ABSTRACT
This study determines prevalence of methicillin-resistant S. aureus from bovine mastitis in dairy cows from October 2012 to June 2013 in Bishoftu town, Ethiopia. In this cross-sectional study, 16 dairy farms were included and 384 lactating cows sampled. Clinical examination and California mastitis test (CMT) were performed to diagnose clinical and subclinical mastitis, respectively. Milk samples were obtained from the quarters that reacted positively to California mastitis test and cultured to isolate S. aureus. Antimicrobial sensitivity test was conducted on the isolates using antibiotics including penicillin G (10 IU), amoxicillin (25 μg), streptomycin (10 μg), erythromycin (15 μg), oxacillin (1 μg), chloroamphenicol (30 μg), vancomycin (30 μg) and ampicillin (10 μg), all from Oxoid and tetracycline (30 μg) and gentamicin (10 μg) with agar diffusion technique. Data were analyzed using Statistical Package for Social Science. Results reveal that of the 384 milk samples collected and subjected to CMT examination, 177(46.09%) were found to be mastitis positive, of which 23(12.99%) and 154(87%) showed clinical and subclinical mastitis, respectively. Of the 177(46.09%) mastitis positive cases, S. aureus was isolated in 110(28.65%) samples. The antibiotic susceptibility test indicated that the highest resistance was observed for penicillin (100%) followed by oxacillin (65.45%), erythromycin (61.82%) and amoxacillin (59.09%). There was a statistically significant difference (P<0.05) between S. aureus prevalence and risk factors (age and lactation stage). This study indicates that there is high existence of methicillin-resistant S. aureus in dairy cows. Therefore, public awareness on transmission, prevention and control of methicillin-resistant S. aureus is suggested.
Key words: Bishoftu, dairy cows, methicillin-resistant Staphylococcus aureus.
Staphylococcus aureus is a versatile and dangerous pathogen in both humans and animals. It causes skin infections, fatal septicaemia, pneumonia and food poisoning as well as life threatening postsurgical infections. In animals, S. aureus is the most notorious pathogen that causes mastitis in dairy cows (Salyers and Whitt, 2002; Butaye et al., 2007). It is highly resistant to adverse environmental conditions and it resists drying as well as high sodium chloride concentrations. This enables a probably temporary and even permanent colonization of skin and nasal mucosa (Ivanka and Vladimir, 2008).
More than 80% of S. aureus strains produce penicillinases and thus beta-lactam antibiotics such as methicillin, which are resistant to penicillinases are widely used to treat S. aureus infections (Armand-Lefevre et al., 2005). One basic reason for the continuing important role of S. aureus in disease is its propensity to become resistant to antimicrobials. The introduction of penicillin had a profound effect on staphylococcal infections, but penicillin resistance soon followed. Similarly, after the introduction of new antimicrobials such as methicillin, it was not long before methicillin-resistant S. aureus (MRSA) developed (Abraham et al., 2007).
MRSA, initially described in the 1960s, is now endemic in many hospitals settings, veterinary hospitals and clinics and may account for up to 30% of the staphylococcal infections. MRSA is S. aureus strain that is resistant to all classes of beta-lactam antimicrobials, including penicillin, cephalosporin, oxacillin, ampicillin and amoxicillin (Bjorland et al., 2001). Staphylococcal antibiotic resistance has been associated with resistant plasmids that have the ability to mediate the production of drug inactivating enzymes such as beta-lactamases (Hiramatsu et al., 2014) and other functions (King et al., 2006; Diep et al., 2008). MRSA is not only resistant to methicillin but also different antibiotics. This resistance is mediated by the acquisition of Staphylococcal cassette chromosome mec (SCCmec) encoding for meticillin-resistance gene, mecA (Diep et al., 2008).
S. aureus isolates of human and animals have been extensively analyzed with respect to their virulence patterns and clonal relatedness in developed countries but there is only sparse information on the molecular diversity of S. aureus isolates of human from Africa (Schaumburg et al., 2011). In particular, little is known about S. aureus infection and asymptomatic carriers in human and animal in Ethiopia except pronounced reports on the effect of S. aureus infection as a major cause of bovine mastitis which resulted in great economic loses (Mekonnen et al., 2005).
S. aureus remains one of the most significant organisms associated with clinical and subclinical bovine mastitis worldwide. It is evidenced that these infections respond poorly to therapy with antimicrobial agents (Vintov et al., 2003; Rene et al., 2008). Moreover, the public health of this issue is of great importance because antibiotic therapy of infectious diseases in animals poses risk of selection of resistant strains and introduction of these strains into the food chain (Lee, 2003).
Determination of susceptibility or resistance of strains to antibiotics is very important from a clinical and economic point of view. Moreover, it is crucial to conduct a surveillance of S. aureus in neglected sub-Saharan Africa in order to pave way for the control of development and spread of antibiotic resistance strains with special emphasis on MRSA. Therefore, this study was carried out to estimate the prevalence of MRSA from bovine mastitis, assess antibiogram profile of S. aureus to methicillin group of antibiotics and identify associated potential risk factors.
Study area
The study was conducted in Bishoftu town, East Shoa Zone of Oromia Regional State, Ethiopia, which is located at 47 km south east of the capital, Addis Ababa. The area ranges from 9°N latitude and 4°E longitude at an altitude of 1850 m above sea level in the central highlands of Ethiopia. The mean annual rainfall of the area is 866 mm, with mean relative humidity of 61.3% and daily mean maximum and minimum temperatures of 26 and 14°C, respectively of which 84% is in the long rain season (June to September). The dry season extends from October to February (NMSA, 2012).
Study animals
The study animals included cross bred lactating dairy cows found in purposively selected dairy farms in Bishoftu town, comprising age groups of 3 to 9 years and above.
Sample size determination
Sample size was calculated according to the formula of Thrusfield (2005). Accordingly, the total numbers of sample required for this study were 384 (dairy cows screened for mastitis, both clinical and subclinical), taking into account expected prevalence of 50% as no study has been conducted on isolation and identification of MRSA in the study area with 95% confidence interval and 5% level of precision.
Where, n = required sample size; Pexp= expected prevalence; d= precision of the sample estimate. For eventual selection of the individual study animals, 16 study dairy farms were identified with purposive type of sampling method and on the basis of the ease of accessibility, convenience and willingness of the farm owners. The selected dairy farms were clustered into large scale (farms with herd size of 50 and above dairy cows) and small scale or small holders (farms with herd size less than 50 dairy cows). Accordingly, only 3 large scale and 13 small scale dairy farms were identified.
Thus, the calculated 384 lactating cross breed dairy cows were sampled using simple random sampling methods from the 16 dairy farms. For the sake of confidentiality, all the 16 selected dairy farms were designated with alphabetic letters A to P. The alphabetic letters B, G and L represent the large scale groups while the rest 13 represent small scale farms. Moreover, lactating dairy cows were grouped into three categories on the basis of their lactation stages as early, mid and late.
Study design
A cross-sectional study design was employed to investigate the prevalence of methicillin-resistant S. aureus from both clinical and sub-clinical mastitic dairy cows in Bishoftu town.
Clinical examination of the udder
Following clinical examination, clinical mastitis was diagnosed based on visible and palpable signs (hard and swollen quarter, kicking on touching the udder and heat) as previously described by Kivaria et al. (2007). In addition, milk from each quarter was withdrawn and examined for any change (watery secretions, clots in milk and blood-tinged secretions). The size and consistency of mammary quarters were inspected for the presence of any anatomical malformation, such as disproportional symmetry, swelling, firmness and blindness (Quinn et al., 1999).
California mastitis test
The California mastitis test (CMT) was conducted to diagnose the presence of subclinical mastitis. This screening test was performed according to the procedure given by Quinn et al. (2002). Briefly, a squirt of milk, about 2 ml from each quarter was placed in each of the four shallow cups in the CMT paddle. An equal amount of the commercial CMT reagent was added to each cup. A gentle circular motion was applied to the mixtures, in a horizontal plane for 15 s. The result was scored as 0, +1, +2 or +3 depending on the intensity of reaction. Samples with CMT result score of 0 and +1 were considered as negative, while those with a score of +2 or +3 were taken as positive and sampled for bacteriological analysis (Quinn et al., 2002).
Milk sample collection
Milk samples were collected aseptically from quarters diagnosed with CMT ≥2 and clinical cases were submitted for bacteriological examination. Briefly, the udder of the cow was thoroughly cleaned with water and dried with a clean towel. After disinfecting the teats with swabs with 70% ethyl alcohol, milk was collected. The first 3 to 4 streams of milk were discarded, and then, about 10 ml of milk was collected from each teat aseptically in separate universal bottles held at slightly horizontal position in order to avoid contamination from the udder (Singh et al., 2007). Tubes were sealed properly and transported on ice to Microbiology Laboratory of College of Veterinary Medicine and Agriculture, Addis Ababa University, where samples were immediately cultured or kept in a refrigerator at 4°C for a maximum of 24 h until cultured on standard bacteriological media (Singh et al., 2007).
Bacterial isolation and identification
Samples collected were cultured following the standard procedure given by Quinn et al. (2002). Milk samples that had been collected from CMT positive and clinical cases were refrigerated, and dispersion of bacteria and fat, were accomplished by warming the samples at room temperature (25°C) for about an hour and then mixed by shaking. The samples were allowed to stand for a while for the foam to disperse and just before inoculation, the tube was inverted gently. One standard loop (0.01 ml) of milk sample was streaked on 7% sheep blood agar. The plates were examined for gross colony morphology, pigmentation and haemolytic characteristics after 24 to 48 h incubation at 37°C. Presumptive colonies of S. aureus were selected and sub-cultured on nutrient agar (Oxoid, UK) and incubated aerobically at 37°C for 24 to 48 h. After this incubation on nutrient agar, Staphylococcus suspected colonies (based on hemolysis character and pigment production/golden yellow) were taken and subjected to Gram’s stain reaction and catalase test. Furthermore, following primary identification, biochemical tests such as tube coagulase test, mannitol and maltose fermentation were carried out to identify the bacterium to the species level (Quinn et al., 2002).
Antimicrobial sensitivity test
A total of 110 S. aureus isolated from milk sample of clinical and subclinical mastitis cases of dairy cows were subjected to antimicrobial susceptibility test. Antimicrobials used in this study were penicillin G (10 IU), amoxicillin (25 μg), streptomycin (10 μg), erythromycin (15 μg), oxacillin (1 μg), chloroamphenicol (30 μg), vancomycin (30 μg) and ampicillin (10 μg), all from Oxoid and tetracycline (30 μg) and gentamicin (10 μg) from BBL microbiology systems. The selection of the types of antimicrobial agents used was made purposely to investigate sensitivity pattern of S. aureus to methicillin group of antibiotics and antibiotics commonly used in the study of dairy farms. Oxacillin was used in the place of methicillin and the strains of S. aureus which are referred to as MRSA are usually oxacillin resistant S. aureus (ORSA). Though, methicillin and oxacillin are similar antibiotics, MRSA is the usually accepted designation and this approach was preferred in this study (Gustavo et al., 2001; Cheesbrough, 2002).
Agar disc diffusion (Kirby-Bauer method) was used as described by Quinn et al. (2002). The antibiotic disks were applied on the surface of the inoculated Mueller-Hinton agar plates using aseptic techniques. The disks were deposited with center at least 24 mm apart from each other. Each disk was pressed down with sterile forceps tip to ensure complete contact with the agar surface (Quinn et al., 2002). After measuring the zone of inhibition, isolates were classified sensitive, intermediate and resistant. National Committee for Clinical Laboratory Standard (NCCLS) breakpoints was used to interpret the inhibition zone as adapted from Quinn et al. (1994).
Data management and analysis
All obtained data were stored in Microsoft Excel spreadsheet and analyzed using appropriate statistics. While sampling, animal data related to age, lactation stage, names of the dairy farms and type of antibiotics commonly used in the dairy farms were recorded with proper data collection format. Depending on the findings of clinical inspection and CMT results, cases were categorized as either positive or negative and then positive cases were taken to the laboratory for investigations. The age of the study animals was determined from birth records and categorized as young adults (≥ 3 to 5 years), adults (> 6 to ≥ 9 years) and old (> 9 years). The lactation period of lactating dairy cows were obtained from birth records and owners witnessed and categorized them as early lactation (≤3 months), middle lactation (4 to ≤6) and late lactation periods (> 6 months). Logistic regression was used to show the association of the potential risk factors with occurrence of S. aureus using SPSS version 20 statistical software. The final model was fit using step wise logistic regression. Pearson’s Chi-square test was used to analyze the proportions of categorical data. In all the analysis, the level of significance was set at 5%.
Out of the 384 milk samples collected from cross breed lactating cows and subjected to CMT test, 177 (46.09%) were found to be mastitis positive, of which 23 (12.99%) and 154 (87.00%) showed clinical and subclinical mastitis, respectively. Similarly, out of the 177 (46.09%) CMT positive mastitis quarters, S. aureus was isolated in 110 samples with rate of 62.15% positivity. However, the prevalence of S. aureus among the examined 384 cross bred lactating cows is only 110 (28.65%) (Table 1). The prevalence of S. aureus for the age and lactation period of ≤ 5 years and ≤ 3 months, >6 to ≤ 9 years and ≤ 3 months, and > 9 years and ≤ 3 months were 12.5, 28.6 and 20%, respectively.
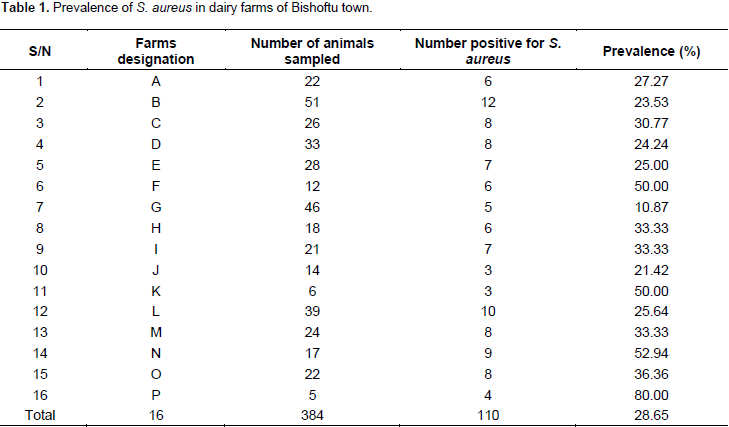
Antimicrobial susceptibility test result
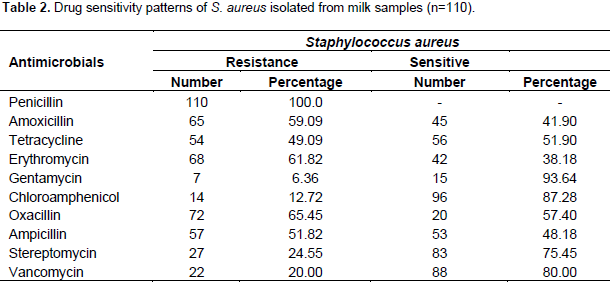
A total of 110 isolates of S. aureus originating from milk samples were tested for susceptibility to methicillin group of antibiotics and other commonly used antimicrobials in the study area. Accordingly, the antimicrobial susceptibility test result showed that S. aureus was highly resistant only to four of the ten antibiotics, specifically, penicillin (100%), oxacillin (65.45%), erythromycin (61.45%) and amoxicillin (59.09%) (Table 2).
Associated risk factors
The prevalence of S. aureus as opposed to specific risk factors was investigated using Chi-square test. There was a statistically significant difference (P<0.05) between the prevalence of S. aureus and risk factors, namely, age and lactation stage. For instance, older cows more than 9 years of age were noticed to be more affected (48%) than adult cows between 6 and 9 years (29.8%). The prevalence of S. aureus on the basis of different lactation stages showed that highest figure was recorded during late stage of lactation (38.1%) followed by mid lactation (21.4%) and early lactation (20%). The above variation in the prevalence of S. aureus on the basis of different lactation stages was statistically significant (P<0.05) (Table 3 and Figure 1).
The present study addressed the status of MRSA from bovine mastitis in Bishoftu town. In line with this, S. aureus isolates were obtained and tested for antimicrobial susceptibility test by using methicillin group of antibiotics. Moreover, potential risk factors associated with mastitis caused by S. aureus were assessed. The rate of isolation of S. aureus from mastitic milk was found to be 28.65%. The isolation rate of S. aureus in this work is slightly similar to that of Hundera et al. (2005) who isolated S. aureus at the prevalence rate of 29.20%.
However, the prevalence rate of S. aureus in the current study was lower than that of the findings of Lakew et al. (2009), Abera et al. (2010), Mekibib et al. (2010) and Sori et al. (2011) in which the isolation rate of S. aureus was 41.4, 42.10, 47.50 and 39.44%, respectively. The relatively high prevalence of S. aureus in previous studies can most likely be attributed to the wide distribution of the organism inside the mammary glands and on the skin of teats and udder (Jones et al., 1998). It is well known that S. aureus has already adapted survival in the udder and can establish chronic and subclinical infections. From there, it is shed into the milk, which serves as source of infection for healthy cows during the milking process (Takele et al., 2017). S. aureus is one of the contagious pathogens that can be easily transmitted from one cow to another during unhygienic milking process (Rowe, 1999).
The findings of the present study (28.65%) was found to be much different from that of Mekonnen et al. (2005) who reported 8% prevalence of S. aureus. It was also greater than the reports of Bitew et al. (2010), Salihu et al. (2011), Daka et al. (2012) and Fufa et al. (2013) who identified 20.3, 22.8, 17.9 and 21.13%, respectively. The difference in the prevalence of S. aureus between the present study and the previous reports could be due to the variation in the hygienic and management systems of the dairy farms or increase in the spread and abundance of S. aureus with time.
The antibiotic susceptibility assay performed for S. aureus isolates from clinical and subclinical mastitis cases in dairy farms from Bishoftu town, revealed higher degree of resistance for penicillin (100%), oxacillin (65.45%), erythromycin (61.45%), amoxicillin (59.09%) and ampicillin (51.82%). The present study also showed that gentamycin, vancomycin and streptomycin were found to be effective antibiotics against S. aureus. This present report is similar to the findings of Addisalem and Mersha (2012) and Sori et al. (2011). In fact, these antibiotics are effective against Gram negative bacteria. However, the findings of this study showed gentamycin, vancomycin and streptomycin to be effective against S. auerus. Probably, these drugs are less circulated in dairy farms of the study area with relatively less chance of resistance development.
Among others, the resistance pattern of penicillin was also in agreement with the reports of Abera et al. (2010) and Shiferaw et al. (2009). The 51.82% resistance recorded for ampicillin in the present study was quite lower than that of Corrales et al. (1995) and Mekonnen et al. (2005) who found 75 and 83% resistance, respectively. This difference in the resistance pattern of S. aureus isolates to ampicillin between the current and previous reports may be associated with the infrequent and or frequent use of the drug in the study area. It has also been documented that MRSA isolates that are resistant to beta-lactam antibiotics may induce cross resistance to vancomycin as stated by Gundogan et al. (2005). In the present study, all the S. aureus isolates originating from milk were resistant to beta-lactam group of antibiotics.
Regarding the risk factors, age of the animals and stage of lactation were found to be statistically significant (P<0.05) with prevalence of S. aureus. The prevalence of S. aureus in the current study showed an increasing pattern of 20, 21.4 and 38.1%, in early, mid and late lactation stages, respectively. Similar increasing prevalence pattern were also reported by Moges et al. (2012) and Mungube et al. (2005) where higher prevalence of S. aureus was observed in mid and late as compared to early stages of lactation. This could be attributed to prolonged risk of exposure to pathogens and contact of the dairy cows with other animals though in different lactation stages. Moreover, it is possible for the dairy cows to acquire S. aureus infection from different dairy personnel working in the farms. Similarly, the prevalence of S. aureus was noted to increase while age advances.
For instance, the prevalence of S. aureus was 20.2, 29.8 and 45% for age groups ≤ 5, > 6 to ≤9 and > 9 years of animals, respectively. Obviously, similar justifications specifically: prolonged risk of exposure to pathogens and contact of the dairy cows with other animals could be cited for increasing prevalence of S. aureus with age. The present study on S. aureus infection in dairy cows is significantly important with a higher prevalence of 28.6%. Accordingly, S. aureus infection was pointed out to be one of the major dairy animals’ health problems in Bishoftu town in particular, and Central Ethiopia at large. The wide spread distribution of S. aureus imposed greater resistance to methicillin group of antibiotics in dairy cows.
The resistance of S. aureus to multiple drugs belonging to methicillin group, especially penicillin, ampicillin, erythromycin and oxacillin calls for serious and immediate public attention in raw milk consumption. On the contrary, few antibiotics namely, vancomycin, gentamycin, chloroampenicol and streptomycin were observed to be effective against S. aureus, where proper and ethical use of these drugs might help in the control and limitation of resistance to S. aureus. The current work findings also disclosed that the prevalence of S. aureus increased as age and lactation period of the dairy cows increased. Therefore, there is an urgent need to create public awareness at different levels, on transmission, prevention and control of MRSA. It is very important to implement a systematic application of an in vitro antibiotic susceptibility test prior to the actual use of antibiotics both in therapeutic as well as prophylactic schemes. Farm level hygienic and improved management practices should be introduced to small and mid-scale dairy farms. Impact and dynamics of genetic antibiotic resistance determinants should also be investigated. Specific strain(s) of MRSA should be identified and characterized, and thus, the responsible zoonotic strain(s) could be known.
The authors have not declared any conflict of interests.
REFERENCES
|
Abera M, Demie B, Aragaw K, Regassa F, Regassa A (2010). Isolation and identification of Staphylococcus aureus from bovine mastitic milk and their drug resistance patterns in Adama town, Ethiopia. J. Vet. Med. Anim. Health 2:29-34.
|
|
|
|
Abraham J, Morris D, Griffeth G, Shofer F, Rankin S (2007). Surveillance of healthy cats and cats with inflammatory skin disease for colonization of the skin by methicillin-resistant coagulase-positive staphylococci and Staphylococcus schleiferi subspies schleiferi. Vet. Dermatol. 18:252-259.
Crossref
|
|
|
|
|
Addisalem T, Mersha C (2012). Study on the Occurrence of Bovine Mastitis in Addis Ababa Dairy Farms and Associated Risk Factors. Adv. Biol. Res. 6(4):151-158.
|
|
|
|
|
Armand-Lefevre L, Ruimy R, Andremont A (2005). Clonal comparison of Staphylococcus aureus isolates from healthy pig farmers, human control and pigs. Emerg. Infect. Dis. 11:711-714.
Crossref
|
|
|
|
|
Bitew M, Tafere A, Tolosa T (2010). Study on bovine mastitis in dairy farms of Bahir Dar town and its environs. J. Anim. Vet. Adv. 9: 2912-2917.
Crossref
|
|
|
|
|
Bjorland J, Sunde M, Waage S (2001). Plasmid-borne smr gene causes resistance to quaternary ammonium compounds in bovine Staphylococcus aureus. J. Clin. Microbiol. 39:3999-4004.
Crossref
|
|
|
|
|
Butaye P, Struelens M, Vanholme L (2007). Methicillin Resistant S. aureus. Report on Zoonotic Agents in Belgium; Trends and sources by working group on food born infection and intoxications, pp. 59-61.
|
|
|
|
|
Cheesbrough M (2002). District Laboratory Practice in Tropical countries: Cambridge: Cambridge University press: England 2:225-248.
|
|
|
|
|
Corrales J C, Counteras A, Sierra D, Marco J C (1995). Sensibilidad antibiotica invitro de estaflococos y corinebacterias aisladas de mamitis subclinicas caprinas. Med. Vet. 12:16-24.
|
|
|
|
|
Daka D, Solomon G, Dawit Y (2012). Antibiotic-resistance Staphylococcus aureus isolated from cow's milk in the Hawassa area, South Ethiopia. Ann. Clin. Microbiol. Antimicrob. 11:26.
Crossref
|
|
|
|
|
Diep BA, Chambers HF, Graber CJ, Szumewski JD, Miller LG, Han LL, Chen J H, Lin F (2008). Emergence of multi-drug-resistant community associates methicillin-resisitant Staphylococcusaureus. Clone USA 300 in Men who have sex with men. Ann. Intl. Med. 148:1-17.
Crossref
|
|
|
|
|
Fufa A, Gemechis F, Bekele M, Alemayehu R (2013). Bovine Mastitis: Prevalence, Risk Factors and Bacterial Isolation in Small-Holder Dairy Farms in Addis Ababa City, Ethiopia. Glob. Vet. 10:647-652.
|
|
|
|
|
Gundogan N, Citak S, Yucel N, Devren A (2005). A note on the incidence and antibiotic resistance of Staphylococcus aureus isolated from meat and chicken samples. Meat Sci. 69:807-810.
Crossref
|
|
|
|
|
Gustavo PK, Marines DV, Igor MM (2001). High frequency of colonization and absence of identifiable risk factors for MRSA in intensive care unit in Brazil. Braz. J. Infect. Dis. 5:1-7.
Crossref
|
|
|
|
|
Hiramatsu K, Katayama Y, Matsuo M, Sasaki T, Morimoto Y, Sekiguchi A, Baba T (2014). Multi-drug-resistant Staphylococcus aureus and future chemotherapy. J. Infect. Chemother. 20:593-601.
Crossref
|
|
|
|
|
Hundera S, Ademe Z, Sintayehu A (2005). Dairy cattle mastitis in and around Sebeta, Ethiopia. Intl. J. Appl. Vet. Med. 2:1525-1530.
|
|
|
|
|
Ivanka M, Vladimir J (2008). Current knowledge of methicillin-resistant staphylococcus aureus and community-associated methicillin-resistant Staphylococcus aureus. Biomedical Papers 152:191-202.
Crossref
|
|
|
|
|
Jones GM, Bailey TL, Roberson JR (1998). Staphylococcus aureus mastitis: cause, detection and control. Virginia Cooperative Extension, Pub. Num. Virginia pp. 404-229.
|
|
|
|
|
King MD, Humphrey BJ, Wang YF, Kourbalova EV, Ray SM, Blumbrg HM (2006). Emergence of community acquired methicillin-resistant Staphylococcus aureus USA 300 clone as the predominant cause of skin and soft tissue infections. Ann. Intl. Med. 144:309-317.
Crossref
|
|
|
|
|
Kivaria F M, Noordhuizen J P, Msami H M (2007). Risk factors associated with the incidence rate of clinical mastitis in smallholder dairy cows in the Dares Salaam region of Tanzania. Vet. J. 173:623-629.
Crossref
|
|
|
|
|
Lakew M, Tolosa T, Worku T (2009). Prevalence and major bacterial causes of bovine mastitis in Asella, South eastern Ethiopia. Trop. Anim. Health Prod. 41:1525-1530.
Crossref
|
|
|
|
|
Lee HL (2003). Methicillin (oxacillin)-resistant Staphylococcus aureus strains isolated from major food animals and their potential transmission to humans. Appl. Environ. Microbiol. 69:6489-6494.
Crossref
|
|
|
|
|
Mekibib B, Furgasa M, Abunna F, Megersa B, Regassa A (2010). Bovine Mastitis: Prevalence, Risk Factors and Major Pathogens in Dairy Farms of Holeta Town, Central Ethiopia. Vet. World 3:397-400.
Crossref
|
|
|
|
|
Mekonnen H, Workineh S, Bayleyegn M, Moges A, Tadele K (2005). Antimicrobial susceptibility profiles of mastitis isolates from cows in three major Ethiopian dairies. Med. Vet.176:391-394.
|
|
|
|
|
Moges N, Asfaw Y, Belihu K, Tadessa A (2012). Antimicrobial Susceptibility of Mastitis Pathogens from Smallholder Dairy Herds in and around Gondar, Ethiopia. J. Anim. Vet. Adv. 10:1616-1622.
|
|
|
|
|
Mungube EO, Tenhagen BA, Regassa F, Kyule M, Shiferaw YN, Kassa MP, Baumann L (2005). Reduced Milk Production in Udder Quarters with Subclinical Mastitis and Associated Economic Losses in Crossbred Dairy Cows in Ethiopia. J. Trop. Anim. Health Prod. 37:503-512.
Crossref
|
|
|
|
|
National Meteorological Services Agency of Ethiopia (NMSA)(2012). Rainfall and temperature data of Debre Zeit, National Metrological Service Agency, Addis Ababa, Ethiopia.
|
|
|
|
|
Quinn PJ, Carter ME, Marke BK, Carter GR (1999). Clinical Veterinary Microbiology. Mosby International Limited, London, pp. 254-266.
|
|
|
|
|
Quinn PJ, Carter ME, Markey BK, Carter GR (1994). Mastitis: In Clinical Veterinary Microbiology. Mosby International Limited, London, pp. 327-344.
|
|
|
|
|
Quinn PJ, Markey BK, Carter ME, Donelly WJ, Leonard FC (2002). Bacterial cause of bovine mastitis.Veterinary Microbiology and Microbial Diseases, Blackwell Science Ltd, a Blackwell publishing company, pp. 465-475.
|
|
|
|
|
Rene SH, Dik JM, Andreas S, Christopher T, Danièle M, Patrick B, Alessia F, Andra U, Alice A, Miguel M, Christina G, Katharina S, Christian B, Anna L M, Dariusz W, Marianne S, Frank MA (2008). Prevalence of antimicrobial resistance among bacterial pathogens isolated from cattle in different European countries: 2002–2004. Acta Veterinaria Scandinavica. 50:28.
Crossref
|
|
|
|
|
Rowe JD (1999). Milk quality and mastitis. Small Ruminants for Mixed Practitioner. Western Vet. Conference. Lasvagas, pp. 152-156.
|
|
|
|
|
Salihu MD, Junaidu AU, Tambuwal FM, Magaji AA, Jaafaru S (2011). Prevalence of Mastitis in lactating cows in some selected commercial dairy farms in Sekoto Metropolis. Adv. Appl. Sci. Res. 2:290-294.
|
|
|
|
|
Salyers AA, Whitt, DD (2002). A molecular approach Bacterial pathogenesis, 2nd edn. Washington, DC: ASM Press pp. 53-100.
|
|
|
|
|
Schaumburg F, Ateba-Ngoga U, Kosters K, Kock R, Adegnika A A, Kremsner P A, Lell B, Peters G, Mellmann A, Becker K (2011). Virulence factors and genotypes of Staphylococcus aureus from infection and carriage in Gabon. Clin. Microbiol. Infect. 17:1507-1513.
Crossref
|
|
|
|
|
Shiferaw A, Ejeta G, Asfaw Y (2009). Bacterial causes of septicaemia and antibiogram profile in cattle from Debre Zeit, Ethiopia. Rev. de. Med. Vet. 160:204-208.
|
|
|
|
|
Singh R, Singh KP, Chauhan RS (2007). A text book of diagnostic pathology. Centre for animal disease research and diagnosis, Indian Veterinary Research Institute, pp. 109-112.
|
|
|
|
|
Sori T, Hussein J, Bitaw M (2011). Prevalence and Susceptibility Assay on Staphylococcus aureus iolated from Bovine Masititis in dairy Farms of Jimma Town, South west Ethiopia. J. Anim. Vet. Adv. 10:745-749.
Crossref
|
|
|
|
|
Takele B, Halefom H, Fikru G, Ashenafi FB, Fufa A, Bedasso M, Dinka A, Hika W, Reta DA (2017). Determination of the sources and antimicrobial resistance patterns of Salmonella isolated from the poultry industry in Southern Ethiopia. BMC Infect. Dis. 17(1):352.
Crossref
|
|
|
|
|
Thrusfield M (2005). Veterinary Epidemiology.3rd edition Blackwell Science Ltd. UK., pp. 233-250.
|
|
|
|
|
Vintov J, Aarestrup FM, Zinn CE, Olsen JE (2003). Association between phage types and antimicrobial resistance among bovine Staphylococcus aureus from 10 countries. Vet. Microbiol. 95:133-147.
Crossref
|
|