the ability of the T. viride to reduce or inhibit the penetration of the pathogen in tubers with an average penetration of the pathogenic agent equal to 1.65 mm (by injection), whereas the treatment by discs gives an average penetration equal to 1.81 mm. It can be concluded that the mechanisms placed by T. viride in-vitro will be the same as that demonstrated in-vivo, namely, mycoparasitic secretion of volatiles, antibiosis, and competition for space.
The authors have not declared any conflict of interest
REFERENCES
|
Benhamou N, Chet I (1996).Parasitism of Sclerotia of Scleratium rolfsii by Trichoderma harzianum: ultrastructural and cytochemical aspects of the interaction. Phytopathology 86:405-416.
Crossref
|
|
|
|
Bohl W H, Johnson S B (2010). Commercial potato production in North America. The Potato Association of America Handbook. 2 ed. The Potato Association of America, Orono, ME.
|
|
|
|
Chet I (1990). Mycroparasitism, recognition, physiology and ecology. Pages725-733 in: New direction in Biological Control: Altematives for Suppressing Agricultural Pests and Diseases. R. Baker and P. Dunn, eds. R. Alan, Liss Inc. New York.
|
|
|
|
Elad Y, Kôhl J, Fokkema N J (1994). Control of infection and sporulation of Botrytis cineria on bean and tomato by saprophytic bacteria and fungi. Euro. J. Plant Pathol.100:315-33.6
|
|
|
|
Howel CR l (2003).Mechanisms employed by Trichoderma species in the biological control of plant diseases: the history and evolution of current concepts. Plant Dis. 87:4-10.
Crossref
|
|
|
|
Kerroum F, Karkachi N, Henni JE, Kihal M (2015). Antagonistic effect of Trichoderma harzianum against Phytophthora infestans in the North-west of Algeria. Int. J. Agron. Agric. Res. 6(4):44-53.
|
|
|
|
Moayedi G, Mostowfizadeh-Ghalamfarsa R (2010). Antagonistic Activities of Trichoderma spp. on Phytophthora Root Rot of Sugar Beet. Iran Agric. Res. 29(2):21-38.
|
|
|
|
Singh A, Islam MN (2010). In vitro evaluation of Trichoderma spp. against Phytophthora nicotianae Int. J. Expt. Agric. 1(1):20-25.
|
|
|
|
Sonnewald S, Sonnewald U (2014). Regulation of potato tuber sprouting. Planta 239(1):27-38.
Crossref
|
|
|
|
Struik P (2007). Response of the potato plant to temperature. pp367-394. In: Vreugdenhil D, Bradshaw JE, Gebhardt C, Govers F, Mackerron DK L, Taylor MA, Ross HA (Eds.). Potato biology and Biotechnology: Advances and perspectives. Elsevier, Oxford, UK.
Crossref
|
|
|
|
Timothy L, Widmer (2014). Screening Trichoderma species for biological control activity against Phytophthora ramorum in soil. Biol. Control 79:43-48.
Crossref
|
|
|
|
Yang L, Song R, Deng X, Gao Y (2013). Screening, Identification and Biological Characteristics of Trichoderma Strain on Biological Control of Phytophthora infestans. Forestry Protection Institute of Heilongjiang Forestry Academy.
|