ABSTRACT
Rhizoctonia solani is the causative agent of rice sheath blight, which has become a major problem in rice production. Seaweed provides a rich source of structurally diverse and biologically active secondary metabolite and is proved to be better in decreasing foliar fungal diseases which ultimately increase its fertility and help in the growth of plants. The use of natural products is the ultimate way of combating this disease. In this context, five different seaweeds such as Sargassum wightii, Dictyota bartyrensiana, Ulva reticulate, Gelidiella acerosa and Odonus niger were used together with the fish powder extract in the control of sheath blight disease in rice were studied. Evaluation of marine products against R. solani was carried out by paper disc assay; agar well method and mycelial dry weight. Among the five marine extracts tested, extracts of S. wightii [brown seaweed algae] at a high concentration (20%) was found to be the best in the reduction of spore germination (19.60%). The leaf extracts of S. wightii [brown seaweed algae] at highest concentration of 20% showed a maximum reduction in both paper disc method and agar well method with 44.65 and 45.90% zone of inhibition, respectively. The antifungal compounds were identified through gas chromatography mass spectroscopy. The results revealed that, 18 compounds were present in S. wightii and among that n-hexadecanoic acid which was closely related to 9, 12-octadecadienoic acid may be responsible for the inhibition of the growth of R. solani. The present study revealed that the efficacy of seaweed extracts against fungal pathogens may be due to higher levels and early accumulation of phenolics and phytoalexins, and the pot study proved that R. solani can be controlled by the application of marine products which may be further used for field study.
Key words: Seaweeds, Rhizoctonia solani, antifungal compounds, rice.
Rhizoctonia solani Kuhn is the causal agent of rice sheath blight, which has become a major constraint to rice production during the last two decades (Kobayashi et al., 1997). The intensification of rice cropping systems with the development of new short stature, high tillering, high yielding varieties, high plant density and an increase in nitrogen fertilization (Gangopadhay and Chakrabarthi, 1982; Ou, 1985) has seen the “emergence of R. solani as an economically important rice pathogen”.
This pathogen can survive in soil for many years by producing small (1-3 mm diameter) irregular shaped, brown to black sclerotia in soil and on plant tissues. The ability of R. solani to produce sclerotia with a thick outer layer allows them to float and survive in water. R. solani also survives as mycelium by colonizing soil organic matter as a saprophyte, particularly as a result of plant pathogenic activity (Ghaffar, 1988). The sclerotia present in the soil and/or on plant tissue germinate to produce vegetative threads (hyphae) of the fungus that can attack a wide range of food and fibre crops.
Presently, sheath blight disease management is mainly achieved through systemic fungicides (Pal et al., 2005) and the bacterial bio-control agents like plant growth-promoting rhizobacteria (PGPR) offer a promising means of controlling plant diseases (Mew and Rosales, 1992). Brown seaweeds contain bio-control properties and contain many organic compounds and growth regulators such as auxins, gibberellins and precursor of ethylene and betaine which affect plant growth. Seaweed extracts have been reported to increase plant resistance to diseases, plant growth, yield and quality (Jolivet et al., 1991). Thus, seaweeds are bestowed with varied sources of bioactive natural products that exhibit biomedical and antimicrobial properties (Kumar et al., 2005; Karthikeyan and Shanmugam, 2016). Peres (2012) was the first to observe antifungal substances in seaweeds. The seaweed is commercially available and some reports have indicated enhanced plant yield and health in different crops following application, although the mechanisms of action have not been determined (Norrie et al., 2002; Colapietra and Alexander, 2006).
Application of seaweed extracts is proved to be better to decrease the foliar fungal diseases which ultimately increase its fertility and help the growth of plants (Jayaraj et al., 2008). Kumar et al. (2005) evaluated the bioactive potential of seaweeds against plant pathogenic bacterium Xanthomonas oryzae pv. oryzae. Kumar et al. (2008) tested crude seaweeds extracts against the phytopathogenic bacterium, Pseudomonas syringae causing leaf spot disease of the medicinal plant, Gymnema sylvestris. The use of anti-microbial drugs (Arioli et al., 2015) has certain limitations due to changing patterns of resistance in pathogens and side effects they produce.
Seaweeds provide a rich source of structurally diverse and biologically active secondary metabolites. The functions of these secondary metabolites are defense mechanism against herbivores, fouling organ Figures and pathogens for example, grazer-induced mechanical damage triggers the production of chemicals that act as feeding detergents or toxins in seaweeds (Ammirato, 1986). They contain all major and minor plant nutrients as well as biocontrol properties and many organic compounds such as auxins, gibberellins and precursors of ethylene and betaine which affect plant growth (Wu et al., 1997).
Seaweeds are benthic marine macro algae mainly used for the production of agar, alginate, liquid fertilizers and manures (Sivakumar, 2014). Most of the secondary metabolites are the bactericidal or the antimicrobial compounds derived from seaweeds which consist of diverse groups of bacteriostatic properties such as brominates phenols, oxygen heterocyclic; Terpenols, Sterols, Polysaccharides, dibutenolides peptides and proteins. Although, most of the antibiotics found from terrestrial sources are used as therapeutic agents to treat various diseases, the oceans have enormous biodiversity and potential to provide novel compounds with commercial value (Anderson et al., 2006). In this context, the present study was carried out to evaluate the various marine products against Rhizoctonia solani under in vitro condition.
Evaluation of marine products against R. solani in vitro
The efficacy of the marine products listed in Table 1 was tested against R. solani
Preparation of marine products
Preparation of crude seaweeds extracts (Vallianayagam et al., 2009)
Each 1 kg of live, healthy and matured samples (Brown and red seaweeds) of each seaweed collected along the Coast of Pamban (Rameswaram (9°14`N; 79°14`E), Gulf of Mannar, Tamil Nadu, India) were washed thoroughly in seawater followed by tap water to remove extraneous particles and epiphytes. Then, they were air dried under shade in the aboratory for 3 days. The shade-dried samples were chopped and pulverized. Each 50 g powdered sample was separately extracted for 7 days thrice in 500 ml of 1:1 (v/v) chloroform: methanol using 1 L Erlenmeyer conical flask under dark condition. The extractants were pooled and concentrated by using flask evaporator under reduced pressure at 45°C and weighed stored at 0°C (Plates 3, 4 and 5).
Preparation of fish powder extracts (Suji, 2004)
Two marine fish species (trash fish and edible fish) were processed at a local processing plant, using 3.5% sucrose and 0.15% phosphate as cryoprotectants. The frozen blocks were transported to the laboratory and stored at 18°C until drying. A 500 g block of each fish was dried using a Labconco Freeze Dry System at a temperature of 40°C until the moisture content reached 5%. The samples were milled and sieved using a 40 mm screen mesh. The resulting powder was vacuum packed and stored at 4°C. Powdered samples were soaked in chloroform (1:4 w/v) and extracted for 2 days at room temperature and the extracts were collected and concentrated.
Evaluation of marine products against R. solani
Paper disc assay
Various concentrations like 5, 10, 15 and 20% of seaweed extracts and fish powder extracts were made. 20 ml of PDA medium was seeded with 3 ml of sclerotial suspension (1x106 sclerotia/ml) of the fungus and solidified. Sterile filter paper discs (10 mm) were dipped separately in known concentration of treatments and placed equidistantly over the seeded medium. Three replications were maintained. The plates were incubated at 28±2°C for 48 h. The inhibition zone of the fungal growth around the treated paper discs was measured and recorded. The paper disc dipped in sterile distilled water served as control (Plate 9) (Saha et al., 1995).
Agar well method
Seaweed extracts and Fish powder extracts like 5, 10, 15 and 20% individually (10 ml) were added to the sterilized potato dextrose agar medium and thoroughly mixed just before plating. 20 ml of these mixtures individually were immediately poured into sterilized Petri plates and were allowed to solidify. A 9 mm of PDA disc was removed by using cork borer to form wells; 1 ml of spore suspension was poured into the well. All these were carried out under aseptic conditions. The plates were incubated at 28±2°C for 10 days. Potato dextrose agar medium without natural product served as the control. Three replications were maintained. The radial growth of the colony was measured. The percent inhibition of the growth was calculated (Thongson et al., 2004).
Mycelial dry weight
Potato dextrose broth was prepared in 250 ml Erlenmeyer flasks and autoclaved. Seaweed extracts and Fish powder extracts at 2.5, 5, 7.5 and 10 ml concentrations were added to 47.5, 45, 42.5 and 40 ml broth in flasks so as to get final concentrations of 5, 10, 15 and 20% of the filtrate in the broth. Similar procedure has been followed for taking the mycelial dry weight as stated earlier.
Identification of antifungal compounds
Analysis of antifungal compound through gas chromatography mass spectroscopy (GCeMS) (NIST Version. 2.0, 2005)
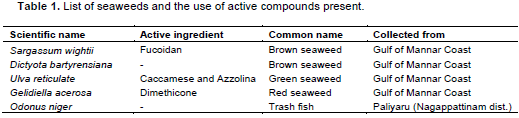
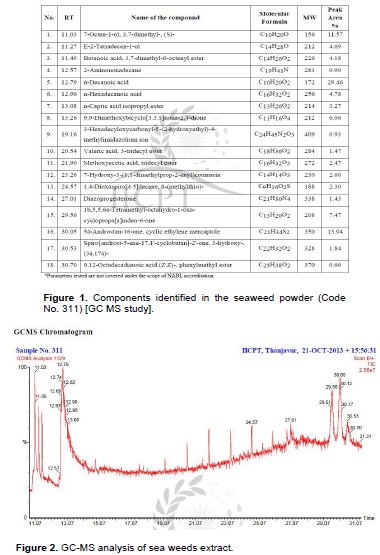
Based on the growth inhibition studies, seaweed extract was selected and chemical constituents were determined with a GC Clarus 500 Perkin Elmer Gas chromatography equipped with a mass detector. Turbo mass gold containing an Elite-1 (100% dimethyl poly siloxane), 30 m × 0.25 mm ID employed were the following: Carrier gas, helium (1 mL/min); oven temperature program 110°C (2 min) to 280°C (9 min); injector temperature (250°C); total GC time (36 min). The water extract was injected into the chromatograph in 2.0 Ml aliquots. The major constituents were identiï¬ed with the aid of a computer-driven algorithm and then by matching the mass spectrum of the analysis with that of a library (NIST Version. 2.0, year 2005). Software used for gas chromatography mass spectroscopy (GCeMS) was Turbo mass-5.1. This work was carried out in Indian Institute of Crop Processing Technology (IICPT), Thanjavur (Figures 1 and 2).
In vitro evaluation of marine products against R. solani
Paper disc method and well method
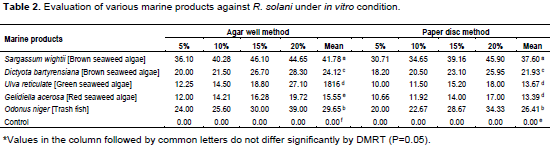
Various marine products were selected and evaluated for the antimicrobial activity by two methods, such as paper disc and agar well method. The leaf extracts of Sargassum wightii [brown seaweed algae] at the highest concentration (20%) was found to be the maximum reduction in both paper disc method and agar well method recorded was 44.65 and 45.90% inhibition zone, respectively. It was followed by a highest concentration (20%) of Odonus niger [trash fish] which recorded 39.00 and 34.33% inhibition zone in paper disc method and agar well method, respectively. All the concentrations of G. acerosa [red seaweed algae] recorded the minimum percent inhibition zone than all other extracts (Table 2).
The result of the experiment revealed the superiority of Sargassum wightii. Hence, the same was used for further studies.
Gas chromatography mass spectroscopy (GCeMS) analysis
On the basis of performance of marine products in the preceding in vitro studies, S. wightii (brown seaweed) was tested to determine the nature of chemical compound (s) present in the seaweed extract. The results revealed that 18 compounds were present in S. wightii.
The molecular weights, name of the compound, chemical formula, retention time and peak area percentage are given in Figure 1. Among these, n-Hexadecanoic acid which was closely related to 9, 12-Octadecadienoic acid may be responsible for the inhibition of the growth of R. Solani (Figure 2).
The seaweeds and the prepared marine products has significant role in the control of the R. solani in in vitro condition. Generally, all the marine products inhibited the mycelial growth of pathogen in the present study of which, S. wightii [brown seaweed algae] @ 20% exhibited the highest level of inhibition of R. solani. This statement has been confirmed by several workers. Sultana et al. (2007), reported that brown, green and red seaweeds were highly effective against R. Solani in- vitro and vivo conditions. Several workers have reported on the efficacy of seaweed extracts against fungal pathogens (Norrie et al., 2002; Jayara et al., 2008). This may be due to higher levels and early accumulation of phenolics and phytoalexins (Garcia-Mina et al., 2004). The above results lend supports to the present findings and helpful for further study in the treatment of sheath blight caused by R. solani in rice plant.
The authors have not declared any conflict of interest.
The authors thank the authorities of the Science and Engineering Board (SERB) for their financial support and acknowledge the co-operation of the Department of Plant Pathology, Faculty of Agriculture, Annamalai University for the successful completion of the research work.
REFERENCES
|
Ammirato P (1986). Morphogenesis and clonal propagation. In: Plant tissue culture and its agricultural application. (Eds): Withers L, Alderson P. Butterworth, London. pp. 21-47.
|
|
|
|
Anderson MX, Kourtchenko O, Dangl JS, Mackey D, Elerstrom M (2006). Phospholipase dependent signaling during the AvrRpml and AvrRpt2-induced disease resistance response in Arabidopsis thaliana. Plant J. 47:947-949.
Crossref
|
|
|
|
Arunkumar A, Vijayababu MR, Kanagaraj P, Balasubramanian K, Aruldhas MM (2005). Growth suppressing effect of Garlic compound Diallyldisulfide on prostate cancer cell line (pc.3) in vitro. Biol. Pharm. Bull. 28:740-743.
Crossref
|
|
|
|
Baker KF (1990). Types of Rhizoctina diseases and their occurrence. In parameter, JB Jr (ed) Rhizoctina solani, biology and pathology pp125-48. Univ of Calif. Press, Berkeley, Los Angeles and London.
|
|
|
|
Colapietra M, Alexander A (2006). Effect of foliar fertilizer on yield and quality of table grapes. (proc. VthIS on mineral nutrition of fruit plants, Eds. J.B. Retamales and G.A.Lobos,) Acta Hort. 721:213-218.
|
|
|
|
Gangopadhay S Chakrabarti NK (1982). Sheath blight of rice. Rev. Plant Pathol. 61:451-460.
|
|
|
|
Garcia-Mina JM, Antolin MC, Sanchez-Diaz M (2004). Metal humic complexes and plant Micro-nutrient uptake: a study based on different plant species cultivated in diverse soil types. Plant Soil. 258:57-68.
Crossref
|
|
|
|
Ghaffar A (1988). Soil borne disease research. Final Research Report. Department of Botany, University of Karachi 75270, Pakistan, 111 p.
|
|
|
|
Jayaraj J, Wan A, Rahman M, Punja ZK (2008). Seaweed extract reduces foliar fungal disease on carrot. Crop Protect. 27:1360-1366.
Crossref
|
|
|
|
Jolivet l, Huchon P, Brun J, Pichon X, Chamot Rooke N, Thomas J (1991). Arc deformation and marginal basin opening: Japan sea as a case study. J. Geophys. Res. 96:1-10.
|
|
|
|
Karthikeyan K, Shanmugam M (2016). Development of a protocol for the application of commercial bio-stimulant manufactured from kappaphycus alvarezii in selected vegetable crops. J. Exp. Biol. Agric. Sci. 4:92-102.
|
|
|
|
Kobayashi T, Mew TW, Harshiba T (1997). Relationship between incidence of rice sheath blight and primary inoculum in the Philippines: mycelia in plant debris and sclerotia. Ann. Phytopathol. Sco. Jpn. 63:324-327.
Crossref
|
|
|
|
Mew TW, Rosales AM (1992). Control of Rhizoctonia solani sheath blight and other diseases by rice seed bacterization. Biol. control plant dis. pp. 113-123.
|
|
|
|
Norrie J, Branson T, Kethley PE (2002). Marine plants extract impact on grape yield and quality. In: Int. Symp. Foliar Nutr. Perennial Fruit Plants 594:315-319.
Crossref
|
|
|
|
Pal R, Chakrabarthi K, Chakraborthy A, Chowdhury A (2005). Dissipation of pencyeuron in rice plant. J. Zhejiang. Univ. Sci. 613(8):756-758.
Crossref
|
|
|
|
Saha BP, Saha K, Mukherjee PK, Mandal SC, Pal M (1995). Antibacterial activity of leucas lavandulaefolia rees. Indian Drugs 32: 402-404.
|
|
|
|
Sivakumar SR (2014). Antimicrobial potential of white crystalline solid from red algae Porteiriahornemanii against the plant pathogenic bacteria. Afr. J. Agric. Res. 9(17):1353-1357.
|
|
|
|
Suji HA (2004). Microbiological, biochemical, biophysical and sensory assessment of quality in value added products developed from marine fish (Sufflamen capistratus). M.Phil Thesis. CMST. pp. 80.
|
|
|
|
Thongson C, Davidson PM, Mahakarrchanakul W, Weiss J (2004). Antimicrobial activity of ultrasound – assisted solvent – extracted species. Letters Appl. Microbial. 39:401-406.
Crossref
|
|
|
|
Wu Y, Jenkins T, Bluden G, Von Mende N, Hankins SD (1997). Suppression of fecundity of the root knot nematode, Melodogynejavanica in monoxenic cultures of Arabidopsis thaliana treated with an alkaline extract of Ascophyllum nodosum. J. Appl. Phycol. 10:91-94.
Crossref
|