ABSTRACT
Water contamination is one of the leading causes of deaths and diseases worldwide, accounting for the deaths of more than 14,000 people daily, majority being children under 5 years old, therefore periodic monitoring of municipal water supplies are necessary. Comparative bacteriological analyses of twenty five samples of stored borehole water from five hostels in a tertiary institution in Oyo, Oyo State, was carried out using standard microbiological methods between March and April, 2018 to determine their bacteriological content. The hostels were: University Female Hostel (UFH), Diocese of Lagos West Hostel (DLW), Joseph Adetiloye Hostel (JAH), Diocese of Lagos Hostel (LAG) and Peter Jasper Akinola Hostel (IBD). A total of thirteen (13) different bacteria genera were isolated and identified as: Aeromonas (17%); Escherichia (4%); Staphylococcus (9%); Pseudomonas (9%); Lactobacillus (13%); Corynebacterium (27%); Micrococcus (6%); Streptococcus (3%); Serratia (4%); Klebsiella (1%); Citrobacter (3%); Shigella (3%); and Salmonella (1%). The total viable count ranged from 5.0×103 cfu/mL (IBD) to 1.23×105 cfu/mL (DLW). Total coliform bacteria counts of the stored water ranged from 0 to 28 MPN/100 mL. The bacteria load and presence of coliforms and pathogenic organisms raised grave concerns as WHO permissible limits for total viable count and coliforms are 100 cfu/mL and 0 MPN/100 mL, respectively. The organisms isolated are of public health significance as ingestion of water contaminated by them could result in gastroenteritis, especially Escherichia that indicates possible fecal contamination.
Key words: Bacteriological investigations, pathogens, groundwater, water quality, Oyo.
The importance of water to human existence and ecological sustainability cannot be overrated as it is essential for life. It is found in virtually all living cells and is paramount to life. Although a human can do without food for up to twenty eight days, man cannot go without water for three days (Ukpong et al., 2013; Akin-Osanaiye et al., 2018).
Studies have proven that over one billion people in the world lack access to safe drinking water and about 2.5 billion people do not have access to adequate sanitation services at all (Tar et al., 2009). In developing countries such as Nigeria, clean pipe borne water availability is not available in almost all the states. Due to the inability of Government to meet the ever increasing water demand, people resort to ground water sources such as shallow wells and boreholes as alternative water resources (LAWMA, 2000). Natural groundwater is usually of good quality but this can deteriorate due to inadequate source of protection and poor resource management (Sadiya et al., 2018).
Groundwater is found beneath the earth’s surface where it collects in voids of rocks and soil and it forms the ultimate source of water for springs, wells and boreholes. A borehole is a hydraulic structure which allows the withdrawal of water from an aquifer or groundwater resource (NWRI, 1997). Borehole water serves as the major source of drinking water in the local population of Nigeria (Akpoveta et al., 2011). Unfortunately, borehole water is not entirely pure and its purity depends on the geological conditions of the soil and in particular anthropogenic activities in the area which include: improper waste disposal, close proximity of septic to groundwater supply and leachate from landfills and dumpsites often polluting groundwater supply, thereby resulting in the transmission of bacteria and diseases (Boutaleb et al., 2008; Onwughara, et al., 2010).
Collected and stored borehole water microbial contamination is caused not only by the collection and use of faecally contaminated water that was not safe, to begin with, but also by the contamination of water (that was microbiologically safe initially), during storage. Unhygienic and imperfectly protected (poorly covered or open) water collection and containers for storage, unhealthy means of dispensing water from storage containers, including faecally contaminated dippers, hands, tools, lack of protection against vectors (flies, cockroaches, rodents, etc.) and inadequate cleaning of storage container to prevent biofilm formation and accumulation of sediments and pathogens, all are factors contributing to this problem (Steiner et al., 2006; Onwughara et al., 2010; Akpoveta et al., 2011).
Microorganisms play an important role in water quality and the microorganisms that are concerned with water borne diseases are Salmonella species, Shigella species, Escherichia coli and Vibrio cholera. The presence of faecal coliforms of Escherichia coli and those listed earlier are indicators of contaminated water (Adetunde and Glover, 2010).
Globally, water related diseases remain a major health concern. 1.8 million children die every year from waterborne diseases (that is to say 1 every 15 s). Worldwide, waterborne diseases are the most implicated killers of children under five years old and more people die annually from unsafe water than from violence (including war). Unsafe or inadequate water, sanitation, and hygiene account for 3.1% of all deaths worldwide. Unsafe water causes 4 billion cases of diarrhea each year, and results in 2.2 million deaths, mostly of children under five; a child dying every 15 s (UNESCO, 2017; WHO, 2019; Denchak, 2018).
The objectives of this study are therefore to determine the bacteriological enumeration, investigate the bacteriological content and compare bacteriological patterns of stored borehole water gotten from five different hostels water supplies in a tertiary institution within Oyo town of Oyo State, Nigeria.
Study area
The study was carried out in a tertiary institution in Oyo, Oyo State, Nigeria. The institution is located on the Oyo-Ogbomoso road in Atiba Local Government Area (LGA) of Oyo State between longitude 3.9351°E and latitude 7.8371°N. The campus can be reached within 1 h by road from Ibadan and Ogbomoso, in 1.5 h from Ilorin and Ile-Ife and in 2.5 h from Lagos. The institution has five hostels, in which two (2) are allocated to females and three (3) to males. The five hostels are:
(a) University Female Hostel (UFH);
(b) Diocese of Lagos West Female Hostel (DLW);
(c) Joseph Adetiloye Hostel (JAH);
(d) Diocese of Lagos Hostel (LAG);
(e) Peter Jasper Akinola / Ibadan Hostel (IBD);
Each of the hostels has a source of borehole from which water is pumped into plastic storage tanks and then to an overhead tank. The hostel residents make use of the stored water for different purposes including drinking, washing and bathing. The water content of the tanks is used up daily due to the population of residents.
Sample collection
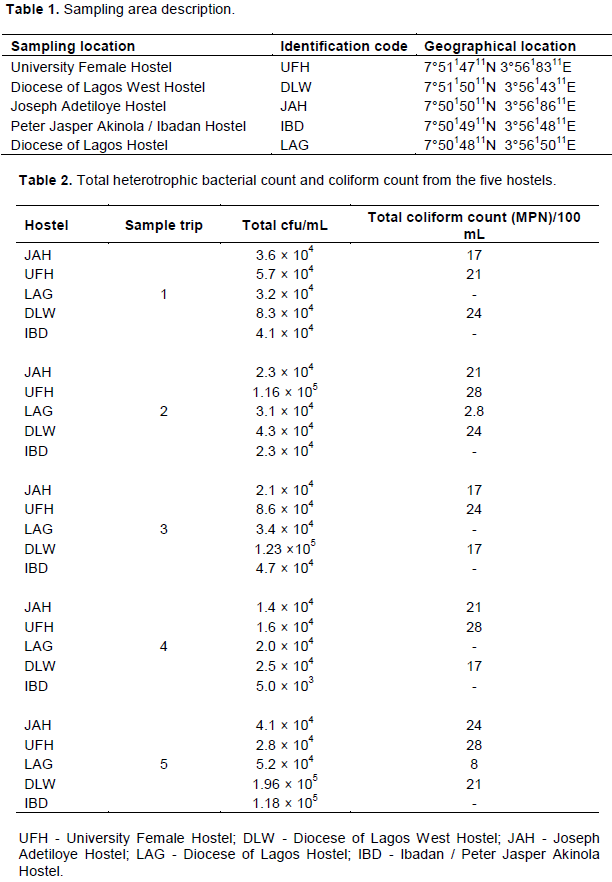
Water samples were collected from five (5) different storage tanks, holding water, pumped from individual boreholes of the hostels. The water samples were collected weekly (on Mondays) between the months of March and April 2018 which is a peak period in the use of the hostels. A total of five (5) samples were collected from each storage tank on five respective sampling trips which gave rise to twenty five (25) water samples in all. The average sampling time was 8 am each sampling day. Table 1 shows the description of the sampling areas with their geographical location. Samples were collected into 20 mL sterile sample bottles, screw capped and labeled. Samples were collected and transported using ice bag immediately to the laboratory for analysis.
Media used
Media for the multiple tube fermentation and plate counts were prepared according to the manufacturer’s instructions. The media used were Nutrient Agar (Himedia), MacConkey Agar (Himedia), Lactose broth (Lab M), and Eosin Methylene Blue (Himedia) agar. All were prepared according to the manufacturer’s instruction and sterilized in an autoclave at 121°C for 15 min.
Bacteriological analysis of water
Using the pour plate method, total heterotrophic bacteria in the water samples were obtained (Dhawale and LaMaster, 2003). Serial dilution was carried out and 1 mL aliquots of dilutions of 10-1 and 10-3 of each representative sample were inoculated into sterile Petri dishes. 10 mL of molten Nutrient agar (NA) was then introduced in the Petri dish over the samples. These were then swirled to attain even distribution and incubated at 37°C for 24 h. Petri-dishes from dilutions containing between 30 and 300 discrete colonies were counted and was made in cfu/mL (colony forming unit) (APHA, 2017). The colony forming units per millimeter (cfu/mL) was calculated by dividing the average number of colonies per dilution with the dilution factor.
A sterile inoculating loop was ascetically used to pick a loopful of each water sample. This was then streaked across the already set solid agar surface using the quadrant method of streaking. The inoculating loop was flamed between streaks and eventually after use; the plates were incubated at 37°C for 24 h.
Enumeration of total coliform bacteria
Multiple tube fermentation test
Multiple tube fermentation tests were conducted to enumerate total and faecal coliform (APHA, 2017). Total coliform count was determined with the aid of the three tube assay of the Most Probable Number (MPN) method.
Presumptive test
Presumptive coliform test was carried out using lactose broth. The first set of the three tubes had sterile 10 mL double strength lactose broth (DSLB) and the second and third sets had 10 mL single strength lactose broth (SSLB). Durham tubes were inserted in test tubes prior to sterilization. The three sets of the tubes received 1, 0.1 and 0.01 mL of water samples using sterile pipettes. All tubes were then incubated at 37°C for 24 to 48 h for estimation of total coliforms and examined afterwards for acid and gas production. The MPN was then determined from the MPN table for the three sets of tube (APHA, 2017).
Confirmed test
A loopful of culture was transferred from a positive tube from presumptive test into a tube of Lactose broth with Durham tube to carry out Confirmed test. The tubes were incubated at 37°C for 24 to 48 h for total coliform and 44.5 for faecal coliforms and observed for gas production.
Completed test
For the completed test a loopful of broth from a positive tube is streaked on Eosine Methylene Blue (EMB) agar plate for pure colonies. The plates were incubated at 37°C for 24 to 48 h. Colonies developing on EMB agar were further identified. Colonies with green metallic sheen were confirmed to be faecal coliform bacteria with rods shape.
Organisms observed with different morphology (mixed growth) were sub-cultured on Nutrient agar and Eosin Methylene Blue agar and incubated at 37°C for 24 h to get pure cultures.
After development of bacterial growth colony on the agar surface, cultural characteristics of the isolates on different solid agar were examined. Growth characteristics including colonial morphology, color pigmentation, form, deviation, margin, surface, and optical characters were recorded, following Bergey's manual of systematic bacteriology (Ryan and Ray, 2008).
Biochemical tests for identification of isolates
Biochemical tests carried out for identification of isolates were: Gram stain, Endospore test, Catalase test, oxidase, Sugar fermentation (TSI), Hydrogen sulphide, motility, Indole test; and Mannitol fermentation test.
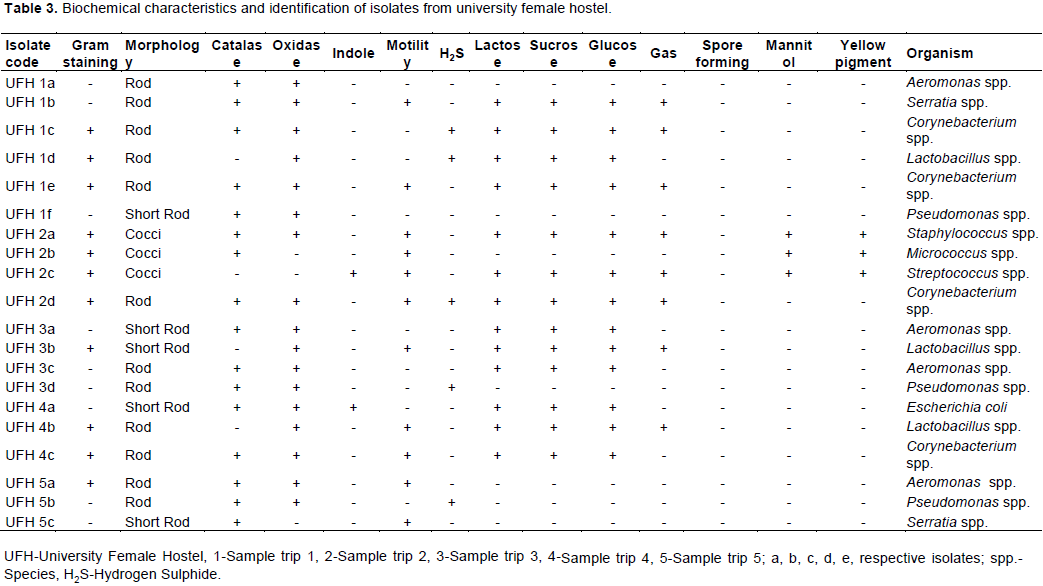
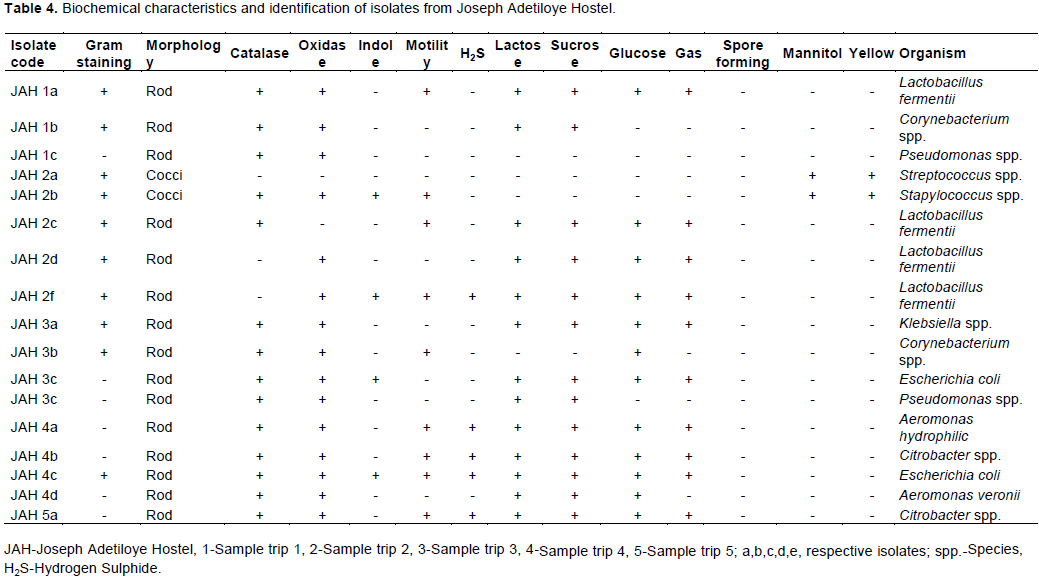
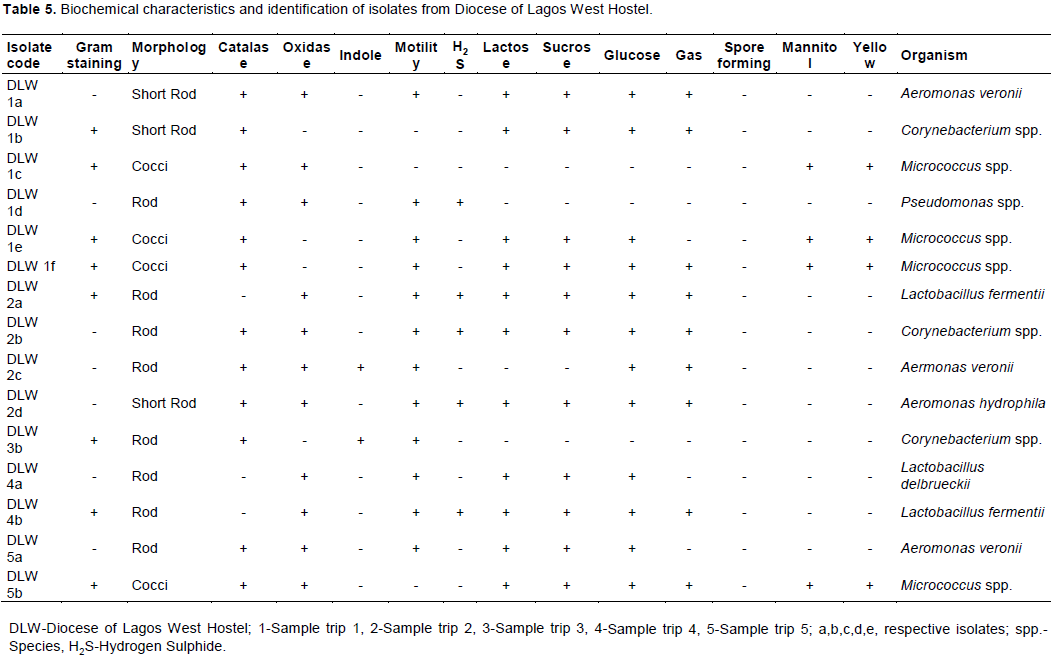
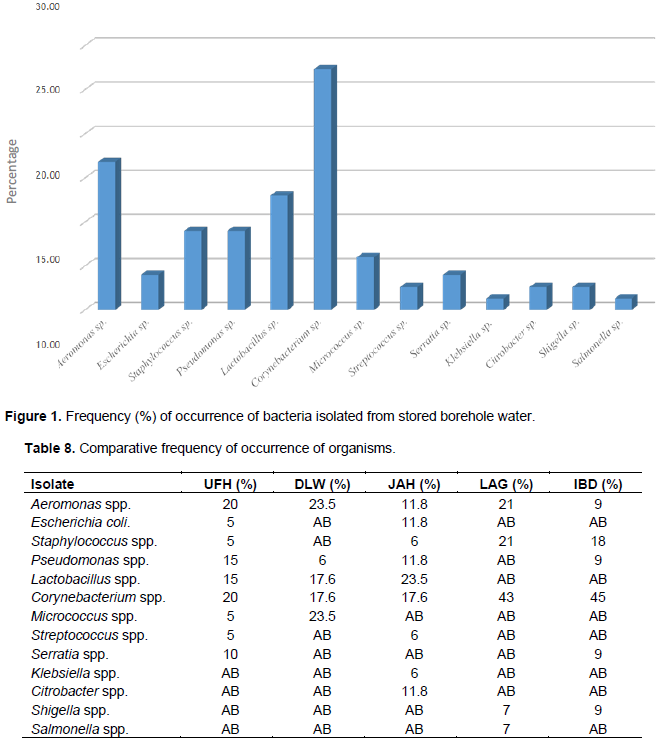
The total heterotrophic counts of bacteria from stored borehole water samples of the various hostel ranged from 5.0 × 103 (IBD) to 1.23 × 105 cfu/mL (DLW) while the total coliform count of the stored water samples ranged from 0 (IBD) to 28 (UFH) MPN/100 mL (Table 2). Only Ibadan Hostel recorded 0 total coliform counts all through the study, next in line was Lagos Hostel which recorded low coliform counts on only 2 trips out of 5 UFH; however, had the highest number of coliform counts on four trips out of five as shown in Table 2. A total of 77 bacteria isolates were obtained in this study (Tables 3 to 7), 32 (41.6%) were Gram negative, while 45 of them (58.4%) were Gram positive bacteria. The most number of organisms were isolated from UFH, followed by LAG, next was JAH, then DLW and IBD had the least number of isolates (Tables 3 to 7). The 77 organisms isolated belonged to only 13 different bacterial genera which were characterized and identified as: Corynebacterium (27%), Aeromonas (17%), Lactobacillus (13%), Escherichia (4%), Psuedomonas (9%), Staphylococcus (9%), Micrococcus (6%), Serratia (4%), Citrobacter (3%), Shigella (3%), Streptococcus (3%), Klebsiella (1%), and Salmonella (1%) as shown in Figure 1. Table 8 shows a comparative representation of the different isolates from the hostels and reveals that UFH hostel had the most number of isolates, while LAG hostel had the least number of organisms.
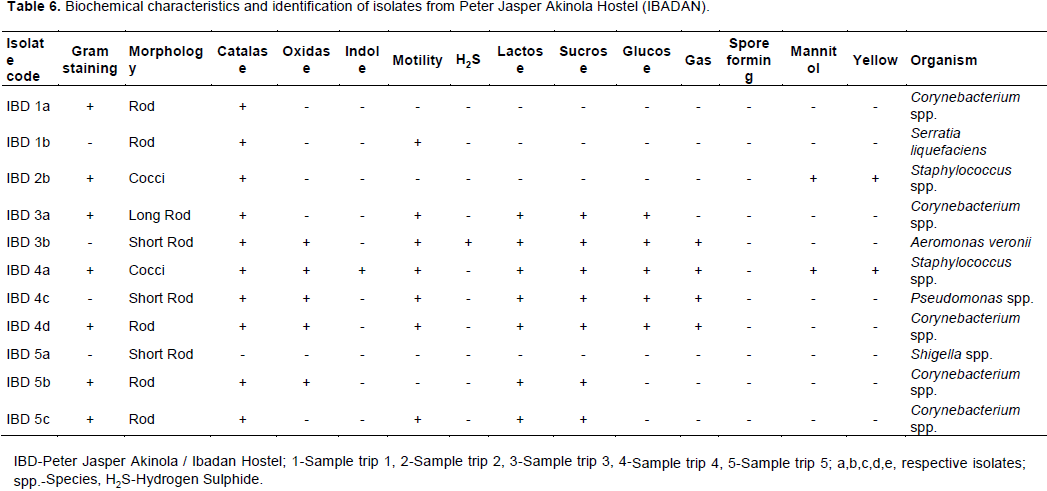
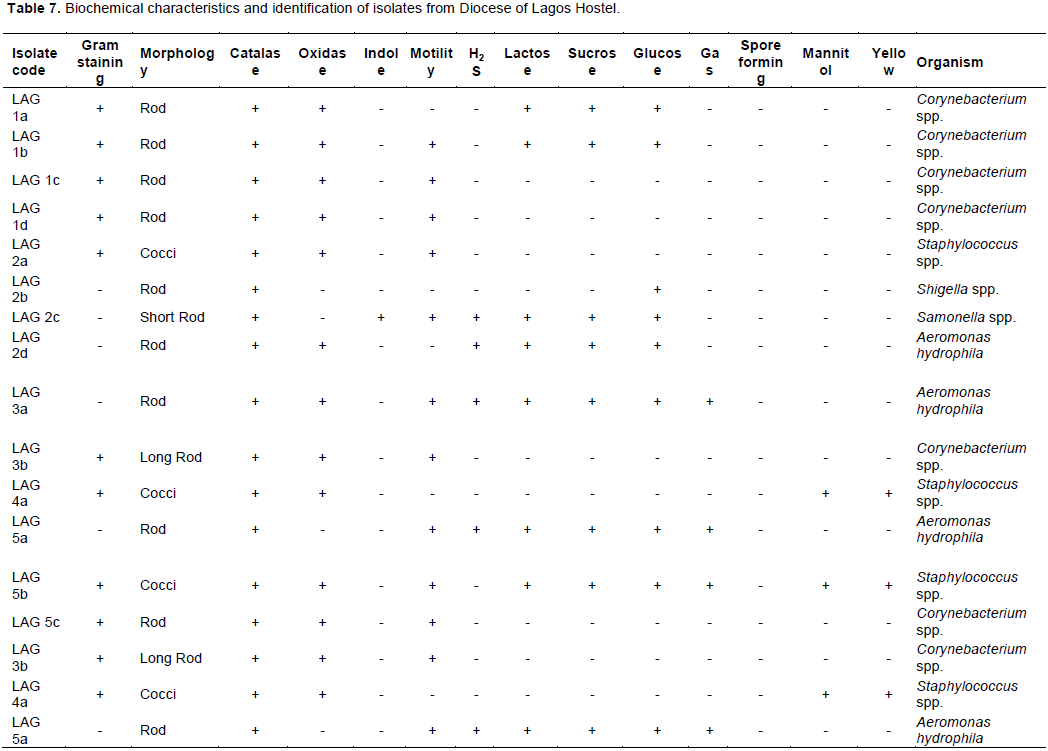
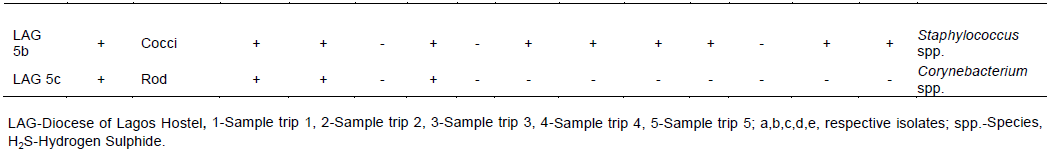
AB-Absent, UFH-University Female Hostel, DLW-Diocese of Lagos West Hostel, JAH- Joseph Akintiloye Hostel, LAG-Diocese of Lagos Hostel, IB-Peter Jasper Akinola Hostel (Ibadan).
Data shown in Table 8 further reveal the following occurrence of organisms according to site of isolation: Aeromonas species and Corynebacterium species were isolated from all five hostels studied (UFH, DLW, JAH, LAG and IBD). Staphylococcus species were isolated from UFH, JAH, LAG and IBD hostels, Pseudomonas species from UFH, DLW, JAH, and IBD hostels. Lactobacillus species found occurrence in UFH, DLW, and JAH hostels. E. coli and Streptococcus species were isolated from UFH and JAH hostels, Micrococcus species from UFH and DLW hostels, Serratia species from UFH and IBD hostels, Shigella species from LAG and IBD hostels. Klebsiella and Citrobacter species each found occurrence only in JAH hostel, while Salmonella species was isolated only from LAG hostel.
The total heterotrophic bacteria count from the five hostels indicated that none of the stored water samples fell within the 100 cfu/mL limit allowed by WHO (2006) for potable water. The high viable bacteria count of the stored water can be attributed to lack of water treatment and hygienic care (for example, washing) of the storage tanks. Most of the sampled waters recorded total coliform counts exceeding the WHO recommended standards which stipulate that total coliform counts should not exceed 1-3/100 mL of potable water and 0/100 mL of thermotolerant coliforms (WHO, 2006) and SON standards that recommend 0/100 mL of coliforms in portable water. Coliforms are indicator organisms and their presence is indicative of other disease causing organisms in the sampled water (Bello et al., 2013). The total coliform count obtained implies contamination, possibly from piping leakages within the water system network, unsanitary conditions or groundwater contamination.
Three members of the coliform bacteria group were isolated in this study (Escherichia, Citrobacter and Klebsiella). Coliforms are important markers for bacteriological water quality as they are established causes for human gastroenteritis and their presence in potable water makes it unsafe for consumption. The most implicated coliform, E. coli indicates recent faecal contamination. E. coli, a normal intestinal gut flora, is found in the gut of humans and animals, where it is harmless (WHO, 2017). However, in other parts of the body, E. coli can cause serious disease. Two particular pathogenic serotypes of E. coli namely E. coli O157:H7 and E. coli O104:H4 are known causes of diseases in humans (Ateba and Bezeuidenhout, 2008). A severe outbreak of illness caused by E. coli O157:H7 was recorded in Walkerton community, Ontario, Canada resulting in 7 deaths and over 2300 cases of illness (Aboh et al., 2015). Coliforms have been isolated from soil and water and their presence in the samples tested suggests possible breakage of the piping system. Moreover, the presence of coliforms in tested water samples implies a failure to meet up with WHO standards.
Worldwide, 80 to 165 million cases of infection from Shigella are reported, causing 600,000 deaths annually largely in developing countries, the most vulnerable group being children below 10 years (WHO, 2017), which makes the presence of Shigella unacceptable among others. The incidence of high numbers of Pseudomonas in potable water often elicits foul odour, taste, and high turbidity levels. Salmonella also isolated in this study clinically manifests in gastroenteritis in humans and one of its serotype-typhi causes typhoid fever with devastating public health implications (WHO, 2017). These organisms are commonly found in the environment with some species being host specific particularly in animals and also humans. Their presence in tested samples suggest possible compromise of nearby sewage system, water piping leakages and perhaps questionable personal hygiene of the operators.
Micrococcus and Aeromonas are known water contaminants that easily proliferate in waters exposed to contaminated air, dust and where water holding vessels are not cleaned regularly.
They are opportunistic pathogens that are known to induce chronic diarrhea in humans when water polluted by them is ingested (Igbinosa et al., 2012; Pavan et al., 2013). There is a possibility that the storage tanks were not properly covered or covers may have depreciated due to weathering.
Most of the organisms isolated are important human pathogens associated with variety of infectious diseases and outbreaks including: gastroenteritis, typhoid fever, dysentery, cholera, urinary tract infection among others, especially where such bacteria possess virulence factor genes (Orji et al., 2006; Uzoigwe and Agwa, 2012; Bello et al., 2013). They belong to the family of Enterobacteriaceae, and members of this family are generally spread via the faecal-oral route, and their presence in the samples indicates contamination of the water supplies. If such water is consumed without treatment, it poses grave health hazards to humans (WHO, 2006; SON, 2015).
This study also reveals disparities in organisms isolated from the different hostels. This indicates differing sanitary and environmental conditions of the storage tanks in the various hostels. It also implies that the contamination source is peculiar to prevailing conditions of the respective sampling sites.
This study isolated pathogenic organisms from stored borehole water supplies in a tertiary institution. These findings prove that all the stored water tested failed to meet up with approved SON and WHO standards for portable water. Consumption of water from these supplies without treatment may pose serious health risks to the consumers.
There is a need to sensitize operators of boreholes and end users of the stored water on the importance of maintaining clean and hygienic environmental conditions around the borehole and water storage tanks to prevent contamination. Further investigation of the waste disposal system of hostels where coliforms were isolated from samples is necessary, together with proper monitoring of environmental conditions of the water systems in all hostels. Adequate water disinfection and treatment of all storage water tanks is advocated to prevent any adverse effect to end users of the water supplies. Regular water system monitoring and analysis will be instrumental in ensuring water supplies fall within approved limits.
The authors have not declared any conflict of interests.
REFERENCES
|
Aboh EA, Giwa FJ, Giwa A (2015). Microbiological assessment of well waters in Samaru, Zaria, Kaduna, State, Nigeria. Annals of African Media 14:32-38.
Crossref
|
|
|
|
Adetunde LA, Glover RLK (2010). Bacteriological quality of borehole water used by students' of University for Development Studies, Navrongo Campus in Upper-East Region of Ghana. Current Research Journal of Biological Sciences 2(6):361-364.
|
|
|
|
|
Akin-Osanaiye BC, Mohammed SE, Echoki J (2018). Comparative analysis of pipe borne water and other sources of water in Gwagwalada Area Council, Federal Capital Territory, Abuja, Nigeria. Journal of Biology and Genetic Research 4(1):2545-5710.
|
|
|
|
|
Akpoveta OV, Okoh BE, Osakwe SA (2011). Quality assessment of borehole water used in the vicinities of Benin, Edo State and Agbor, Delta State of Nigeria. Current Research in Chemistry, 3(1):62-67.
Crossref
|
|
|
|
|
American Public Health Association (APHA) (2017). Standard Methods for the Examination of Water and Wastewater, 21st Edn., APHA, Washington DC. Available online at
View
|
|
|
|
|
Ateba CN, Bezuidenhout CC (2008). Characterisation of Escherichia coli O157 strains from humans, cattle and pigs in the North-West Province, South Africa. International Journal of Food Microbiology 128(2):181-8.
Crossref
|
|
|
|
|
Bello OO, Osho A, Bankole SA, Bello TK (2013). Bacteriological and physicochemical analyses of borehole and well water sources in Ijebu-Ode, Southwestern Nigeria. International Journal of Pharmacy and Biological Sciences 8:18-25.
Crossref
|
|
|
|
|
Boutaleb S, Boualoul M, Bouchaou L, Oudra M (2008). Application of remote sensing and surface geophysics for groundwater prospecting in a hard terrain, Morocco. In S.Adelana and A.MacDonald (Ed.) Applied groundwater studies - IAH selected papers on hydrogeology. Taylor and Francis Group, London. 13:215-226.
Crossref
|
|
|
|
|
Denchak M (2018). Water pollution: Everything you need to know. Retrieved online on 10:11:18 from:
View
|
|
|
|
|
Dhawale S, LaMaster A (2003). Microbiology Laboratory manual. The mcHill Company. Inc. USA. p. 226.
|
|
|
|
|
Igbinosa IH, Igumbor EU, Aghdasi F, Tom M, Okoh AI (2012). Emerging Aeromonas species infections and their significance in public health. Scientific World Journal 2012:625023.
Crossref
|
|
|
|
|
Lagos State Waste Management Authority (LAWMA) (2000). Management of landfill wastes: The Journey So Far. L.E.P.A. Handbook.
|
|
|
|
|
National Water Research Institute, NWRI (1997). Training Guide for Basic Water Treatment Operations. Kaduna. National Water Resources Institute. 1.
|
|
|
|
|
Onwughara IN, Nnorom IC, Kanno OC (2010): Issues of roadside disposal habit of municipal solid waste, environmental impacts and implementation of sound management practices in developing country: Nigeria. International Journal of Environmental Science and Development 1(5):409-417.
Crossref
|
|
|
|
|
Orji MU, Ezenwaje EE, Anyaegbunam BC (2006). Spatial appraisal of shallow well water pollution in Awka, Nigeria. Nigerian Journal of Microbiology 20(3):1389-1384.
|
|
|
|
|
Pavan KP, Raghuveer YP, Shiva SA (2013). Identification of opportunistic pathogenic bacteria in drinking water samples of different rural health centers and their clinical impacts on humans. BioMed Research International 2013(2):348250.
Crossref
|
|
|
|
|
Ryan KJ, Ray CG (2008). Sherris Medical Microbiology, 4th edition. McGraw Hill pp. 370-380.
|
|
|
|
|
Sadiya A, Chukwuma CO, Olatunbosun OA, Onyinye FN (2018). Comparative study of the physicochemical and bacteriological qualities of some drinking water sources in Abuja, Nigeria. Global Journal of Pure and Applied Sciences 24: 91-98.
Crossref
|
|
|
|
|
Standards Organization of Nigeria (SON) (2015). Nigerian Standard for Drinking Water Quality NIS 554:2015. Retrieved online on 15:03:19 from:
View
|
|
|
|
|
Steiner TS, Samie A, Guerrant RL (2006). Infectious diarrhea: new pathogens and new challenges in developed and developing areas. Clinical Infectious Diseases 43:408-410.
Crossref
|
|
|
|
|
Tar A, Eneji I, Ande S, Oketunde F, Ande S, Shaaton R (2009). An assessment of heavy metals loading in River Benue in the Makurdi Metropolitan Area in Central Nigeria. Nigerian Journal of Chemical Society of Nigeria 34:56-62.
|
|
|
|
|
Ukpong EC, Ogarekpe NM, Bejor ES (2013). Comparative analysis of water quality in hand dug well and borehole in Calabar South Local Government Area in Nigeria. The International Journal of Engineering and Science 2(8):95-101.
|
|
|
|
|
United Nations Educational, Scientific and Cultural Organization (UNESCO) (2017). Facts and Figures. Wastewater: The untapped Resource. The United Nations World Water Development Report 2017. Retrieved online on 30:03:2019 from:
View
|
|
|
|
|
Uzoigwe CI, Agwa OK (2012). Microbiological quality of water collected from boreholes sited near refuse dumpsites in Port Harcourt, Nigeria. African Journal of Biotechnology 11(13):3135-3139.
Crossref
|
|
|
|
|
World Health Organization (WHO) (2006). Guidelines for Drinking Water Quality. Geneva. Addendum to the 3rd Vol. 1 Recommendations. World Health Organization 1:23-48.
|
|
|
|
|
World Health Organization (WHO) (2017). Guidelines for Drinking-Water Quality: Fourth Edition Incorporating the First Addendum. Geneva: World Health Organization; 2017. Retrieved online from:
View
|
|
|
|
|
World Health Organization (WHO) (2019). Sanitation. Retrieved on 28:10:18 from:
View.
|
|