ABSTRACT
The study was carried out with the aim to isolate Staphylococcus aureus from camel and goat raw milk and determine their antimicrobial susceptibility pattern. 204 raw milk samples were collected from randomly selected lactating camels (n=62) and lactating goats (n=142) in live-stock producing pastoralists’ areas of Somali region for isolation and identification of S. aureus. Antibiotic susceptibility tests were performed on all S. aureus isolates to 16 antibiotics by Kirby-Bauer disk diffusion method. Twenty three (11.27% of the total) S. aureus strains were isolated. Four (6.45%) strains were isolated from camel raw milk samples and 19 (13.34%) from goat raw milk samples. S. aureus isolates showed resistance to Nalidixic acid, Polymixin B, and Penicillin G. S. aureus isolates were sensitive to vancomycin, ciprofloxacin, cefoxitin, cephalotin, gentamycin, Doxycycline, kanamycin, trimethoprim-sulfamethoxazole, chloramphenicol, norfloxacin, and erythromycin. Multi drug resistance was detected in 69.2% of the isolates. The present study has demonstrated the existence of alarmingly high level of multiple antimicrobial resistances of S. aureus among camel and goat milks.
Key words: Staphylococcus aureus, camel and goat milk, antimicrobial susceptibility test, Ethiopia.
Staphylococcus aureus is a ubiquitous human and animal pathogen and a common cause of invasive and life threatening infections. Human illness through S. aureus range from minor skin infection such as pimples, boils, cellulites, toxic shock syndrome, impetigo, and abscesses to life threatening disease such as pneumonia, meningitis, endocarditis, and septicaemia (Daka et al., 2012; Thaker et al., 2013; Mekuria et al., 2013). S. aureus is also a major causative pathogen of clinical and subclinical mastitis in animals (Adwan et al., 2005; Mekuria et al., 2013). On top of it, it is also an important food borne pathogen. Milk has been reported as a common food which is a source of staphylococcal infections and a cause of staphylococcal poisoning (Lowy, 1998; Le loir et al., 2003). About 50% strain of S. aureus are able to produce enterotoxins associated with food poisoning (Payne and Wood, 1974).
The natural ecological niches of S. aureus are the nasal cavity and the skin of warm-blooded animals. The skin, mucosa membranes, teats and udders of milking animals are the most important reservoir of this contaminant. S. aureus is frequently isolated from raw milk manually draw from individual animals, bulk raw milk and naturally from milk of dairy cattle suffering from mastitis. (MedveÄová and Valík, 2012).
Contamination of milk and milk products with pathogenic bacteria is mainly due to processing, handling and unhygienic environment. The occurrence of these pathogenic bacteria in milk and milk products can cause severe health hazards to people as they are highly susceptible to variety of microorganism because of high nutritive value and toxic nature caused by, or thought to be caused by the complex chemical composition (Thaker et al., 2013).
The indiscriminate use of antibiotics for the treatment of animal and human diseases as well as preservatives for milk has led to the development of multiple antibiotic resistances thereby rendering the antibiotic treatment ineffective. S. aureus has been reported to frequently show multiple antimicrobial resistance patterns (Enright, 2003). There is a limited number of studies on prevalence and antimicrobial resistance of S. aureus originated from camel and goat milk in the pastoralist areas of Ethiopia. Thus, this study aimed to isolate S. aureus from milk of healthy lactating camel and goat in Somali region of Ethiopia and found out its prevalence and antimicrobial resistance pattern.
Study design and area description
A cross-sectional study was conducted from November 2013 to April 2014 in Somali region which is located in Eastern part of Ethiopia. The area was selected for its potential production of milk by pastoralist and semi-pastoralists. Pastoralists/semi- pastoralists were included using systematic random sampling methods to meet our total sample number requirement. The areas are geographically found at a latitude of 9°21’N and a longitude of 42°48’ E and characterized by unreliable and erratic rainfall with a precipitation ranging from 300 to 600 mm per annum, high ambient temperature of 30°C, sparsely distributed vegetation dominated by Acacia species, cactus and bushy woodlands. These are arid and semi-arid lowlands lying at an elevation of 500 to 1500 m above sea level and are not suitable for crop production (Tafesse, 2001).
Dairy pastoralists/semi-pastoralists’ household selection
Zonal and woreda agriculture bureau were contacted to obtain the important base-line data about the potential milk production of dairy pastoralists/semi-pastoralists living in each kebeles (the smallest administrative divisions in each region of Ethiopia) and get guidance during sample collection. Based on this base-line data, rural Kebeles with milk production potentials were selected using purposive sampling.
Thirteen kebeles were included in the study from four woredas (Larger administrative division in Ethiopia and comprises around 20-25 kebeles) and, pastoralist/semi- pastoralist households who own local camel and goat breeds from each rural Kebeles were selected. The local camel breeds were one humped African camels (Bos indices) and the goat breeds were Short-eared Somali and Long-eared Somali Milk samples were collected from pastoralist/semi- pastoralist households based on availability. Househods which have one lactating camels and/or goats were taken as milk sources.
Study population and sampling techniques
A total of 204 raw milk samples were collected from randomly selected lactating camels (n=62) and lactating goats (n=142) in live-stock producing pastoralists’ areas of Somali region that were kept under traditional management from different woredas (Larger administrative division in Ethiopia and comprises around 20-25 kebeles) in Somali, eastern Ethiopia. The total number of samples were determined based on previously reported prevalence of S. aureus which were 4.2% in camel milk of Jijiga town (Husein et al., 2013) and 12.8% in goats milk of Adami Tulu areas (Molla et al., 2006) in Ethiopia, respectively. The study included major local camel and goat breeds of Somali region of Ethiopia for sources of raw milk. The lactaing camels and goats were included from the selected zones and woredas of the region based on randomly selected households.
Sample collection and transportation methods
From the randomly selected dairy pastoralist/semi-pastoralist’ households, milk samples were collected based on availability after gathering information about the local breed types they own. Raw milk was directly drawn from udder of lactating camels and goats which were found in the selected pastoralist/semi-pastoralist’ households in each kebeles. A total of 204 raw milk samples were collected using sterile plastic test tubes. For collection of raw milk samples, the udder was washed with antiseptic solution, wiped dry with clean cloth and then disinfected with 70% alcohol, the foremilk was discarded and 20-40 ml of pooled milk were collected. Milk samples were transported in an ice box with ice to Ethiopian Biodiversity Institute, Addis Ababa, Ethiopia for further bacterial identification.
Isolation and identification of S. aureus
About 0.1 ml of milk sample was streaked on Mannitol Salt Agar (MSA) (Hi-Media Laboratories, Mumbai, India) plate and incubated at 37°C for 24-48 h for the isolation of S. aureus. Suspected colonies of S. aureus that were yellow in colour on each MSA plate were further purified by sub-culturing onto MSA plates and incubated aerobically at 37°C for 18–24 h (Daka et al., 2012; Sharma et al., 2011).
Bacterial identification was performed by gram staining, microscopic examination of the morphology and biochemical tests. Gram-positive cocci that occurred in clusters under the microscope were subjected to preliminary biochemical tests (the catalase and oxidase tests). The identities of the isolates were confirmed based on positive results for the DNase test, beta-haemolytic patterns on blood agar enriched with 5% sheep blood and the coagulase slide and coagulase tube test for S. aureus (Bergey’s Manual of Systematic Bacteriology, 2009; Daka et al., 2012).
Antimicrobial susceptibility test
Antibiotic susceptibility tests were performed by the Kirby-Bauer disk diffusion method (CLSI, 2008) on all S. aureus isolates (n=23) using Mueller Hinton agar (supplied by Oxoid, UK). Fresh overnight cultures were prepared and used for antibiotic sensitivity tests. An aliquot (100 μL) from each isolate suspension was spread plated on Mueller Hinton agar (supplied by Oxoid Company). Susceptibilities of the isolates to a panel of sixteen different antibiotic discs (6 μm in diameter, Mast group LTD MERSEY SIDE, UK) were determined.
The following antimicrobial disks (Oxoid disks) with their corresponding concentration were used: Penicillin G (10 units), Amoxicillin (25 mcg), Cephalothin (30 mcg), Cefoxitin (30 mcg), Gentamicin (10 mcg), Kanamycin (30mcg), Nalidixic acid (30 mcg), Ciprofloxacin (5 mcg), Norfloxacin (10mcg), Trimethoprim-Sulfamethoxazole (25 mcg), Polymixin B (300unit), Erythromycin (15 mcg), Vancomycin (30 mcg), Chloramphenicol (30 mcg), Doxycycline (30 mcg) and Tetracycline (30 mcg). Antibiotic discs were gently pressed onto the inoculated Mueller Hinton agar to ensure intimate contact with the surface and the plates were incubated aerobically at 37°C for 18h-24 h (CLSI, 2008). Inhibition zone diameters were measured and value obtained from Clinical and Laboratory Standards Institute (CLSI, 2008) was used to interpret the results obtained. S. aureus isolates were then classified as resistant, intermediate-resistant or susceptible to a particular antibiotic. Multiple drug resistant (MDR) phenotypes were recorded for isolates showing resistance to three and more antibiotics (CLSI, 2008; Rota et al., 1996).
Data analysis
Statistical analyses were carried out using STATA version 11 and WHONETs version 5.6 statistical software packages after exporting the raw data which were entered into excel spread sheet. Descriptive statistics such as percentages and frequency distribution was used to describe bacterial isolates and antimicrobial susceptibility which was expressed as percent of resistant, intermediate and susceptible. In addition, the proportion of bacteria resistant to at least one of the sixteen antibiotics and resistant two or more were calculated.
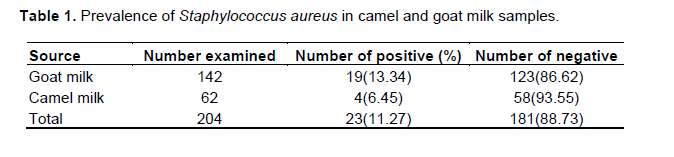
Prevalence of S. aureus in camel and goat milk samples
Of the total 204 examined goat and camel raw milk samples, 23 (11.27%) were positive for S. aureus. Four (6.45%) S. aureus were isolated from the 62 camel raw milk samples, and 19 (13.34%) were isolated from 142 goat raw milk samples (Table 1). The highest isolation of S. aureus was from goat milk (13.34%) while less was from camel milk (6.45%) (P value=0.15).
Antibiotic susceptibility test results
All 23 S. aureus isolates were subjected to antibiotic susceptibility tests. Sixteen different antibiotics were used. In vitro antimicrobial resistance pattern of S. aureus isolates to the 16 antibiotics was investigated.
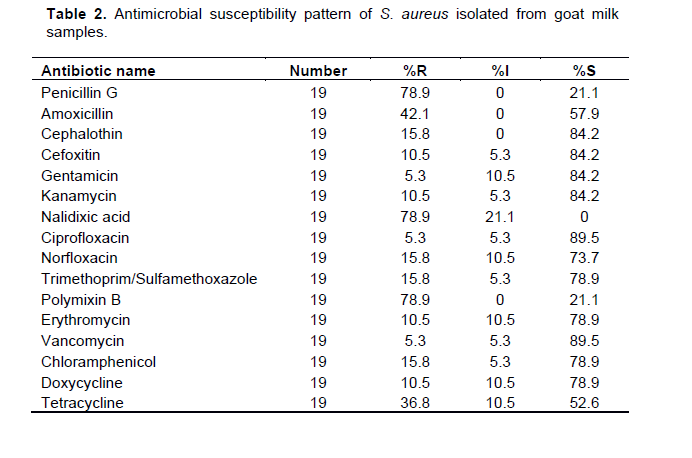
Antimicrobial susceptibility pattern of S. aureus isolated from goat milk samples
Results of antimicrobial susceptibility test against S.aureus showed high susceptibility to ciprofloxacin (89.5%) and vancomycin (89.5%) followed by cephalothin (84.2%), cefoxitin (84.2%), kanamycin (84.2%), gentamicin (84.2%), trimethoprim-sulfamethoxazole (78.9%), erythromycin (78.9%), doxycycline (78.9%), and chloramphenicol (78.9%) (Table 2) whereas high level of resistance was recorded against Penicillin G (78.9%), nalidixic acid (78.9%), polymixin B (78.9%), and tetracycline (36.8%) (Table 2).
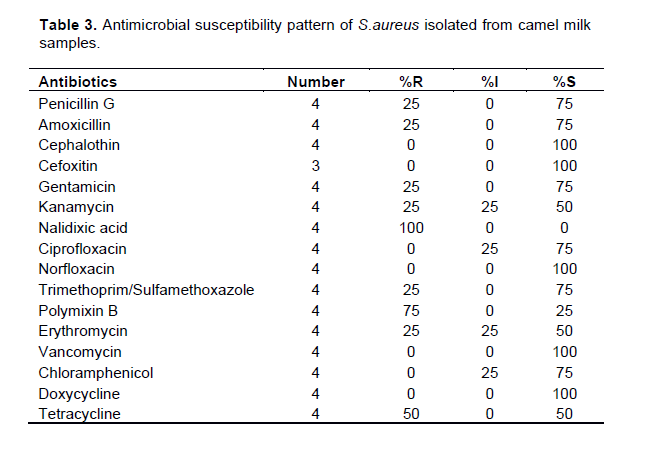
Antimicrobial susceptibility pattern of S. aureus isolated from camel milk samples
Results of antimicrobial susceptibility test against S.aureus showed high susceptibility to vancomycin (100%), cephalothin (100%), cefoxitin (100%), norfloxacin (100%), doxycycline (100%) followed by gentamicin (75%), trimethoprim-sulfamethoxazole (75%), penicillin G (75%), Amoxicillin (75%), ciprofloxacin (75%), and Chloramphenicol (75%) (Table 3) whereas high level of resistance was recorded against Nalidixic acid (100%), Polymixin B (75%), and Tetracycline (50%) (Table 3).
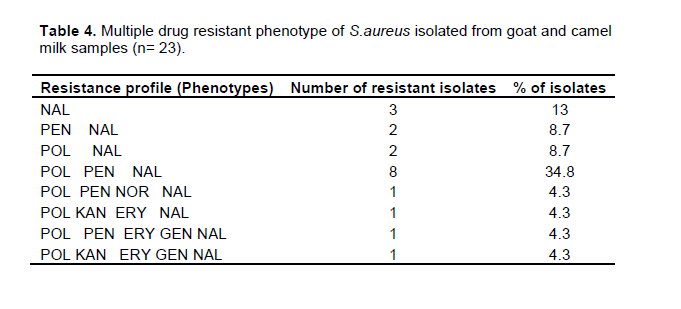
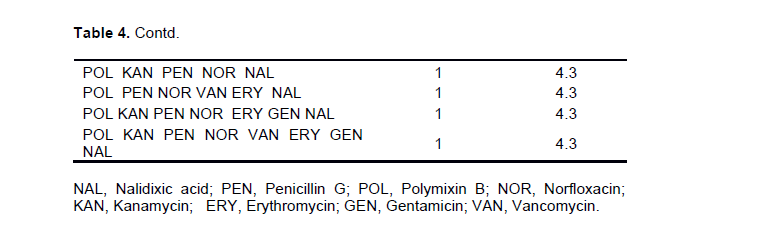
Multiple drug resistant phenotypes of S.aureus
The present study demonstrated multiple drug resistance of S.aureus isolates (Table 4). All of the 23 confirmed S. aureus isolates showed resistance to one or more antimicrobial agents. Three isolates (13.0%) were resistant to single antibiotic and four isolates (17.4%) showed resistance to two antimicrobial agents. Multiple drug resistance (MDR) which is defined as resistance to three or more antimicrobial agents was found in 69.6% of S. aureus isolates. The predominant MDR phenotypes of S. aureus isolated from this study area was POL-PEN-NAL in 34.8% of the isolates. Furthermore, MDR phenotypes POL-PEN-NOR-NAL, POL-KAN-ERY-NAL, POL-PEN-ERY-GEN-NAL, POL-KAN-ERY-GEN-NAL, POL-KAN-PEN-NOR-NAL, POL-PEN-NOR-VAN-ERY- NAL, POL-KAN-PEN-NOR-ERY-GEN-NAL, and POL-KAN-PEN-NOR-VAN-ERY-GEN-NAL were obtained in 4.3% of the isolates each.
The observed prevalence of S. aureus in camel and goat milk found in the present study is in line with the findings of Husein et al. (2013) who found 4.2% isolation rate in camel milk in and around Jijiga town and Molla et al. (2006) who isolated 12.8% from goat milk in Adami Tulu town in Ethiopia. Similarly, it was closely comparable with the findings of Ebrahim et al. (2013) who reported 3.4 and 7.5% prevalence of S. aureus in camel and goat milk in Iran, respectively. However, the present findings are lower than that of Abdurahman et al. (2006) who reported 12.7% in Errer valley of eastern Ethiopia and Abera et al. (2010) who showed a 26.3% prevalence of S. aureus from mastitis milk of camel at Jijiga, eastern Ethiopia. This high isolation rate of S.aureus from camel milk is due to the fact that the collected milk unlike ours is from clinicaly diseased camel suffering from mastitis which is mainly caused by S.aureus.
Studies show that susceptibility patterns of Staphylococcus aureus to antimicrobial agent have varied worldwide, but isolates were usually susceptible to kanamycin, ciprofloxacin, vancomycin, and gentamicin (Alian et al., 2012; Daka et al., 2012; Thaker et al., 2013; Mekuria et al., 2013). S. aureus isolates in the present study also showed high sensitivity towards vancomycin, ciprofloxacin, cefoxitin, cephalotin, gentamycin, chloramphenicol and kanamycin.
The antimicrobial susceptibility result in the present study is comparable with the results obtained in Alamin et al. (2013) where S.aureus isolates were found to be sensitive for the tested antibiotics in the following percentage: ciprofloxacin (77.8%), gentamycin (88.8%), tetracycline (77.8%), amoxicillin (66.7%). In Mekuria et al., (2013) study S. aureus isolates were sensitive to vancomycin (88.2%) and Trimethoprim-Sulfamethoxazole (66.7%). High antibiotic susceptibility of S. aureus to some of the drugs is also reported in Tofaily et al. (2011). Tofaily et al. (2011) showed in their findings that S. aureus isolates were sensitive to amoxicillin (83.3%), tetracycline (83.3%), chloramphenicol (83.3%), ciprofloxacin (100%), doxycycline (100%), trimethoprim-sulfamethoxazole (83.3%) which has much similarity with the present study result.
In contrast to the present study, the reported sensitivity of S. aureus to some antibiotics is much different. For instance, Mekuria et al. (2013) reporetd that the susceptibility rate of S. aureus isolates to Erythromycin was 21.6%. Alamin et al. (2013) reported a susceptibility rate of 33.3% to Trimethoprim-Sulfamethoxazole and Tofaily et al. (2011) cited a sensitivity percentage of 16.6% to Erythromycin. S. aureus isolated in the present study found to be highly susceptible to these antibiotics. This might be that these antibiotics are not frequently used in the study area in veterinary services and perhaps in human medicine.
The high percent of antimicrobial resitance exihibited to Nalidixic acid, Polymixin B , and Penicillin G. in this study is in line with the findings of Tariku et al. (2011) who reported 87.2% resistance to penicillin in Ethiopia, 64.3% of resistance to penicillin G (Daka et al., 2012) in Hawassa area of Ethiopia and 80% resistance to penicillin which is reported in Sweden by Landin (2006). This is in contrast to findings observed by Adesiyun (1994) who reported 23% of resistance to pencillin G in West India and Alian et al. (2012) who reported 17.4% of resistance to pencillin G in Iran.
The probable explanation to the presence of high antibiotic resistant S. aureus to nalidixic acid, polymixin B, penicillin and tetracyclin is the indescriminate and repeated use of these antibiotics in animal and human health facilities of the present study area. Penicillin and tetracycline are the most commonly used antimicrobials in the treatment of infections in the livestock sector in Ethiopia.
Moreover, tetracycline is widely used as growth factors in Veterinary Medicine for livestock rearing in addition to the treatment of bacterial infections. The present study has demonstrated the existence of alarmingly high level of multiple antimicrobial resistances of S. aureus. 69.2% of the isolated S. aureus developed MDR. This result is in line with Daka et al. (2012) who reported 62.8% multidrug resistance rate of S. aureus isolates from cow’s milk in Hawassa town which is located in southern Ethiopia.
Chao et al. (2007) similarly reported a higher multidrug resistance rate (79%) of S. aureus. This is also comparable with findings of Sharma et al. (2011) who reported 70% MDR S. aureus from raw milk of dairy cattle in India. A special attention must be given to the high prevalence of multidrug resistant S.aureus indicated in the present study area among dairy goat and camel which has great risk for consumers and individuals who have contact with animals. If these strains do cause diseases, treatment with the antibiotics that they are resistant to might not be useful. It is also a concern if those isolates get transmitted to humans and cause disease (Zunita et al., 2008).
The prevalence of S. aureus in camel and milk is found to be 6.45 and 13.34% in Somali region of Ethipia. A large proportion of the isolates were resistant to three or more antibiotics which showed the existence of MDR S. aureus in the husbandry. Resistance to Vancomycin is shown in some S. aureus isolates. Thus, it needs an attention since Vancomycin is one of the last choice in the treatment of S. aureus infections. The antimicrobial resistance tests carried out in this study indicated the high resistance of Staphylococcus species to nalidistic acid, polymixin B and penicillin G. The high level of MDR S. aureus needs further investigation based on molecular methods to study its impacts and dynamics of genetic antibiotic determinants.
The authors have not declared any conflict of interests.
REFERENCES
|
Abdurahman OASh (2006). Udder health and milk quality among camels in the Errer valley of eastern Ethiopia. Livestock Res. Rural Dev. 18:3-11.
|
|
|
|
Abera M, Abdi O, Abunna F, Megersa B (2010). Udder health problems and major bacterial causes of camel mastitis in Jijiga, Eastern Ethiopia: implication for impacting food security. Trop. Anim. Health Prod. 42:341-347.
Crossref
|
|
|
|
|
Adesiyun A (1994). Characteristics of Staphylococcus aureus strains isolated from bovine mastitic milk: Bacteriophage and antimicrobial agent susceptibility and enterotoxigenecity. J. Vet. Med. 42:129-139.
Crossref
|
|
|
|
|
Adwan G, Abu-Shanab B, Adwan K (2005). Enterotoxigenic Staphylococcus aureus in raw milk in North of Palestine. Turk. J. Biol. 29: -229-232.
|
|
|
|
|
Alamin MA, Alqurashi AM, Elsheikh AS, Yasin TE (2013). Mastitis incidence and bacterial causative agents isolated from lactating camel (Camelus dromedaries). IOSR J. Afr. Vet. Sci. 2(3):07-10.
|
|
|
|
|
Alian F, Rahimi E, Shakerian A, Momtaz H, Riahi M, and Momeni M (2012). Antimicrobial Resistance of Staphylococcus aureus Isolated from Bovine, Sheep and Goat Raw Milk. Glob. Vet. 8(2):111-114.
|
|
|
|
|
Bergey's Manual of Systematic Bacteriology (2009). Second Edition, Volume Three. The Firmicutes ISBN: 978-0-387-95041-9 Springer Dordrecht Heidelberg London New York.
|
|
|
|
|
Chao G, Zhou X, Jiao X (2007). Prevalence and antimicrobial resistance of foodborne pathogens isolated from food products in China. Foodborne Pathog. Dis. 4:277-284.
Crossref
|
|
|
|
|
CLSI (2008). Performance Standards for Antimicrobial Disk and Dilution Susceptibility Tests for Bacteria Isolated From Animals. Clin. Lab. Stand. Inst. 28:M31-A3.
|
|
|
|
|
Daka D, G/silassie S, Yihdego D (2012). Antibiotic-resistance Staphylococcus aureus isolated from cow's milk in the Hawassa area, South Ethiopia. Ann. Clin. Microbiol. Antimicrob. 11:26.
Crossref
|
|
|
|
|
Ebrahim R, Forough A (2013). Presence of enterotoxigenic Staphylococcus aureus in cow, camel, sheep, goat, and buffalo bulk tank milk. Vet. Arch. 83(1):23-30.
|
|
|
|
|
Enright MC (2003). The evolution of resistant pathogen the case of MRSA. Curr. Opin. Pharmacol. 3:474-479.
Crossref
|
|
|
|
|
Husein A, Haftu B, Hunde A, Tesfay A (2013). Prevalence of camel (Camelus dromedaries) mastitis in Jijiga Town, Ethiopia. Afr. J. Agric. Res. 8(24):3113- 3120.
|
|
|
|
|
Landin H (2006). Treatment of mastitis in Swedish dairy production (in Swedish with English summary). Svensk Veterinärtidning 58:19-25.
|
|
|
|
|
Le loir Y, Baron F, Guatier M 2003. Staphylococcus aureus and food poisoning. Genet. Mol. Res. 2:63-76.
|
|
|
|
|
Lowy FD (1998). Staphylococcus aureus infections. N. Engl. J. Med. 339:520-532.
Crossref
|
|
|
|
|
MedveÄová A, Valík Ľ (2012). Staphylococcus aureus: Characterisation and Quantitative Growth Description in Milk and Artisanal Raw Milk Cheese Production.
Crossref
|
|
|
|
|
Mekuria A, Asrat D, Woldeamanuel Y, Tefera G (2013). Identification and antimicrobial susceptibility of Staphylococcus aureus isolated from milk samples of dairy cows and nasal swabs of farm workers in selected dairy farms around Addis Ababa, Ethiopia. Afr. J. Microbiol. Res. 7(27): 3501-3510.
|
|
|
|
|
Molla B, Wakwoya A, Belihu K, Josef K, Hildebrandt G (2006). A Cross-Sectional Study on the Prevalence, Antimicrobial Susceptibility Patterns, and Associated Bacterial Pathogens of Goat Mastitis in Adami Tulu Oromia Ethiopia. Int. J. Appl. Res. Vet. Med. 4(2):169.
|
|
|
|
|
Payne DN, Wood JM (1974). The incidence of enterotoxin production in strains of Staphylococcus aureus isolated from food. J. Appl. Bacteriol. 37(3): 319-325.
Crossref
|
|
|
|
|
Rota C, Yanguela J, Blanco D, Carraminana JJ, Arino A, Herrera A (1996). High prevalence of multiple resistances to antibiotics in 144 Listeria isolates from Spanish dairy and meat products. J. Food Prot. 59:938-943.
|
|
|
|
|
Sharma D, Sharma PK, Malik A (2011). Prevalence and Antimicrobial Susceptibility of Drug Resistant Staphylococcus aureus in Raw Milk of Dairy Cattle. Int. Res. J. Microbiol. 2:466-470.
|
|
|
|
|
Tafesse B (2001). Studies on Cephalopinatitillator, the case of 'Senegal' in camels (Camelus dromedarius) in semi-arid areas of Somali state, Ethiopia. Trop. Anim. Health Prod. 33:489-500.
Crossref
|
|
|
|
|
Tariku S, Jemal H, Molalegne B (2011). Prevalence and susceptibility assay of Staphylococcus aureus isolated from bovine mastitis in dairy farms in Jimma town South West Ethiopia. J. Anim. Vet. Adv. 10:745-749.
Crossref
|
|
|
|
|
Thaker HC, Brahmbhatt MN, Nayak JB (2013). Isolation and identification of Staphylococcus aureus from milk and milk products and their drug resistance patterns in Anand, Gujarat. Vet World. 6(1):10-13.
Crossref
|
|
|
|
|
Tofaily YI, Kh AL-M, Alrodhan AN (2011). Study on Clinical Mastitis (Bacteriological) in She-Camels(Camelus dromedarius) in Some Areas of Middle Euphrates in Iraq. J. Vet. Med. Sci. 10:2.
|
|
|
|
|
Zunita Z, Bashir A, Hafizal A (2008). Occurrence of Multidrug Resistant Staphylococcus aureus in horses in Malaysia. Vet. World 1(6):165-167.
|
|