ABSTRACT
The present study was conducted to determine the seroprevalence of yellow fever virus in patients with clinical signs of febrile jaundice giving suspicion of yellow fever and to evaluate diagnostic techniques for surveillance of yellow fever in the Republic from Chad. From January 2015 to July 2020, an observational study of virological markers was conducted in jaundice patients in Chad. Of the 1730 patients included in this study, a seroprevalence of 0.28% of yellow fever virus was determined. The distribution of pathogens responsible for diseases associated with yellow fever was: 49.47% (Plasmodium falciparum), 2% (hepatitis E virus), 4.62% (hepatitis C virus), and 29.00% (hepatitis virus B), respectively. Discrepancies in the results between the regional reference laboratories and the national laboratory of the Republic of Chad were observed. All genders and age groups were affected. Of the 1730 samples taken, 55.49% were female and 44.51% male (p = 0.01, a significant difference in favor of the female sex). The male/female sex ratio was 1.25. This study, the first, made it possible to determine the rate of the yellow fever virus in the absence of an outbreak in forest areas and with heavy rainfall and to evaluate the MAC-ELISA-CDC techniques used between the national laboratory of the Republic of Chad and those regional references. It was recommended that real-time polymerization chain reaction techniques be made available to national laboratories and reagents from the same manufacturing company in order to carry out effective monitoring of yellow fever.
Key words: Evaluation, surveillance, seroprevalence, techniques, yellow fever.
Yellow fever (YF), sometimes called black vomit, American plague, fever or typhus amaril caused by a Flavivirus, is a serious disease for which there is currently no drug treatment (Hunsperger et al., 2016; WHO, 2016b). It is an arbovirus of equatorial forest monkeys and is transmitted from monkey to monkey by various mosquitoes; the mosquito playing the role of reservoir and vector and the monkey that of biological amplifying host (WHO, 2018). The causative agent of yellow fever is yellow fever virus, and transmission to humans is through a mosquito (Aedes aegypti) which bites mostly at dusk and dawn (Paules and Fauci, 2017; WHO, 2016b). It is a hemorrhagic fever with liver damage accompanied by jaundice, or yellowing of the skin and mucous membranes, kidneys with a tendency to bleeding and the nervous system, fatal in 20 to 60% of the cases. The number of yellow fever cases is estimated each year between 18 and 50/100,000 people living in areas at risk of transmission (WHO, 2016b). The disease exists in many countries in Africa, South America and Europe (Abeera et al., 2019; Amraoui et al., 2016).
An observational study was performed in the absence of an outbreak in patients with signs of febrile jaundice suspected of yellow fever.
The current outbreaks of yellow fever worldwide have demonstrated that rapid laboratory confirmation of suspected yellow fever cases is a key part of an effective response (WHO, 2016a). The best way to detect these outbreaks early enough is surveillance. The surveillance system that is currently in place in almost all African countries focuses on the human component only and is based on clinical examination for jaundice, blood sampling and research immunoglobulins M (IgM) by national laboratories with confirmation by a regional laboratory (Santiago et al., 2018; www.signalement-moustique.fr, 2017). This way of proceeding, although strongly recommended, remains limited because it often only gives alert when disease transmission is already well established in the community. It is probably too late for a disease such as yellow fever, which is characterized by a high proportion of asymptomatic cases (WHO, 2016b; HCSP, 2017).
Routine screening for yellow fever virus on all suspected blood samples has helped delineate areas of transmission and reduced the risk of infection with yellow fever virus. There remains, however, a residual risk of transmission of the yellow fever virus; this risk could be due to two factors: a technical error and a viral variant not recognized by certain reagents.
The aim of this study was to determine the seroprevalence of yellow fever virus in patients with signs of febrile jaundice associated with yellow fever during or in the absence of the epidemic outbreak and to assess the performance of yellow fever virus detection techniques between the national laboratory of the Republic of Chad and the regional reference laboratories of Yaoundé (Cameroon) and Dakar (Senegal).
The results of this work will allow corrective measures to be taken on yellow fever diagnostic techniques to ensure the quality of results between laboratories and set up an effective surveillance system for the fight against yellow fever in the Republic of Chad and in the sub-region.
This study was carried out in agreement with the integrated epidemiological surveillance service of the Chadian Ministry of Public Health, which through the World Health Organization provided the necessary materials for the analysis of blood samples. During this study, we insisted on the confidentiality and informed consent of the participants.
Framework of the study and progress of the work
The study took place in N’Djamena from January 2015 to July 2020 (Chad), Dakar (Senegal), Amsterdam (Holland) and Yaoundé (Cameroon):
(1) Virology Unit of the Laboratory of the National Reference University Hospital (CHU-RN) of N’Djamena (Chad);
(2) Institute Pasteur in Dakar/Senegal where all stages of molecular, immunochemical and serological diagnosis were carried out;
(3) National Laboratory of the Health District of Amtiman (DSA), Province of Salamat (Chad);
(4) Sanquin Amsterdam Laboratory of Holland (LSAH), The Netherlands where the ELISA test with IgM + / IgG + titers greater than 15 was performed on all samples tested with HEV.
(5) Pasteur Center in Yaoundé, Cameroon where all the stages of molecular, immunochemical and serological diagnosis were carried out.
Choice of samples
The screening strategy for the selection of blood samples was summarized in four markers of viral communicable diseases: yellow fever virus, hepatitis B virus, C and E. The selected blood samples were tested either on the system of immuno-serological test with VIDAS (hepatitis B virus) or immuno-chromatographic tests (hepatitis C and E viruses) for the detection of Ag/Ac and an immunochemical test for the detection of yellow fever virus (ELISA).
Inclusion and exclusion criteria
Included was anyone with clinically visible signs: yellow discoloration of the conjunctiva or yellow discoloration of the skin (suggesting jaundice) with hyperthermia at 40°C. Not included were people who did not have signs suggestive of jaundice.
Sample collection
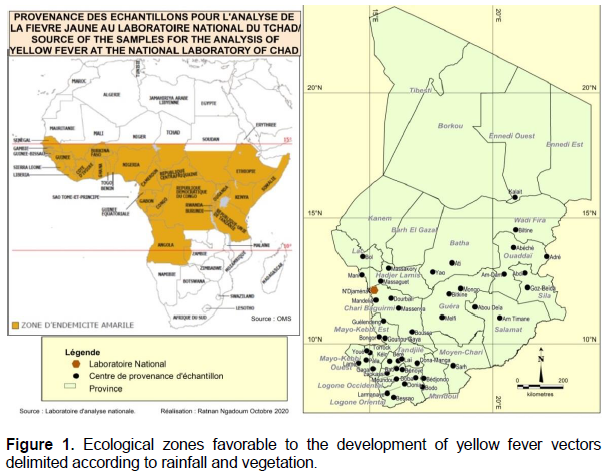
Blood samples were taken systematically from people with signs suggestive of jaundice in towns and villages in the various provinces of the country (Figure 1) after completing the identification forms.
The study population was at least 6 months old to 75 years old, from all occupations and social categories combined. Whole blood was collected in 4 mL EDTA tubes from January 2015 to July 2020 in towns and villages in the various provinces of Chad. Whole blood was collected in 4 mL EDTA tubes from January 2015 to July 2020 in towns and villages in the various provinces of Chad. 5 µL of whole blood were taken from each sample for the systematic performance of the rapid diagnostic test (RDT) for Plasmodium falciparum malaria.
The blood serum obtained after centrifugation at 3000 revolutions for 5 min was used for the detection of the HBs antigen of the hepatitis B virus with the VIDAS machine. The serum was also packaged in 1.8 mL cryotubes and stored at -80°C for shipment to regional reference laboratories for quality control. The following variables were considered in the people to be sampled such as: sex, age, fever, jaundice, hemorrhage, vaccination status (has the patient received at least one dose of yellow fever vaccine?), marital status, profession, risky behaviors (previous transfusion, surgical procedures, multiple sexual partners, etc.), the system used for serological screening, date of collection, dispatch and receipt of blood samples at the laboratory, areas at risk of yellow fever transmission (presence of mosquitoes). Analysis of these parameters provided a better understanding of the risks of transmission of YF and viral hepatitis.
Data processing
Fisher and Yates Chi-square (c²) test was used to compare qualitative variables with a significance level set at 5%.
Microbiological analysis
Presentation of the tests
One Step SD Bioline: The One Step SD Bioline rapid tests were used for the detection of hepatitis C virus (HCV) and P. falciparum.
One step anti-HCV SD bioline VHC (standard diagnostics, INC): The MP Diagnostics (MPD) Assure Device (in cassette form) is a rapid immunochromatographic test for the detection of IgM antibodies to hepatitis E virus (HEV). The test was performed according to the manufacturer’s instruction. The test is specific for HCV in human serum, plasma and whole blood. The colloidal conjugate and the serum migrate by chromatography along the membrane to the test region (T) and form an antigen-antibody-antigen complex of high sensitivity and specificity which manifests itself as a visible line. This test consists of two marks T (test line) and C (control line), neither of these lines is visible before the test. The control line is used for procedural control and should appear each time if the test is performed correctly.
HEV detection with MP Assure Device HEV IgM rapid diagnostic test: The MP Diagnostics (MPD) Assure Device (in cassette form) is a rapid immunochromatographic test for the detection of IgM antibodies to hepatitis E virus (HEV). The test was performed according to the manufacturer’s instruction. The test is specific for HEV in human serum, plasma and whole blood. The colloidal conjugate and the serum migrate by chromatography along the membrane to the test region (T) and form an antigen-antibody-antigen complex of high sensitivity and specificity which manifests itself as a visible line. This test consists of two marks T (test line) and C (control line), neither of these lines is visible before the test. The control line is used for procedural control and should appear each time if the test is performed correctly.
General principle of SD Bioline Plasmodium rapid diagnostic test: The SD Bioline Rapid Diagnostic Tests (RDTs) for malaria detect specific antigens produced by plasmodial species. These antigens are: Histidine Rich Protein 2 (HRP2), specific for P. falciparum; Lactate dehydrogenase (pLDH) and aldolase produced by all species of Plasmodiun.
Qualitative detection of P. falciparum antigen by the SD Bioline P. falciparum rapid diagnostic test: The choice for detection of P. falciparum is the fact that this species represents 98% of all Plasmodium species in Chad (ENIPT, 2017). The P. falciparum (whole blood) rapid malaria test device is a qualitative membrane test for the detection of P. falciparum in whole blood. The membrane is pre-coated with P. falciparum. During the test, the whole blood sample reacts with the dye conjugate, which has been precoated in the test strip. The mixture then migrates over the membrane by capillary action and reacts with the P. falciparum on the membrane, at the level of the test line. If the sample contains the P. falciparum antigen, a pink line appears in the test area. The absence of a pink line in the test area indicates that the sample does not contain P. falciparum. A pink line always appears in the control area; it indicates that an adequate volume of sample has been added and the membrane has been impregnated.
Qualitative determination of HBsAg by the VIDAS machine (BioMérieux)
The VIDAS machine was used for the detection of HBs antigen of hepatitis B virus. The composition and concentration of reagents for HBsAg are: S1: 1 vial containing 1 mL of HBsAg (red cap): standard calibration; C1: 1 vial containing 1.9 mL of HBsAg (white cap): control; C2: 1 vial containing 1.9 mL of HBsAg (white cap): control; Cartridge or strip of HBsAg (single use) containing 10 wells, one of which is for the sample and 9 for the conjugates; HBsAg cone (single use): AC-Ag binding.
The BioMérieux VIDAS machine was used for the detection of Antigen-HBs. A positive and negative quality control system for each run is available to validate a system test kit and internal control for each sample. Single-use, barcode, ready-to-use (reconstitution for some) reagents were used.
The software supplied with the VIDAS system includes programs for analysis and data management. A two-way computer interface automatically transfers results to the user's Laboratory Information System (LIS) and to various product and patient reports. This avoids human errors in reading the results. A quality control system is available to validate a VIDAS system test kit.
As part of the work, VIDAS HBsAg cartridges (BioMérieux) were used for the detection of HBs antigen. The VIDAS has 5 compartments. After collection of whole blood in a dry tube or lithium tube and centrifugation, 150 µl of serum was taken and transferred to the first well of the VIDAS HBsAg cartridge. The cartridge is placed in the corresponding compartment of the VIDAS. The automaton is started by clicking on the appropriate compartment and the test result is expected in 1 h and 21 min.
Qualitative determination of yellow fever virus by ELISA chain
The main test necessary for the confirmation at the national laboratory of the Republic of Chad of yellow fever in an outbreak situation or in the absence of an epidemic outbreak was the enzyme-linked immunosorbent assay (MAC-ELISA-CDC) for the detection of IgM virus, yellow fever. The test was performed following the protocol developed by the American Center for Disease Control and Prevention (CDC) enacted by the World Health Organization.
Origin of samples and mapping of areas at risk for yellow fever
Figure 1 illustrates the geographical origin of blood samples for the detection of yellow fever virus at the national laboratory in Chad. The areas at risk for yellow fever transmission are located between the 10 and 15th parallels. These are forested areas with heavy rainfall containing rivers, lakes, streams favorable to the development of mosquitoes, vectors of yellow fever and associated diseases. In these areas, the virus can circulate continuously and silently between mosquito and monkey populations. It can circulate throughout the year because climatic conditions remain favorable for mosquito activity. In these areas eco-geographic (Figure 1), the mosquito acts as a reservoir and which maintains the presence of the virus by vertical transmission.
Distribution of samples in terms of the category of persons
All sexes are affected. Of the 1730 cases seen and collected in the various health structures of the country, 55.49% (960/1730) were female and 44.51% (770/1730) male (x2 = 6.904> x20> 3, 84, p = 0.01, dof = 1), there is a significant difference in favor of the female sex. The male/female sex ratio in the present study is 1.25 (960/770). All ages were also affected with extremes of 6 days and 75 years. The most affected age group was 15 to 45 years old with 887 cases (51%). It is the working population, the most mobile and certainly the most exposed. Under 15 years (Pediatrics) represent 46% (Figure 2). Note that out of all cases, 29 pregnant women were recorded.
Distribution of proportions of the main symptoms of suspected yellow fever cases
Headache comes first, followed by fever and vomiting. This breakdown showed that the signs suggestive of jaundice are not always linked to yellow fever but associated with other infectious viral and parasitic agents (Table 1).
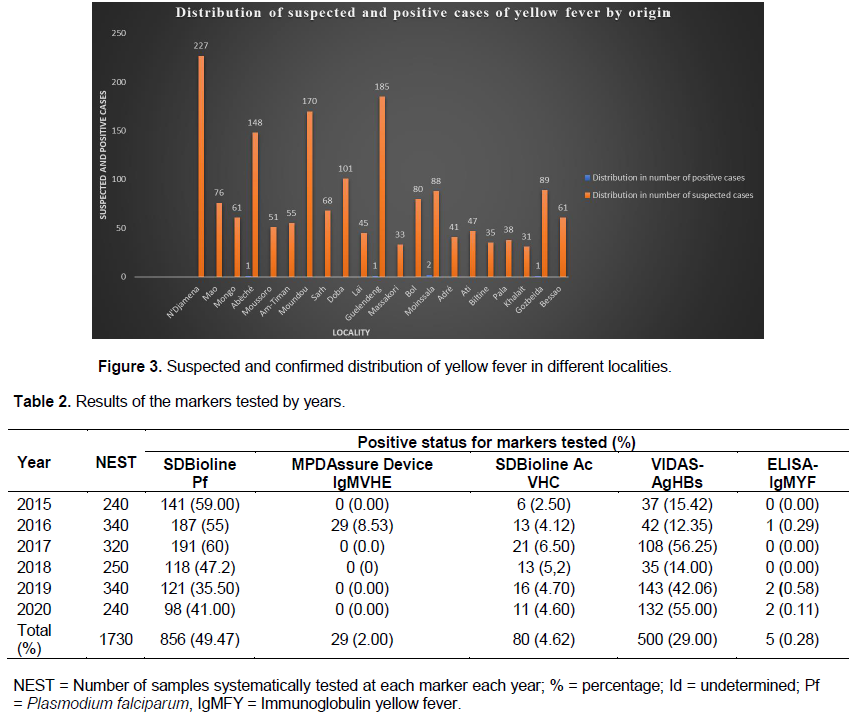
Distribution of suspected and positive cases of yellow fever by origin
Figure 3 illustrates the frequencies of receipt of suspected cases and confirmed positive cases by the National Laboratory of the Republic of Chad during the study period. The localities of Guelendeng, Laï, Moundou and N’Djamena located in the forest and marshy areas of the Republic of Chad (Figure 1), reported large numbers of suspicious samples: 185, 165, 150 and 127, respectively. The locality of Moinssalla notified two presumptive positive cases followed by Guelendeng, Laï and Bol with one positive case each (Figure 3).
Distribution of the proportions of diseases associated with yellow fever without proof of competence by year
A total, 1730 blood samples were collected for the detection of yellow fever. It emerges from this study that 5/1730 (0.28%) were detected presumptive positive for ELISA-IgMYF by the National Laboratory of Chad, 856/1730 (49.47%) positive for P. falciparum, 29/1730 (2%) positive for HEV, 80/1730 (4.62%) positive for HCV and 500/1730 (29%) for HBV, respectively (Table 2). Of the two cases of yellow fever confirmed positive (2/1730 (0.11%)) with the seroneutralization test technique by reduction of lysis plaque (PRNT), one was confirmed positive at the regional reference laboratory, Center Pasteur of Cameroon (LRRCPC) and the other by the regional reference laboratory, Institute Pasteur de Dakar, Senegal (LRRIPDS). Of the five presumptive positive yellow fever cases, four were unvaccinated and one was of unknown vaccination status. A death was recorded in two cases in the locality of Moinssala as a result of complication of the patient's disease, a case imported from Cameroon since the patient claimed to be back after spending ten years in southern Cameroon and that he was ill only two weeks after arriving in the locality of Nguelendeng in Chad (Figure 3). This proportion showed that malaria was the main cause of consultation in the centers of health facilities in Chad. In 2016, out of a total of 340 samples analyzed, 1 (0.29%) IgMYF was detected by the ELISA chain, 55.00% (malaria), 8.53% (hepatitis E), 4.12% (hepatitis C and 12.35% (hepatitis B) were associated with suspected yellow fever cases, respectively, and infectious markers of hepatitis B, C, E viruses were also noted with yellow fever virus (Table 2).
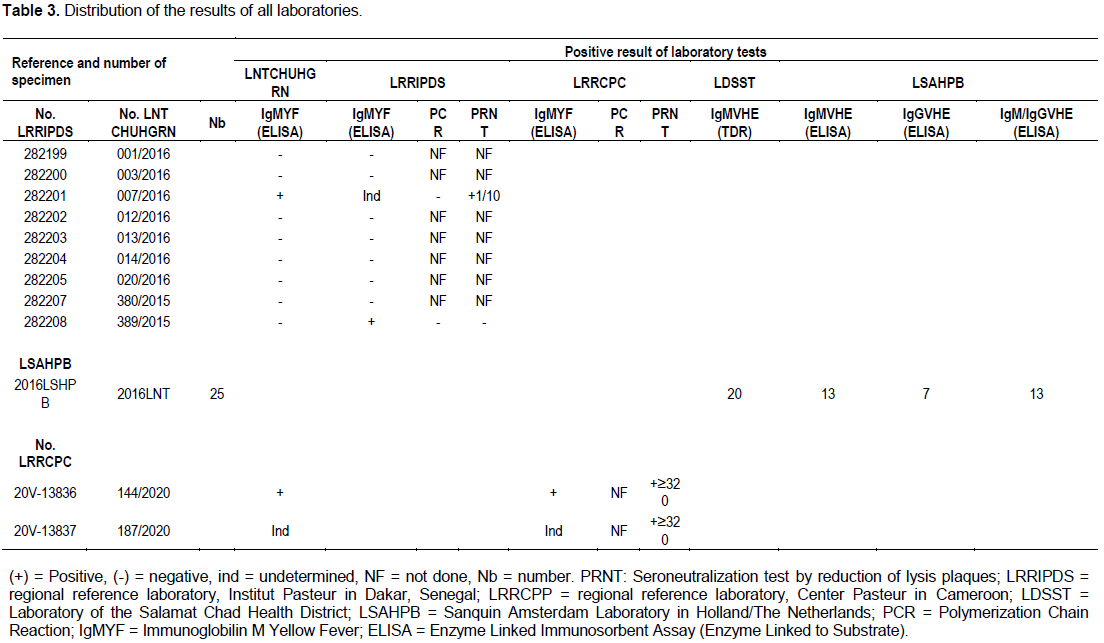
Distribution of the results of all laboratories
The consistency analysis carried out on the results obtained with the reagents having been used by five laboratories (four techniques concerned), made it possible to identify a certain number of discordant values.
A total of 1,730 whole blood samples were collected and systematically analyzed at the National Reference Laboratory (LNR) of the National Reference University Hospital (CHU-RN) of N'Djamena for the detection of yellow fever, malaria and the virus hepatitis B, C, E. Of the 1,730 samples, nine (9) samples, including three (3) positive, were sent for confirmation to the Regional Reference Laboratory in Dakar, Senegal, but the results obtained from these two laboratories showed no match. Two (2) samples, one of which was detected presumptively positive and the other undetermined by the national laboratory of Chad were confirmed positive and undetermined by the Regional Reference Laboratory of the Pasteur Center of Cameroon and yet the three laboratories (LNT, LRRIPDS and LRRCPC) used the same ELISA technique (Table 3). Twenty-five (25) samples which tested positive in the rapid diagnostic test (MPD Assure Device IgMVHE) were sent to the Sanquin Laboratory in Amsterdam for the confirmation of the hepatitis E virus by ELISA, of which 13 were confirmed positive (IgM), 7 (IgG) and 13 both IgM/IgG, respectively. Sample 007/2016 was detected IgMYF positive on ELISA by the National Reference Laboratory (LNR), undetermined (ELISA), negative (PCR) but positive on the seroneutralization test by reduction of lysis ranges by the Regional Laboratory of Reference (LRR) of Dakar. On the other hand, the sample 389/2015 was declared negative on the ELISA-IgMYF by the National Laboratory of Chad and it was detected positive by ELISA-IgMYF of the Regional Reference Laboratory of Dakar, and seven samples were declared negative by the ELISA-IgMYF by the LNR and were confirmed negative by the Dakar LRR (Table 3).
On October 11, 2016 (Week 38), the Amtiman Health District, a locality in the Salamat province of Chad experienced an outbreak of febrile jaundice, the first case of which was reported on August 1, 2016 (week 31). The Amtiman Health District supported by Médecin Sans Frontière Holland (MSF-H) decided to conduct active research on cases of febrile jaundice in the community and health structures with rapid diagnostic tests (TDR Assure Device IgM) for the detection of the Hepatitis E virus by the laboratory of the Amtiman hospital. This outbreak of febrile jaundice caused an overall case fatality of 4.16% (5/120). From week 37, out of a total of 120 cases of febrile jaundice, 20 RDTs were positive. Samples from the 20 RDT positive cases were sent to N’Djamena to the national surveillance laboratory for confirmation. Faced with information about the lack of a confirmatory test in Chad, and the need to send samples for confirmation outside the country, MSF-H had sent the 20 samples and 5 others to the Sanquin Laboratory in Amsterdam. Of the total of 25 samples, 13 were confirmed IgG and IgM positive by ELISA for Hepatitis E virus (Table 3), 5 samples were negative (IgG and IgM) and 7 samples were negative for IgM but IgG positive indicative of HEV infection.
At the end of this study, which focused on the surveillance assessment of the seroprevalence of yellow fever in Chad, it appears that 5/1730 (0.28%) were detected presumptive positive. This rate is lower than those obtained during yellow fever epidemics by other authors (Kean, 2017; Sylva et al., 2020). For the sample (007/2016), two (2) isolated values ??were observed for the same technique (MAC-ELISA-CDC) with a titer (1/10) of seroneutralization by reduction of the lysis plaques. Furthermore, there are discrepancies in the results between the laboratories (Poisson et al., 2011). This discrepancy could also be due to pipetting errors by the technicians. On the other hand, for the sample (187/2020), there was no discrepancy for the technique (MAC-ELISA-CDC) with seroneutralization by reduction of the lysis plaques to a high titer (≥320). This result corroborates with laboratory performance evaluation work for the detection of Hepatitis B Virus DNA (HBV-DNA) in terms of inter-laboratory variability (WHO, 2016a; Monath et al., 2015).
This study showed a strong participation of female donors. Female donors were 55.49% and 525 (44.51%) donors were male. The massive participation of women in the study could be explained by the high level of compassion of women towards a sick relative and also by the proportion of women of 52% in Chad (RGPH3, 2014).
The results (Table 2) have sufficiently shown that there are mosquitoes involved in diseases associated with yellow fever in Chad, but without any proof of their competence as vectors. The malaria rate (49.47%) obtained from this study corroborates with the prevalence of malaria in the general population (40.9%) in Chad (ENIPT, 2017). The proportions of seropositivity for viral hepatitis B (29.00%) and C (4.62%) obtained in suspects with jaundice signs could testify to the association of yellow fever with other diseases in terms of markers tested and symptoms (Tables 1 and 2). These results corroborate with the results of previous work carried out by Bessimbaye et al. (2014) on viral hepatitis B and C which reported similar proportions. In addition, several authors have indexed the involvement of mosquitoes other than Aedes aegypti in the urban-rural transmission cycle of yellow fever with association of other diseases (Kean, 2017). The discrepancy in the laboratory results (LNR and LRR) would be linked to these factors (vaccination coverage, cross reactions with other Flaviviruses) which could influence the analysis of the samples since the two laboratories use the same MAC-ELISA technique CDC (Table 3). On the other hand, the tests used did not make it possible to distinguish the antibodies generated against the wild virus (natural immunity) from those aroused by the vaccine virus (acquired immunity), including the seroneutralization test by reduction of the lysis plaques, however more specific. The low frequency of the detection rate for the circulation of the yellow fever virus in Chad could be linked either to the delay in sending samples from most of the different localities (towns and villages) to the National Laboratory, or to the vaccination coverage of certain study areas.
As a result, the usefulness of serological surveys is less in places where vaccination coverage is high. Therefore, one should either avoid sampling where immunization coverage is high or determine by calculation what size of the sample would avoid this problem.
Antibodies from cross-reactions are more likely to be present in older people because older people have probably had more opportunity to come into contact with Flaviviruses in their lifetime than in older people, children, who are less likely to be exposed. Younger children may have antibodies to maternal immunoglobulin G (IgG).
The low frequency of the detection rate of the circulation of the yellow fever virus in Chad could be linked either to the delay in sending samples from the different localities (towns and villages) to the National Laboratory, or to the vaccination coverage of certain areas of the study (Figures 1 and 3). In addition, transport time, vaccination coverage and sample handling have also reported the impact of these factors on the quality of diagnostic results (WHO, 2014; Ruta et al., 2020). As a result, the usefulness of serological surveys is less in places where vaccination coverage is high. Therefore, one should either avoid sampling where immunization coverage is high or determine by calculation how large the sample is to avoid this problem. For localities where the survey could not detect the presence of yellow fever virus, it would be wise to test for the presence of yellow fever virus in mosquitoes (detected by real-time PCR) to isolate the virus.
This is the first National Quality Control operation dedicated to the search for RNA of the yellow fever virus. It involved 4 laboratories which all returned a reply slip.
Overall, there is a number of outlier or isolated inter-laboratory values, showing heterogeneity in the results obtained for the same technique.
Detection of the isolated yellow fever virus remains a major challenge for developing countries and in Chad. The evaluation of the circulation of the yellow fever virus requires, on one hand, a better selection and a better loyalty of the places where the vaccination coverage is less or absent in order to carry out a serological survey and on the other hand, by the method which would make it possible to distinguish the antibodies generated against the wild virus (natural immunity) from those aroused by the vaccine virus (acquired immunity). It would be desirable to avoid sampling where the vaccination coverage is high, or to determine by calculation what is the size of the sample which makes it possible to avoid this problem and also to promote vaccination against the virus, in particular from ages 9 and above, and depending on gender.
To the WHO, CDC Atlanta and CDC Africa which are involved in the research of the yellow fever virus, it is recommended that they should make available the standard MAC-ELISA-CDC kits to the national laboratories to carry out adequate serological analyses for the detection of yellow fever virus IgM and/or RT-PCR tests containing the same reagents with the same concentrations and from the same manufacturing company to perform differential tests (such as dengue viruses, West Nile virus and Zika virus) in order to minimize discrepancies results between national and regional reference laboratories for fever.
The authors have not declared any conflict of interests.
The authors thank all the people who allowed and facilitated the realization of this investigation in the Health Districts of the different provinces: Yellow fever surveillance focal points for the collection and delivery of samples to the national laboratory; WHO-Chad and MSF-Holland, for sending samples to regional laboratories for quality control and confirmation of results; Mr. RATNAN for drawing up the map of yellow fever for the study areas.
REFERENCES
|
Abeera A, Fahim A, Yasir R, Nasir A R, Shugufta Y, Irfan N S (2019). Epidemiology of dengue fever and utility of dengue ns1 antigen rapid diagnostic point of care test at combined military hospital malir. Pak Armed Forces Medicine Journal 70 (4):1019-1023.
|
|
|
|
Amraoui F, Vazeille M, Failloux AB (2016). French Aedes albopictus are able to transmit yellow fever virus. Euro Surveillance 21(39):30361.
Crossref
|
|
|
|
|
Bessimbaye N, Moussa AM, Mbanga D, Tidjani A, Mahamat SO, Ngawara MN, Ngarnayal G, Fissou HY, Sangare L, Ndoutamia G, Barro N (2014). HBsAg and Anti-HCV antibody seroprevalence in people infected with HIV1 in N'Djamena, Chad. Bulletin Société Pathologie Exotique 107:327-331.
Crossref
|
|
|
|
|
ENIPT (2017). National Survey on Malaria Indicators in Chad 2017. 165p.
|
|
|
|
|
HCSP (2017). Notice on what to do with an imported or indigenous case of yellow fever. Bulletin épidémiologique hebdomadaire. pp. 1-26.
|
|
|
|
|
Hunsperger EA, Muñoz-Jordán J, Beltran M, Colón CJ, Carrión J, Vazquez J, Acosta LN, Medina-Izquierdo JF, Horiuchi K, Biggerstaff BJ, Margolis HS (2016). Performance of Dengue Diagnostic Tests in a Single-Specimen Diagnostic Algorithm. Journal Infectious Disease 214(6):836-844.
Crossref
|
|
|
|
|
Kean S (2017). On the trail of yellow fever. Science 357(6352):637-641.
Crossref
|
|
|
|
|
Monath TP, Pedro FC, Vasconcelos PFC (2015). Yellow fever. Journal of Clinical Virology 64:160-173.
Crossref
|
|
|
|
|
Paules CI, Fauci AS (2017). Yellow Fever - Once Again on the Radar Screen in the Americas. England Journal Medicine 376:1397-1399.
Crossref
|
|
|
|
|
Poisson F, Cordobes DM, Laperche S (2011). Evaluation of laboratory performance for the detection of Hepatitis B Virus DNA (HBV-DNA) in terms of inter-laboratory variability. Ed AFSSAP. pp. 1-13.
|
|
|
|
|
RGPH 3 (2014) Second General Population and Housing Census. N'Djamena. INSEED, pp. 1-164.
|
|
|
|
|
Ruta K, Meera Modak , Mrunal Gosavi , Dileep Wani , Akhilesh C Mishra , Vidya A Arankalle (2020). Comparative assessment of commercial enzyme-linked immunosorbent assay and rapid diagnostic tests used for dengue diagnosis in India. Indian Journal Medicine 151(1):71-78.
Crossref
|
|
|
|
|
Santiago GA, Vazquez J, Courtney S, Matias KY, Andersen LE, Butler AE, Roulo R, Bowzard J, Villanueva JM, Munoz-Jordan JL (2018). Performance of the Trioplex real-time RT-PCR assay for detection of Zika, dengue, and chikungunya viruses. Nature Communication 9(1):1391-1401.
Crossref
|
|
|
|
|
Sylva NIO, Sacchetto L, de Rezende IM, Trindade GS, LaBeaud AD, de Thoisy B, Drumond BP (2020). Recent sylvatic yellow fever virus transmission in Brazil: the news from an old disease. Virology Journal 17(1):1277-1289.
Crossref
|
|
|
|
|
WHO (2014). Assessment of the risk of circulation of the yellow fever virus in endemic countries 2nd Report: 1-54.
|
|
|
|
|
WHO (2016a). "Yellow fever: epidemiology 2015". Report, pp. 1-38.
View
|
|
|
|
|
WHO (2016b). Laboratory diagnosis of yellow fever in Africa 1:1-7.
|
|
|
|
|
WHO (2018). A global strategy to Eliminate Yellow fever Epidemics (EYE) 2017 - 2026. Geneva: World Health Organization: 1-56. Tiger Mosquito Reporting Portal (Aedes albopictus) .
|
|