ABSTRACT
The development of effective and less toxic antimicrobial agents is required for the treatment of respiratory tract infections. This study was carried out to evaluate the phytochemical and antibacterial activities of Anogeissus leiocarpus (DC.) (Guill. & Perr.) and Terminalia glaucescens (Planch. ex Benth.) against non-tuberculous mycobacteria species. The methanol, dichloromethane and aqueous extracts were screened against five (5) non-tuberculosis mycobacteria (NTM) species by agar diffusion method. Minimum inhibitory concentration (MIC) was determined by agar dilution method while bactericidal studies were done by viable count technique. The methanol and aqueous extracts were active against all the test organisms with zones of inhibition ranging from 10±0.0 to 25±0.5 mm. The MIC and MBC range from 0.3125 to 2.5 and 1.25 to 10 mg/mL, respectively. Bactericidal activities of aqueous extracts against Mycobacterium smegmatis ATCC 19420 revealed a drastic dose-dependent decline in the surviving population after 6 h of exposure accompanied by a total (100%) kill after 24 h of exposure. The antimicrobial activities demonstrated by these plants suggest the presence of therapeutically important antimycobacterial compounds and thus justify as well as support the use of these medicinal plants for the treatment of respiratory tract infections.
Key words: Anogeissus leiocarpus (DC.) (Guill. & Perr.), Terminalia glaucescens (Planch. ex Benth.), antibacterial, nontuberculous mycobacteria species, bactericidal, in vitro.
Rapidly growing mycobacteria (RGM) belong to non-tuberculous mycobacteria group, which is a heterogeneous group of organisms that occasionally are a primary cause of lung diseases and affect patients with underlying chronic lung disease such as bronchiectasis, pneumoconiosis, or healed tuberculosis (Griffith et al., 2007). RGM pulmonary infection is serious and difficult to cure, with improvement or resolution in less than one-half of patients with cancer who have definitive/probable infection. A common feature of all RGM is their resistance to first-line anti-tuberculous agents hence the need for new therapeutic agents that will be effective for the cure of RGM infections. The use of medicinal plants for the treatment of infectious diseases is an age long practice that is on the rise globally. Thus, two medicinal plants used in folklore medicine for the treatment of cough and tuberculosis identified from an ethnobotanical survey were chosen for investigation of their anti-mycobacterial activity. The plants are Anogeissus leiocarpus (DC.) Guill. & Perr. (African birch) and Terminalia glaucescens Planch. ex Benth. (Combretaceae).
Anogeissus is a genus of trees native to South Asia, the Arabian Peninsula, and Africa, belonging to family Combretaceae. The genus has eight species, five native to South Asia, two endemic to the southern Arabian Peninsula, and one native to Africa (Mann et al., 2008). A. leiocarpus is found in Africa from northeastern Ethiopia to Senegal, and its bark is used to produce Anogelline, a substance used in cosmetics (Shuaibu et al., 2008). Traditionally, in most parts of Hausa land (Northern Nigeria), infusion of A. leiocarpus leaves is used in the treatment of cough, wound infections and rashes in small children (Bizimana, 1994). A. leiocarpus is traditionally acclaimed to be effective in treating infectious wounds in man and animals (Dweek, 1997). The pulped roots are applied to wounds and ulcers, and the powdered bark is also rubbed on gums to reduced tooth ache. It is also used as vermifuges and the leaves decoction is used for washing and fumigation (Ibrahim et al., 1997, 2005). The root of the plant when used as chewing stick is known to have antibacterial effects on Lactobacillus sp. (Owoseni and Ogunnusi, HYPERLINK "http://scialert.net/fulltext/?doi=ajbs.2011.570.574"HYPERLINK http://scialert.net/fulltext/?doi=ajbs.2011.570.574and Ogunnusi2006). Antimicrobial activity against a variety of viruses, the malaria parasite and some bacteria has also been demonstrated (Taiwo HYPERLINK "http://scialert.net/fulltext/?doi=ajbs.2011.570.574"et alHYPERLINK "http://scialert.net/fulltext/?doi=ajbs.2011.570.574"., 1999). A. leiocarpus is used medically for the treatment of diabetic ulcers, ascariasis, gonorrhoea, general body pain, blood clots, asthma, coughing, bronchitis, pulmonary disorder, hemoptysis, pneumonia, catarrh, hay-fever and tuberculosis (Mann et al., 2003, 2007, 2008; Barku et al., 2013). Personal interactions with some herb sellers revealed that leaf extracts of this tree are commonly used in the treatment of typhoid fever, diarrhoea, malaria fever, rheumatism, cough and skin infections whether administered singly or in combination with other herbs.
Terminalia is a genus of large trees of the flowering plant family Combretaceae, comprising around 100 species distributed in the tropical regions of the world (Mann et al., 2008). It is traditionally used in the treatment of diabetes (Njomen et al., 2008; Ndukwe, 2005). It is also widely used as a chewing stick in Nigeria, thus various studies have been carried out on its antimicrobial activity against oral pathogens (Ogundiya et al., 2008). Terminalia glaucescens is used in the treatment of dysentery. It has found use as an antimicrobial agent in the last stages of AIDS (Koudou et al., 1995). Antiplasmodial activity of ethanolic extract of the plant was described by Mustofa et al. (2000). Trees of this genus are known to be a source of secondary metabolites, e.g. cyclic triterpenes and their derivatives, flavonoids, tannins and other aromatics. Some of these substances have antifungal, antibacterial, anti-cancer and hepatoprotective effects.
In this study, the inhibitory activities of A. leiocarpus (DC.) (Guill. & Perr.) (leaf, stem bark and root) and T. glaucescens (Planch. ex Benth.) (root) on rapidly growing mycobacteria were investigated, to justify the use of these plants in the treatment of respiratory tract infections, especially tuberculosis.
Plant collection, extraction and preparation of extracts
A. leiocarpus (leaf, stem bark and root) and Terminalia glaucescens (root) plant materials were obtained and identified according to the international WHO guidelines “WHO Guidelines on Good Agricultural and Collection Practices” (WHO, 2003). Voucher specimens were deposited at Forest Research Institute of Nigeria Ibadan, Oyo State (FRIN), where the plants species were identified, authenticated and assigned voucher specimen number FHI 109925 and FHI 108282 for Anogeissus leiocarpus and Terminalia glaucescens, respectively. Plant materials were soaked in methanol and extracted for 72 h with constant agitation. The extraction process was repeated thrice. Extracts were filtered using Whatman No. 1 filter paper and combined, prior to being concentrated to dryness using a rotary evaporator. Fifty grams (50 g) of methanol extracts from the samples was defatted with n-hexane and partitioned into dichloromethane and water. The methanol extract (ME), dichloromethane (DCM) and aqueous (AQ) partitions were dried using a rotary evaporator, weighed and stored at 4°C. Solutions of extracts/partitions were reconstituted in 40% methanol with final concentrations of 1, 2, 10 and 20 mg/mL for the initial screening. Concentrations in the range of 0.156 and 20 mg/mL were also prepared to determine the minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) of the bioactive extracts.
Antimicrobial agents
The chemotherapeutic agent used in the test as a positive/drug control was Rifampicin at 20 and 40 µg/mL (Nicholas Laboratories Limited, England), while the negative/solvent control was 40% methanol.
Phytochemical screening
Phytochemical screening was carried out to detect the presence of secondary metabolites such as anthraquinones, flavonoids, tannins, saponins, alkaloids, phenol among others using methods described by Harborne (1998).
Strains of nontuberculous mycobacteria (NTM)
Five non-tuberculous mycobacteria isolates used for this investigation were Mycobacterium fortuitum ATCC 684, Mycobacterium smegmatis ATCC 19420, Mycobacterium phlei ATCC 19240, Mycobacterium smegmatis and Mycobacterium abscessus.
Susceptibility testing
Susceptibility was determined using the agar cup diffusion technique as previously described (Lawal et al., 2014). Plates were incubated at 37°C for two to three days after which diameters of zones of inhibition (mm) were measured. Methanol (40%) was included in each plate as a solvent control while Rifampicin (20 and 40 µg/mL) was used as positive control.
Determination of minimum inhibitory concentration (MIC)
Minimum inhibitory concentrations (MIC) of bioactive extracts were determined by a modification of standard agar dilution method procedures as previously described (CLSI, 2008). Extracts were tested at various concentrations ranging from 20 to 0.156 mg/mL. The positive/drug control was rifampicin. The MICs were determined after two to three days of incubation at 37°C. The MIC was regarded as the lowest concentration that prevented visible growth of test organisms. The experiments were performed in duplicate.
Determination of minimum bactericidal concentration (MBC)
Minimum bactericidal concentration (MBC) of active plant extracts was determined by a modification of the method of Aibinu et al. (2007). The lowest concentration that prevented bacterial growth after 48 h of incubation was recorded as the minimum bactericidal concentration (MBC). The entire tests were carried out in duplicates to ensure accuracy. Agar plates without extracts and another agar plate without any inoculated organism were also incubated to serve as organism and extract control plates, respectively. Minimum bactericidal concentration (MBC) was also determined for the drug control (Rifampicin).
Determination of bactericidal activity
The viable counting technique previously described (Ogudo et al., 2014) was employed for this purpose. Mycobacterium smegmatis ATCC 19420 was used for this experiment. The procedure was carried out in duplicate. Control plates for negative and positive controls were also incubated. Plates were incubated at 37°C for 24 h before counting the colonies. The numbers of surviving bacterial cells per mL were calculated by taking into consideration the dilution factor and the volume of the inoculum. All the procedures were performed with concentrations equivalent to MIC, 2 × MIC and 4 × MIC. A graph of viable count (Log10) against time (hour) was plotted to show the rate of kill of the test organisms.
The extraction yields for A. leiocarpus leaf, stem bark and root as well as T. glaucescens root are presented in Table 1. Phytochemical screening of the powdered plant samples, aqueous and dichloromethane extracts of A. leiocarpus and T. glaucescens revealed the presence of saponins, flavonoids, tannins, alkaloids, reducing sugar, glycosides and resins in the powdered samples and aqueous partitions (Table 2). The methanol and aqueous extracts were active against all the test organisms with diameter of zone of inhibition ranging between 10±0.0 and 25±0.5 mm (Tables 3 and 4), while the dichloromethane extracts had little/no activity (results not shown). The diameter of the zone of inhibition was concentration-dependent (increased with increase in the concentration of extracts) as shown in Tables 3 and 4. Most of the phytochemical components were absent in the dichloromethane extracts. This explained the reason for the aqueous extracts been more effective than the dichloromethane fractions. Since the dichloromethane extracts had little or no activity on the test organisms they were not studied further. The mechanism of inhibition of the phytochemical components on the mycobacteria species may be due to the impairment of various enzyme systems such as those involved in energy production as well as the interference with the integrity of the cell membrane and structural component synthesis (Huang and Chung, 2003; Okwu and Morah, 2007).
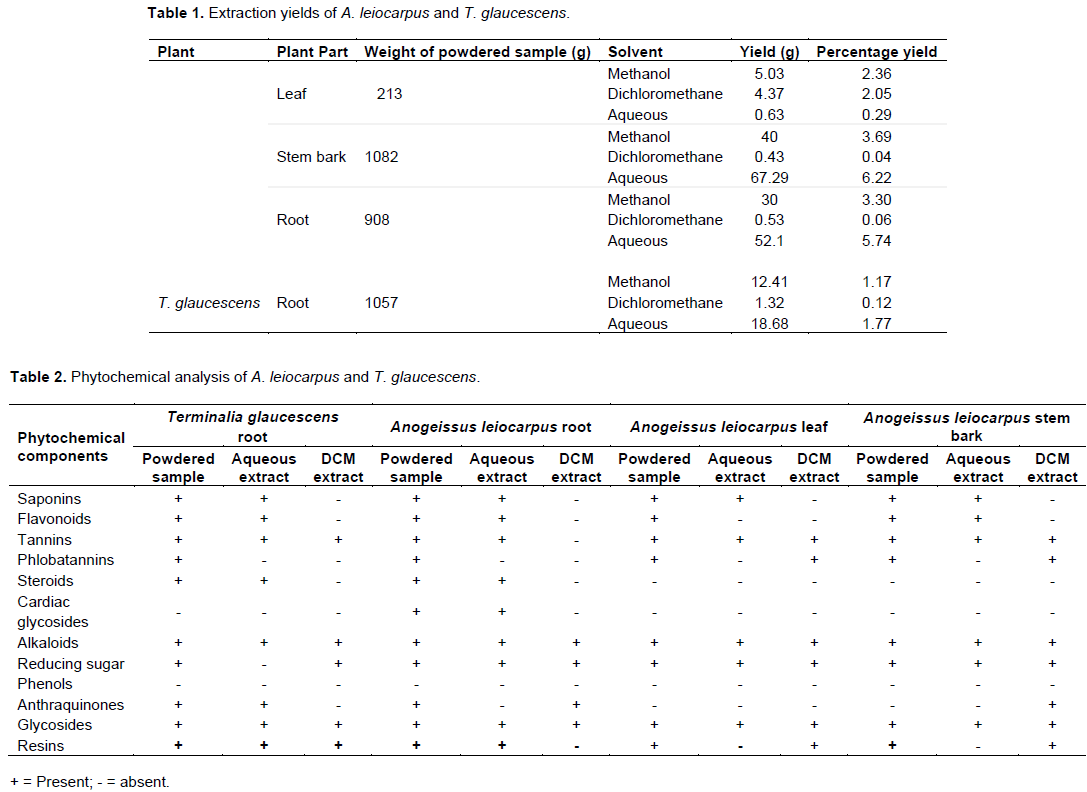
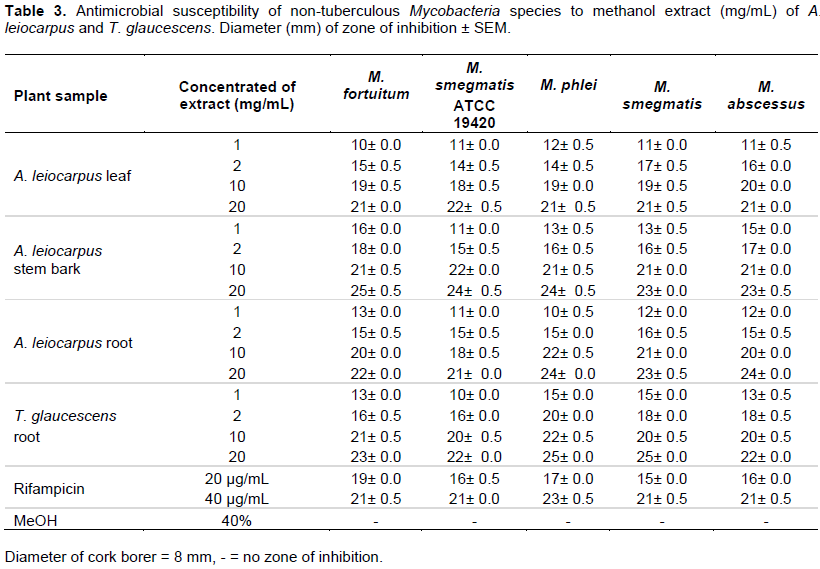
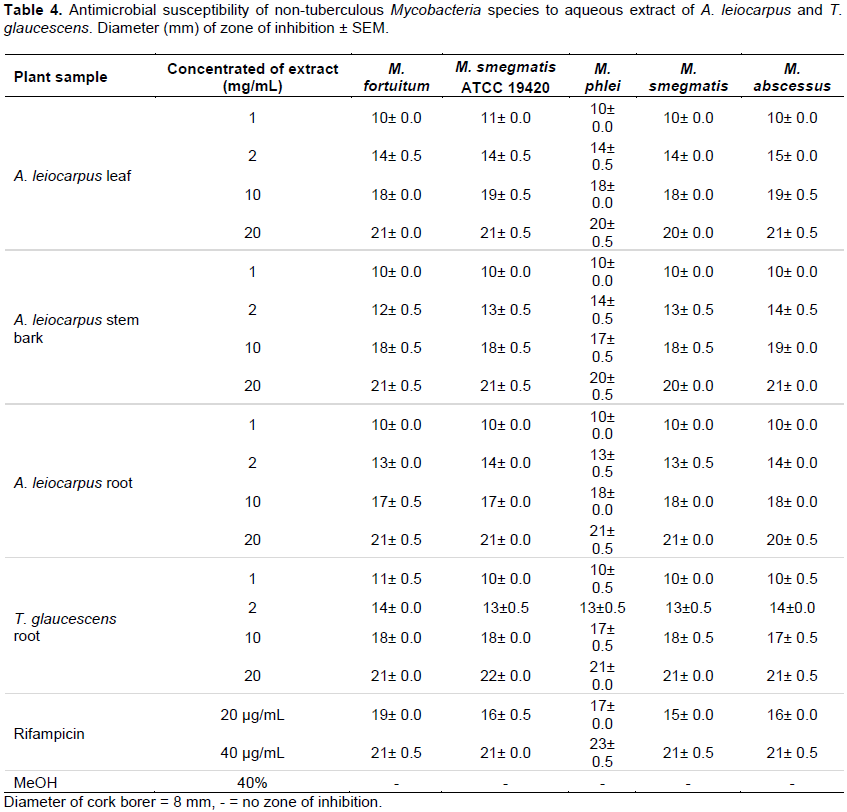
It was also observed that the drug control (Rifampicin) was active on all the isolates tested with the diameter of the zones of inhibition ranging from 15±0.0 to 23±0.0 mm (Tables 3 and 4). The minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) ranged from 0.3125 to 2.5 and 1.25 to 10 mg/mL, respectively (Table 5). The phytochemical components detected in the plant samples have been associated with antimicrobial activity (Marjorie, 1999; Mahajan and Badgujar, 2008) and could be responsible for the observed effects. The inhibitory activities of A. leiocarpus and T. glaucescens observed in this study are in agreement with earlier studies reporting the antimicrobial activities of A. leiocarpus and T. glaucescens (Batawila, 2005; Barku et al., 2013). The plants are used in folklore medicine for the treatment of diabetic ulcers, general body pain, blood clots, asthma, coughs and tuberculosis. Hollist (2004) and Taiwo et al. (1999) reported that both plants are sold as chewing sticks for the prevention or treatment of oral infections in southwest Nigeria. T. glaucescens is one of the plants used in the preparation of the “wonder cure” concoction used in the treatment of tuberculosis in Nigeria. The activity of the plant extracts on Mycobacterium tuberculosis was reported by Adeleye et al. (2008).
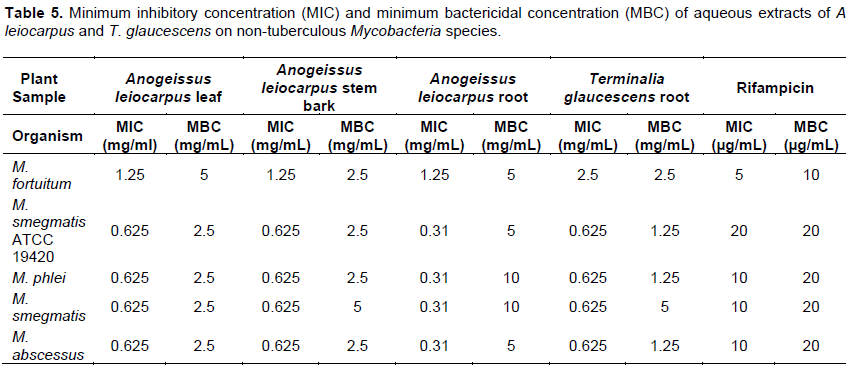
Bactericidal activities of aqueous extracts of A. leiocarpus and T. glaucescens on Mycobacterium smegmatis ATCC 19420 showed a bactericidal activity dependent on the time of exposure and the concentration of each of the extracts as shown in Figures 1 to 4. It was shown that there is a drastic dose-dependent decline in the surviving population after 6 h of exposure accompanied by a total (100%) kill after 24 h of exposure to the aqueous extracts of A. leiocarpus and T. glaucescens at doses equivalent to MIC, 2 × MIC and 4 x MIC (Figures 1 to 4). This result is similar to the kinetics study of the in vitro activities of Zingiber officinale Rosc. (Ginger) and Curcuma longa Linn. (Turmeric) rhizomes against non-tuberculous mycobacteria species (Ogudo et al., 2014). The slightly high MIC values (0.31 to 2.5 mg/mL) recorded and the lower kill rate observed for these organisms as compared to the drug control-Rifampicin (5 to 20 µg/mL) in this study could be explained by the report of Nessar et al. (2012). They observed that resistance shown by most of these organisms, that is, the non-tuberculous mycobacteria species could be either intrinsic, attributed to a combination of the permeability barrier of the complex multilayer cell
envelope, drug export system, antibiotic targets with low affinity and enzymes that neutralize antibiotics in the cytoplasm; or acquired resistance through mutation. The cell envelope of mycobacteria species is usually waxy due to the presence of high lipid content accounting for about 60% of dry weight of the bacteria. This is considered a main factor contributing to their low permeability (Brennan and Nikaido, 1995) hence, the ability of the aqueous extracts to penetrate this barrier is notably significant. From our study, it can be deduced that the aqueous extracts of the test plants contain compounds that could be developed to elicit better anti-mycobacterial activity that will compare favourably with the drug/positive control which killed the organisms within the same contact time though at a lower concentration. This preliminary investigation agreed with the reports of Mann (2007) that the methanol extracts of T. glaucescens and A. leiocarpus inhibited the growth of M. tuberculosis.
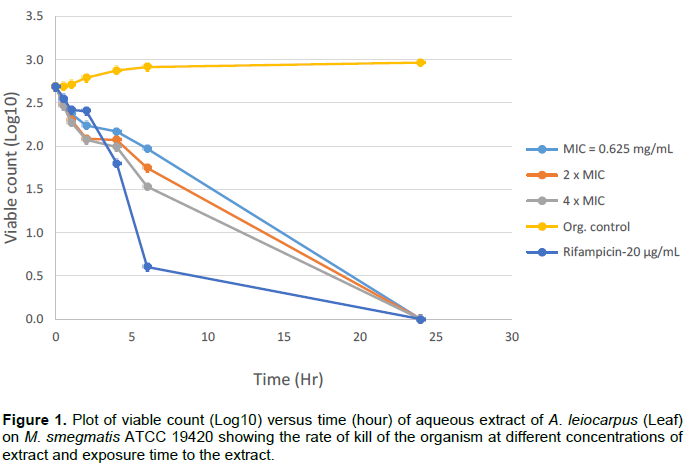
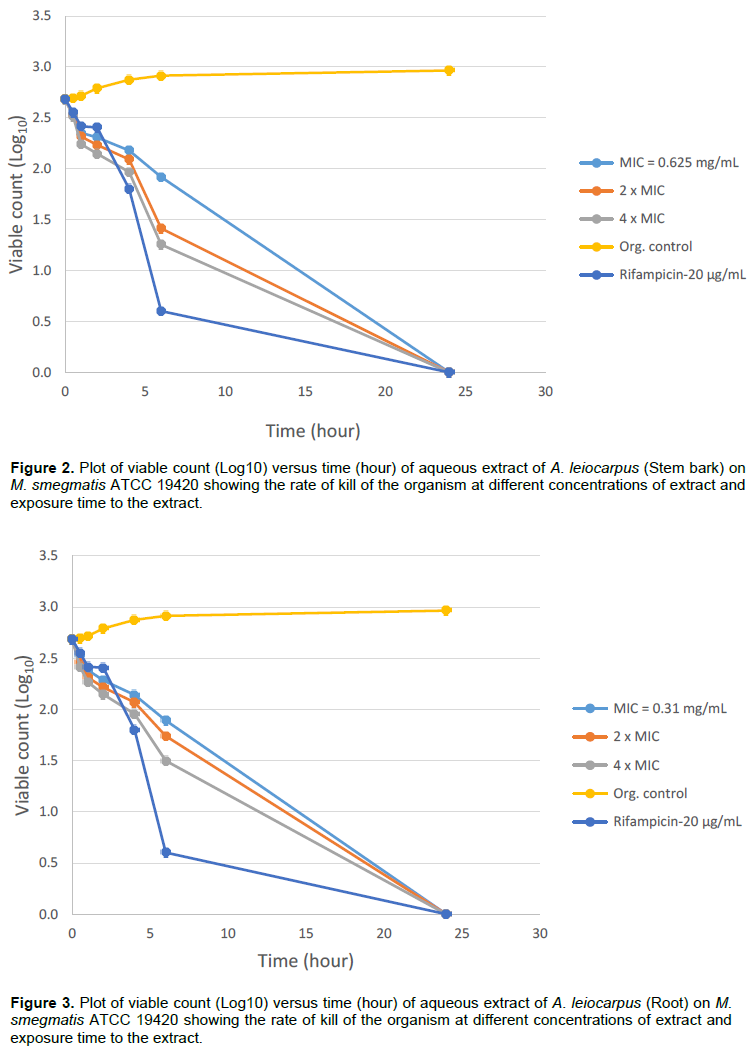
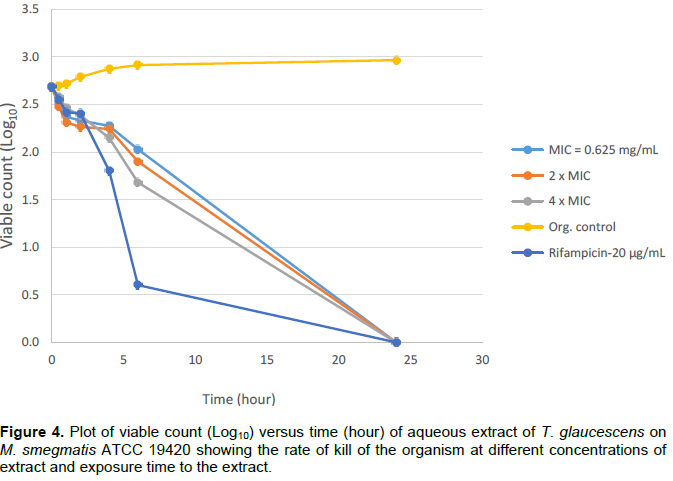
The study investigated antibacterial activities of A. leiocarpus (DC.) (Guill. & Perr.) and T. glaucescens (Planch.) against non tuberculous myco bacteria species. These medicinal plants are used as medicines against infectious diseases including respiratory tract infections. The aqueous fractions of both plants had the highest activity on the test organisms suggesting the presence of bioactive components in the aqueous fractions. These findings support the use of these plants in traditional medicines where the plants are often used in the form of decoctions or are macerated in water for the treatment of various diseases including respiratory tract infections.
The authors have not declared any conflict of interests.
REFERENCES
|
Adeleye IA, Onubogu CC, Ayolabi CI, Isawumi AO, Nshiogu ME (2008). Screening of crude extracts of twelve medicinal plants and "wonder –cure" concoction used in Nigeria unorthodox medicine for activity against Mycobacterium tuberculosis isolated from tuberculosis patients sputum. Afr. J. Biotechnol. 7(18):3182-3187.
|
|
|
|
Aibinu I, Adenipekun T, Adelowotan T, Ogunsanya T, Odugbemi T (2007). Evaluation of antimicrobial property of different parts of Citrus aurantifolia (Lime fruit) as used locally. Afr. J. Tradit. Complement. Altern. Med. 4 (2):185-190.
|
|
|
|
|
Barku VY, Opoku-Boahen Y, Owusu-Ansah E, Dayie NTKD, Mensah FE (2013). In-vitro assessment of antioxidant and antimicrobial activities of methanol extracts of six wound healing medicinal plants. J. Nat. Sci. Res. 3(1):74-80.
|
|
|
|
|
Batawila K (2005). Antifungal activities of five Combretaceae used in Togolese traditional medicine. Fitoterapia 76(2):264-268.
Crossref
|
|
|
|
|
Bizimana N (1994). Traditional Veterinary Practice in Africa. German Technical Cooperation ISBN 3-88085-502-511. Vol. 243. TZ-Verlag-Ges. 917 p.
|
|
|
|
|
Brennan PJ, Nikaido H (1995). The envelope of mycobacteria. Annu. Rev. Biochem. 64:29-63.
Crossref
|
|
|
|
|
CLSI (2008). Performance Standards for Antimicrobial Susceptibility Testing; Ninth Informational Supplement. CLSI document M100-S9. Wayne, PA: Clinical Laboratory Standards Institute. pp. 120-126.
|
|
|
|
|
Dweek AA (1997). Ethnobotanical use of plants. Part 2 African Plants. Cosm. Toil. 112, 4. http://www.dweckdata.co.uk/Published_papers/Africa1.pdf.
|
|
|
|
|
Griffith DE, Aksamit T, Brown-Elliott BA, Antonino Catanzaro, Charles Daley, Fred Gordin, Steven M. Holland, Robert Horsburgh, Gwen Huitt, Michael F. Iademarco, Michael Iseman, Kenneth Olivier, Stephen Ruoss, C. Fordham von Reyn, Richard J. Wallace, Jr., Kevin Winthrop (2007). An Official ATS/IDSA Statement: Diagnosis, treatment and prevention of nontuberculous mycobacterial diseases. Am. J. Respir. Crit. Care Med. 175:367-416.
Crossref
|
|
|
|
|
Harborne JB (1998). Phytochemical methods: A guide to modern techniques of plant analysis. London, UK: Chapman and Hall. p. 279.
|
|
|
|
|
Hollist NO (2004). A collection of traditional Yoruba oral and dental medicaments. Book Builders Publishers Ltd. Ibadan. pp 83-87.
|
|
|
|
|
Huang JW, Chung WC (2003). Management of vegetable crops diseases with plant extracts. Adv. Plant Dis. Manage. 37:153-163.
|
|
|
|
|
Ibrahim K, Nwamba CO, Mann A, Inyang US (2005). A preliminary Investigation into the antibacterial properties of Anogeissus leiocarpus and Piper guineense seeds on Staphylococcus aureus and Pseudomonas aeruginosa. Nig. J. Appl. Arts Sci. 1(1):21-24.
|
|
|
|
|
Koudou J, Roblot G, Wylde R (1995). Tannin constituents of Terminalia glaucescens. Planta Med. 61(5):490- 491.
Crossref
|
|
|
|
|
Lawal TO, Mbanu AE, Adeniyi BA (2014). Inhibitory activities of Ceiba pentandra (L.) Gaertn. and Cordia sebestena Linn. On selected rapidly growing mycobacteria. Afr. J. Microb. Res. 24:2387-2392.
|
|
|
|
|
Mahajan RT, Badgujar SB (2008). Phytochemical Investigations of some Laticiferous plants belonging to Khandesh Region of Maharashtra. Ethnobot. Leaflets 12:1145-1152.
|
|
|
|
|
Mann A (2007). Survey of Ethnomedicine for the treatment of Tuberculosis: Chemistry Perspective. Ayanwola Printing Works, Minna. P 117.
|
|
|
|
|
Mann A, Gbate M, Umar NA (2003). Medicinal and economic plants from Nupeland. Jube-Evans Publisher. P 67.
|
|
|
|
|
Mann A, Yahaya AY, Banso A, Ajayi GO (2008). Phytochemical and antibacterial screening of Anogeissus leiocarpus against some microorganisms associated with infectious wounds. Afr. J. Microb. Res. 2:60-62.
|
|
|
|
|
Marjorie MC (1999). Plant products as antimicrobial agents. Clin. Microbiol. Rev. 12(4):564-582.
|
|
|
|
|
Mustofa AV, Francoise BV, Yves P, Djeneba KB, Mich’ele M (2000). Antiplasmodial activity of plant extracts used in West African traditional medicine. J. Ethnopharm 73:145-151.
Crossref
|
|
|
|
|
Ndukwe KC, Okeke I, Lamikanra A, Adesina SK, Aboderin O (2005). Antimicrobial activity of aqueous extracts of selected chewing sticks. J. Contemp. Dent. Pract. 6(3):86-94.
|
|
|
|
|
Nessar R., Cambau E, Reyrat JM, Murray A, Gicque B (2012). Mycobacterium abscessus: A new antibiotic Nightmare. J. Antimicrob. Chemother. 65(5):1-9.
Crossref
|
|
|
|
|
Njomen SN, Kamgang R, Soua P, Oyono J, Njikam N (2009). Protective effect of methanol-methylene chloride extract of Terminalia glaucescens leaves on streptozotocin-induced diabetes in mice. Trop. J. Pharm. Res. 8(1):19-26.
Crossref
|
|
|
|
|
Ogudo BU, Lawal TO, Adeniyi BA (2014). Extracts of Zingiber officinale Rosc. (Ginger) and Curcuma longa Linn. (Turmeric) Rhizomes inhibited Nontuberculous Mycobacteria in vitro. J. Agric. Biol. Healthc. 4(12):95-103.
|
|
|
|
|
Ogundiya MO, Kolapo AL, Okunade, MB, Adejumobi J (2008). Evaluation of phytochemical composition and antimicrobial activity of Terminalia glaucescens against some oral pathogens. Adv. Nat. Appl. Sci. 2(2):89-93.
|
|
|
|
|
Okwu DE, Morah FNI (2007). Isolation and characterization of flavanone glycoside 4,5,7 trihydroxide flavanone rhamnoglucose from Garcina kola seed. J. Appl. Sci. 7 (2):155-164.
|
|
|
|
|
Owoseni AA, Ogunnusi T (2006). Antibacterial effects of three selected chewing sticks extracts on Lactobacillus sp. Int. J. Trop. Med 1(3):103-106.
|
|
|
|
|
Shuaibu MN (2008). Trypanocidal activity of extracts and compounds from the stem bark of Anogeissus leiocarpus and Terminalia avicennoides. Parasitol. Res. 102(4):697-703.
Crossref
|
|
|
|
|
Taiwo O, Xu HX, Lee SF (1999). Antibacterial activities of extracts from Nigerian chewing sticks. Phytother. Res. 13(8):675-679.
Crossref
|
|
|
|
|
WHO (2003). WHO Guidelines on Good Agricultural and Collection Practices for Medicinal Plants. World Health Organization, Geneva, pp. 7-15.
|
|