ABSTRACT
This study aimed to determine for the first time the levels and patterns of antimicrobial resistance of enterobacteria isolated from poultry and pigs farms in southern Togo. A cross-sectional study was conducted in south Togo in 70 and 47 poultry and pig farms, respectively. Fecal samples were collected once in each farm and enterobacteria isolated according to recommended techniques. Isolates from each sample were tested for susceptibility to 14 antibiotics by disc diffusion method. A total of 109 and 85 strains were recovered from 72.7% (n=64) and 87.93% (n=50) poultry and pig samples respectively. Respectively for Escherichia coli, Klebsiella spp. and Salmonella spp. strains, the most important resistances were observed in poultry/pig farms against tetracycline antibiotic (93.1%/67.6%; 96.2%/78.7% and 100%/100%) and the association sulfoxide-trimethoprim (72.4%/81.1%; 66.7%/78.7% and 100%/100%). In general, resistances were higher against penicillin antibiotics like ampicillin (55.17%/54.05%, 46.15%/38.3% and 50.00%/100%) than cephalosporin antibiotics like ceftazidime (0.00%/0.00%, 5.13%/0.00% and 0.00%/0.00%) resistances where very low or absent. Also, resistance to nalidixic Acid (31.03%/16.22%, 33.33%/29.79, 0.00%/0.00%), first generation quinolones, was relatively high than resistance to norfloxacin (10.3%/10.81%; 20.5%/2.13%; 50%/0.00%) a second generation fluoroquinolone. In poultry, 44.83% of E. coli, 50% of Klebsiella spp. and 100% of Salmonella strains were multi-resistant while in pigs, 37.83% and 27.65% of E. coli and Klebsiella spp. strains showed multi-resistance. In many farms, farmers managed the health of their animals on their own. All surveyed poultry farmers and the majority of pig farmers indicated that they used antibiotics in their farms. This study showed that antimicrobial resistance in animal production in Togo portends a serious problem.
Key words: Antibiotic resistance, Enterobacteria, poultry, pig, Lomé, Togo.
Rapid increase in income and urbanization over the past three decades, combined with population growth have led to increased demand for meat and other animal products in many developing countries (FAO, 2009). To meet increasing daily food demand, economic and technology changes are transforming the livestock sector especially in Africa. Indeed, in Africa and other developing countries, shift in animal production from small holder, mixed crop to intensive, large-scale, and specialized commercialization farms have been observed (Schar et al., 2018). Production of livestock, especially pigs and poultry, is becoming more intensive, geographically concentrated around big towns, linked to supply chains and supported by the use of veterinary drugs like antibiotics (Mensah et al., 2014). Antibiotics used either as curative or preventive treatment against the onset of certain diseases, or even, in extreme cases, to offset poor animal production hygiene in intensive productions is leading to the development of drug resistance. Antibiotic resistance (ABR) is today a worldwide public health concern, with economic, and societal repercussions (Schar et al., 2018). Animals are proofed to be key reservoirs of antibiotic-resistant bacteria that can spread to human through direct contact or food chain.
In Togo, like other Sub-Saharan Africa countries, the use of antibiotics in animal production remains largely undocumented. However, poor control of the use of veterinary pharmaceutical products due to absence or poorly applied legislation to guarantee the quality and the holding of the products released onto the market is reported (Mensah et al., 2014). Inappropriate use of antibiotics as growth promoters by untrained farmers, especially in intensive poultry and pig farms to combat low productivity and high mortality caused by infectious diseases is common due to inadequate legislation. This situation may promote the development of resistance to the antibiotics commonly used in farm animals in these countries. Unfortunately, there is limited data concerning antimicrobial resistance in West African countries due to the absence of monitoring systems (Founou et al., 2018). Recent studies by Vounba et al. (2018, 2019a and b)and Sidibé et al. (2019) in Senegal and Mali respectively, has shown the resistance of some enterobacteria namely Escherichia coli and Salmonella, of avian origin to antibiotics.
In Togo, the poultry sector can be categorized into two: traditional poultry farming, and modern poultry farming. Traditional poultry farming has undergone a remarkable development over the past twenty years as a result of several interventional programs. The results of these programs are reflected in real emergence of a category of farmers adopting improved farming practices (vaccination, housing, improved nutrition, etc.) According to FAO (2015), modern poultry farming is dominated by laying hens for production of eggs for consumption. Poultry farms involve in eggs production account for approximately 95% of current poultry establishments. As in poultry production, pig farming can also be categorized into traditional farming and the modern or semi-intensive farming. Under the traditional farming, pigs are allowed to roam freely. Farmers generally associate livestock with their agricultural or commercial activities. Modern semi-intensive farms exploit exotic breeds (Large White and Landrace), and are characterized by keeping animals in enclosures with rational feeding, and health management including the use of antibiotics (Lhoste, 2009). To date, there are no data on the antibiotic resistance of enterobacterias to antibiotics commonly used in poultry and pig farms in Togo. Thus, the aim of the present study was to provide data on the phenotypic antibiotic resistance of enterobacterias isolated from poultry and pig farms in southern Togo.
Sampling area and sample collection
Sampling area
A cross-sectional study was conducted on private poultry and pig farms located in the peri-urban area of Lomé, in Maritime region (South Togo). The Maritime region is the area of excellence for modern poultry and pig production where commercial poultry and pig farms are mainly located. With more than 80% of modern poultry farms established, this region accommodates more than 90% of the national laying hens’ farms. The maritime region accounts for 50% of the country's urban population with an annual growth of 6.1%. This demographic importance which encourages poultry and pig production in peri-urban areas is due to the concentration of industry and administrative services. Indeed, the Maritime region hosts more than 90% of industrial activity, the largest university and all political institutions.
Sample collection
Fecal samples (88 and 58) were collected (September – October 2019) from 70 and 47 modern poultry and pig farms respectively, based on willingness of the farm owners to participate in the study and accessibility of the farms in the Maritime region of Togo. The sample size is based on the unknown population size (the exact number of poultry and pig farms is unknown in the Maritime region), an expected prevalence of farms with non-susceptible isolates of 50%, a precision of 10% and a confidence level of 90%. The required sample size for prevalence estimation is then estimated to 68 farms using the online WinEpiscope 2.0 (http://www.winepi.net/uk/sample/indice.htm). This 50% expected prevalence of non-susceptible isolates to at least one antibiotic at farm level was used as a conservative approach as no studies had previously estimated the prevalence of poultry or pig farms harbouring resistant enterobacterias. When a farm consisted of one poultry (chicken) house or one building with less than five pig pens, samples were taken from this chicken house or pig building whereas, when there were at least two chicken houses or two pig buildings or one building with more than 5 pens, two samples were collected in two separate chicken houses or pens. In each chicken house, one sample of fresh feces was collected. Each sample consisted of a pool of five samples taken in different parts of the
chicken house. In pig’s farm, each pooled fecal sample was obtained from a pen and consisted of five different fecal samples, one collected from each of the four corners and one from the center of the pen. The pens from which fecal samples were collected were randomly selected from each building. Each farm was visited once. A questionnaire (available in French on request) was completed in each farm at the time of sampling, to collect data relating to biosecurity measures and use of antibiotics on the farm.
Isolation and Identification of targeted bacteria
Necessary laboratory equipment and required media were used to culture the target enterobacteria. The isolation of E.coli was done by the method previously described by Vounba et al. (2019a)and identified by classical gallery tests and API 20 E (Biomerieux). For Salmonella isolation and identification, the method described by Bada-Alambedji et al. (2006)was used. The isolates, which tested positive for E.coli, Klebsiella spp and Salmonella spp, were sub-cultured on nutritive agar for antimicrobial susceptibility testing.
Antimicrobial susceptibility testing
All isolated strains were tested against 14 antibiotics commonly used in veterinary medicine belonging to 06 different antibiotics classes: aminoglycosides [Streptomycin, Gentamicin], Penicillin’s [Ampicillin, Ticarcillin, Amoxicillin + Clavulanic Acid] Cephalosporin’s [Cefuroxime, Cefotaxime, Ceftazidime, Ceftriaxone], Quinolone [Nalidixic Acid; Norfloxacin], and tetracycline’s [Doxycycline; tetracycline], Sulfoxides and Folate pathway inhibitor [Sulfamethazine + Trimethoprim]. Disc diffusion method was performed and interpreted according to the recommendations of the Antibiogram Committee of the French Society of Microbiology (CA-SFM/EUCAST) (Bonnet et al., 2019). Isolates were categorized as susceptible or non-susceptible to each antimicrobial. An isolate was considered susceptible, if it was sensitive to the entire antibiotic tested and non-susceptible if it was resistant or intermediate to this particular antibiotic. The isolate was Multi Drug Resistant (MDR) when it was non-susceptible to at least 1 agent in more than 3 antimicrobial categories as listed by (Magiorakos et al., 2012)when defining multi-drug resistance. Then according to antibiotics tested, 10 antibiotics belonging to 07 categories were used to classify strains as multi-resistant. Indeed a strain was considered multi-resistant when it was resistant to three (03) or more antibiotics belonging to at least three of the following categories: Cephalosporin 2nd generation (Cefuroxime); Cephalosporin 3rd generation (Cefotaxime; Ceftazidime; Ceftriaxone); β-Lactam 3rd generation (Ampicillin) ; β-lactam+ (Amoxicillin + Ac. Clavulanic); Foliate pathway inhibitor (Sulfamethazine + Trimethoprim); Tetracyclines (Tetracycline; Doxycycline); Aminoglycosides (Gentamicin).
Data analyses
Data were entered into Excel 2013 sheet and the prevalence of antibiotic resistance among different groups was calculated by dividing the number of resistant isolates in the group to the number of isolates tested.
Bacterial isolation and antibiotic susceptibility
Number of strains isolated
A total of 109 bacterial strains were identified from poultry samples as described above including 29 E. coli, 78 Klebsiella spp, and 2 Salmonella. Bacterial Strains were recovered from 72.7% of all samples. Klebsiella, E. coli and Salmonella spp isolation rates was 67.1, 20.5 and 2.3%, respectively. In the samples from pig farms, 85 strains were isolated including 37 E. coli, 47 Klebsiella and 01 Salmonella. Global isolation rate of target enterobacteria strains from pig samples was 87.93% with 56.89, 48.28, and 1.72%, respectively for Klebsiella spp, E. coli and Salmonella spp.
Resistance to antibiotics
Resistance to Beta-lactam antibiotics
In this family, resistance was more observed in penicillin antibiotics than cephalosporin antibiotics (Table 1). Indeed, in poultry, E. coli, Klebsiella Spp and Salmonella Spp resistances were high respectively for ampicillin (55.17, 46.15 and 50.00%), ticarcilline (48.28, 41.03 and 50.00%) and amoxicillin+ clavulanic acid (13.79, 21.79 and 0.00%) compared to cefuroxime (17.24, 20.51 and 50.00%), ceftriaxone (0.00, 1.28 and 0.00), ceftazidime (0.00, 5.13 and 0.00%) and cefotaxime (3.45; 1.28 and 0.00%) where relatively low resistance were observed. The same range of resistance was obtained in pigs with resistance being relatively high to ampicillin (54.05; 38.30 and 100%), ticarcilline (35.14; 27.66 and 0.00%) and amoxicillin + clavulanic acid (13.79; 21.79; 0.00%) compared to cefuroxime (2.70; 10.64 and 0.00%), ceftriaxone (5.41; 4.26 and 0.00%), ceftazidime (0.00; 0.00 and 0.00%) and cefotaxime (0.00; 4.26 and 0.00%).
Resistance to quinolones
Either in poultry or pigs, resistance was more observed for first generation quinolone than second generation quinolone. Indeed, in poultry, resistance of E. coli, Klebsiella spp and Salmonella spp to nalidixic acid was respectively, 31.03, 33.33 and 0.00% while resistance to norfloxacin was 10.3, 20.5, and 50.00%. In pigs resistance to nalidixic acid was 16.22, 29.79 and 0.00% higher than resistance observed for norfloxacin which was 10.81, 2.13, 0.00%, respectively for E. coli, Klebsiella spp and Salmonella (Table 2).
Resistance to aminoglycosides
In this class, resistance was more observed for Streptomycin than for Gentamycin. In poultry, resistance for E. coli, Klebsiella spp and Salmonella spp strains to streptomycin was 55.17, 51.28, and 0.00%, respectively; while resistance to gentamycin was 0.00, 6.41, and 0.00%, respectively. Similarly, in pigs, resistance was high to streptomycin (45. 95, 27.66, and 0.00%) than to gentamicin (0.00, 2.13, and 0.00%) (Table 3).
Resistance to other class of antibiotics
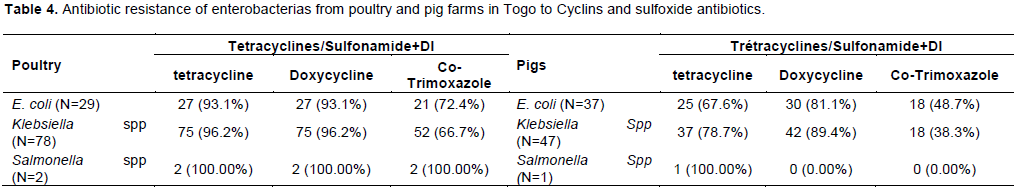
The most important resistance was observed to cyclin antibiotics (tetracycline; doxycycline) and the association sulfoxide – trimethoprim. In poultry, resistance of E.coli, Klebsiella spp and Salmonella strains was the same for tetracycline and doxycycline. Resistance to the association sulfoxide –trimethoprim was 72.41, 66.67, and 100.00%, respectively for E. coli, Klebsiella spp and Salmonella. In pigs, similar resistance was also observed to tetracycline (65.67, 78.72, and 100%), doxycycline (81.08, 89.36, and 0.00%) and Association sulfoxide –trimethoprim (48.65, 38.30, and 0.00%) (Table 4).
Multi –resistance to antibiotics
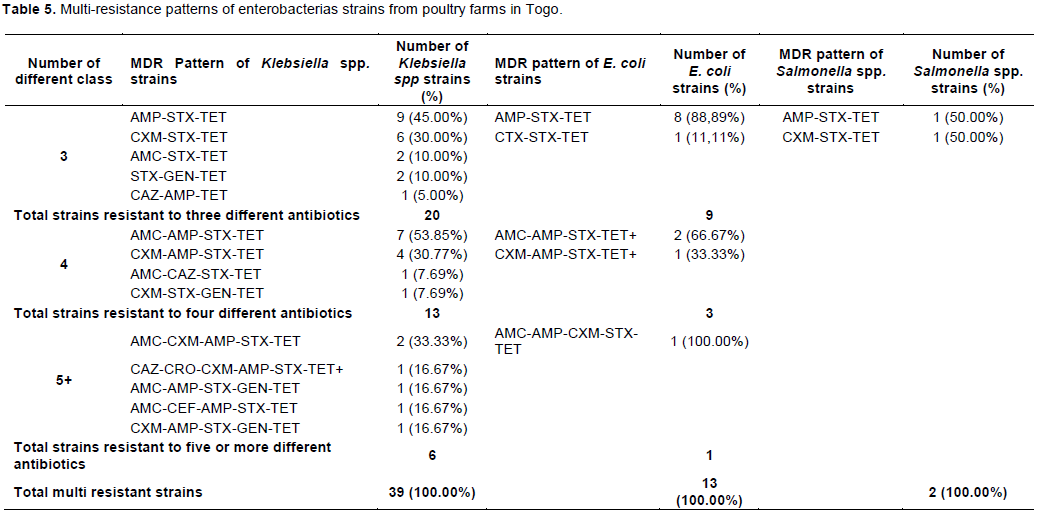
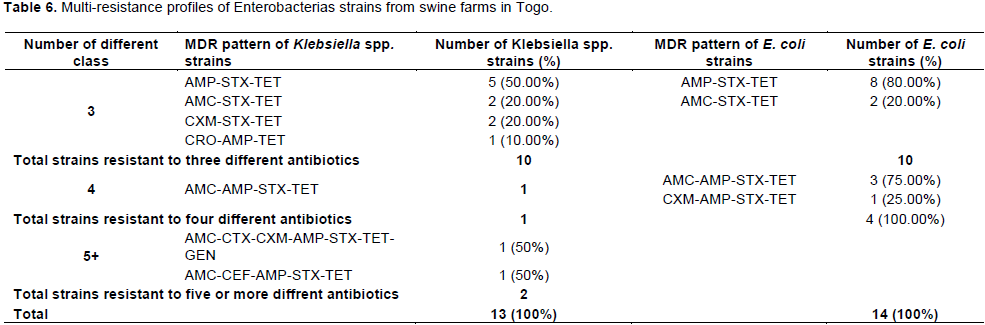
Tables 5 and 6 show multi-Drug resistance pattern of enterobacteria strains. In poultry, 44.83% of E.coli, 50% of Klebsiella spp and 100% of Salmonella strains were multi-resistant. In Pigs, 37.83 and 27.65% of E. coli and Klebsiella spp strains were multi-resistant. Being in bacteria from poultry or pig farms, the most frequent MDR pattern was simultaneous resistance to ampicillin – sulfoxide + trimethoprim -tetracycline.
Antibiotic use in farms
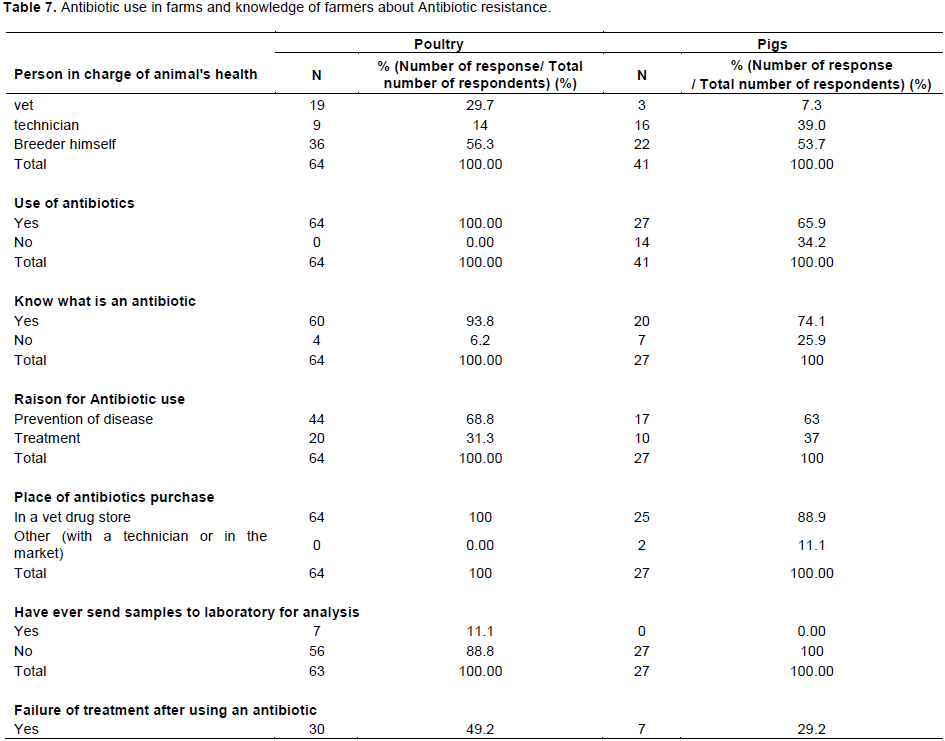
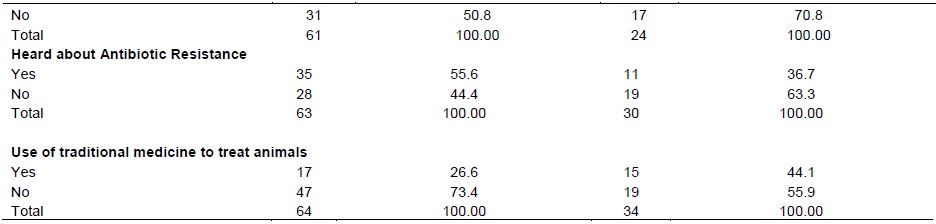
According to the results of the questionnaire reported in Table 7, being in poultry or in pigs farms (56.25 and 53.66% respectively), farmers managed the health of the animals on their own. Only 29.69 and 7.32%, for poultry and pig farms, respectively engaged the services of a veterinarian. All surveyed poultry farmers (100%) and the majority (65%) of pig farmers indicated that they used antibiotics in their farms. Among farmers who used antibiotics, 6.25% (for poultry) and 25.93% (for pig farms) were not able to define an antibiotic or did not know exactly what an antibiotic was among veterinary drugs they commonly used. In addition, 68.75 and 62.96% of poultry and pig farmers indicated that they mainly used antibiotics for prevention. All the antibiotics used in poultry and 88.89% used in pigs were purchased from a veterinary drug store but in the majority of the case without prescription. Hundred percent of pig farmers and 88.89% of poultry farmers had never sent samples for analysis in a laboratory. Forty nine percent and 29.17% of poultry and pig farms experienced a treatment failure after an antibiotic use and only 55.56% of poultry farmers and 36.67% of pig farmers heard about antibiotic resistance. Interestingly, 26.56 and 44.12% of farmers said they used traditional medicine (herbs) sometimes to treat their animals.
Antibiotics are widely used in both humans and livestock and have greatly contributed to better human and animal health. As a consequence, animal health, welfare and productivity have been improved in the livestock sector, and ultimately food safety, food security and nutrition and economic growth have shown positive development. However, the achievements in modern medicine and in the livestock sector due to the discovery and development of antibiotics are threatened by the global emergence of antimicrobial resistance (AMR) (FAO, 2019). Antibiotic use in food animals is highly increasing in many parts of the world (Van Boeckel et al., 2015)and It has been shown that antimicrobial resistance can be transmitted from animals to humans (Manishimwe et al., 2017). Given the context of a One Health approach (that is the perspective that the health of people, animals and the environment are interconnected), the emergence of resistance to antibiotics (antibacterial) in the primary production is an issue and a key task for all livestock sectors is to reduce the inappropriate use of antibiotics, as such use is closely linked to development of AMR in humans (Ozawa et al., 2012).
The focus of this study was to investigate the prevalence of antibiotic resistance of enterobacterias and to assess the use of antibiotics in poultry and pig farms in the peri-urban area of Lome in southern Togo. Although the poultry industry is rapidly evolving in Togo as in other West African countries, knowledge and skills related to biosafety management in poultry production are still low among poultry and pig farmers. This may be the cause of high rates of Enterobacterias strains obtained in the poultry and pig samples are 72.72 and 87.7% respectively. This high prevalence of enterobacterias obtained in farms is a concern as these farms can be the principal source of contamination of poultry or pig meat (Bada-Alambedji et al., 2006).
In general, enterobacterias strains exhibited very high level of resistance to tetracyclines, sulfoxide-trimethoprim corresponding to antibiotics commonly used in veterinary practice in Togo according to survey conducted during sampling followed by increasing resistances to streptomycin, ampicillin and nalidixic acid similar to the finding of Yassin et al. (2017) in China. The level of resistance observed in this study are also similar to other findings reported by some authors in different countries. In Senegal for example, Vounba et al. (2019a)investigated resistance of E.coli strains in poultry and reported high prevalence of non-susceptibility to tetracycline (92.2%), sulfisoxazole (80.8%), trimethoprim-sulfamethoxazole (76.7%), streptomycin (47.7%) and nalidixic acid (44.0%) very close to our findings and those of Sidibé et al. (2019)and Chen et al. (2004)in Mali and China respectively for Salmonella strains.
Among beta-lactam antibiotics tested, like in the study of Yassin et al. (2017)in China, third generation cephalosporins and the association amoxicillin + clavulanic acid remained very active on enterobacterias with low resistance rates recorded. Indeed, high resistances were observed for ampicillin, ticarcilline and cefuroxime compared to amoxicillin+ clavulanic acid, ceftriaxone, ceftazidime, and cefotaxime. This may be due to the fact that third generation cephalosporin’s are not commonly used by farmers. This is a good indicator as third generation cephalosporins constitutes antibiotic of critical importance in veterinary and human health (OIE, 2014; WHO, 2018). Concerning resistance to quinolones and aminoglycosides which also are important antibiotics for veterinary and human health, resistance level was low for second generation quinolone (norfloxacin) and for gentamycin due to the fact that this antibiotics are more expensive and also less used by farmers (Sidibé et al., 2019).
Resistance to at least one antibiotic was common in this study (100% of isolates from poultry). Multi-drug resistance defined by Magiorakos (Magiorakos et al., 2012)as resistance to at least 03 antibiotics belonging to 03 different categories or classes of antibiotics was high and most frequently observed in poultry, where 44.83% of E. coli, 50% of Klebsiella spp and 100% of Salmonella strains were multi-resistant. Potential selection factor for multiple resistance observed may be co-selection, as this is found in other studies (Ozawa et al., 2012). Indeed, it is shown that the usage of antibiotics in livestock promotes the development of antibiotic resistance in farm environments (Heuer et al., 2011). In this context, the resistance detected in enterobacterias isolates from poultry and pigs in this study may have been caused by the selection pressure due to antibiotic use in farms. Indeed, the survey during sampling showed that 100% of poultry farmers and 65.85% of pig farmers used antibiotics. As most of the farmers managed the health of their animals on their own and only few had a veterinarian, antibiotic use without prescription was high with some farmers who used antibiotic without knowing what exactly an antibiotic was. This is of concern because this indicates that veterinary drug shops sell antibiotics to farmers without prescription. The quality of the antibiotics used is another reason for antibiotic resistance as it was observed that there was no adequate measure in place to guarantee the quality of antibiotics imported into the country (Hestbjerg et al., 2002). In the present survey, it was found out that majority of farmers used the manure from livestock for crop production. Manure is a reservoir of resistant bacteria and antibiotic compounds, and its application on agricultural soils is assumed to significantly increase selection of resistant bacteria harboring antibiotic resistance genes in soil (Quaik et al., 2020). The genome location of resistance genes is sometime mobile genetic elements such as plasmids, integrons, and transposable elements and their horizontal transfer to bacteria adapted to soil and their environmental transmission to human without animal’s contact can represent a serious threat to human’s health (Heuer et al., 2011).
This is the first study in Togo to provide information on antibiotic resistance of enterobacterias isolated from different poultry and pig farms. The prevalence of antibiotic resistance of Enterobacterias to tetracyclins and sulfoxide-trimethoprim (more than 50%) was generally high and very low to gentamycin and third generation cephalosporin’s (less than 5%). Use of antibiotic without veterinary prescription among poultry and pig farmers was practiced as farmers managed the health of their animals on their own and ignorantly chose any antibiotic for their animals. Despite its limitations, this study showed that the antimicrobial resistance in the poultry and pig farms in Togo is a serious problem.
For this reason, studies of virulence genes and antimicrobial resistance at molecular level in multi-resistant strains are needed and should be the next step of this preliminary investigation. This will help assess the threat posed by antimicrobial resistance in animal’s production to human health. It is recommended that policies and regulations promoting controlled use of antibiotics be established and enforced in Togo. Food and Agriculture Organization of the United Nations (FAO, 2019)and Word Organization for animal health have provided guidance toward a responsible and prudent use of antimicrobial in pigs and poultry. This guidance can be used as a baseline to sensitize veterinarians and establish contextualized policies and regulations controlling the import, distribution and responsible use of antibiotics in animal production in Togo.
The authors have not declared any conflict of interests.
The authors appreciate the direction of animal production of Togo for authorization to collect samples in maritime region and laboratory of LAMICODA (Laboratoire de Microbiologie et de Contrôle des Denrées alimentaires) in Togo for facilitating sample conservation and processing. They are also grateful to laboratory of MIPI (Microbiologie Immunologie Pathologie Infectieuse) of EISMV for hosting the research program.
REFERENCES
|
Bada-Alambedji R, Fofana A, Seydi M, Ayayi Akakpo J (2006). Antimicrobial resistance of salmonella isolated from poultry carcasses in Dakar (Senegal). Brazilian Journal of Microbiology 37:510-515.
Crossref
|
|
|
|
Bonnet R, Bru J-P, Caron F, Cattoir V, Courvalin P, Dubreuil L, Jarlier V, Lina G, Merens A, Plesiat P, Ploy MC, Soussy CJ, Varon E (2019). Comité de l'antibiogramme de la Société Française de Microbiologie.
View
|
|
|
|
|
Chen S, Zhao S, White DG, Schroeder CM, Lu R, Yang H, McDermott P F, Ayers S, Meng J (2004). Characterization of Multiple-Antimicrobial-Resistant Salmonella Serovars Isolated from Retail Meats. Applied and Environmental Microbiology 70(1):1-7.
Crossref
|
|
|
|
|
Food and Agriculture Organization (FAO) (2015). revues nationales de l'élevage SECTEUR AVICOLE Togo.
View
|
|
|
|
|
Food and Agriculture Organization (FAO) (2009). The state of agriculture and Food, Livestock and the environment.
Crossref
|
|
|
|
|
Food and Agriculture Organization (FAO) (2019). Prudent and efficient use of antimicrobials in pigs and poultry FAO Animal production and health manual.
View
|
|
|
|
|
Founou LL, Amoako DG, Founou RC, Essack SY (2018). Antibiotic Resistance in Food Animals in Africa: A Systematic Review and Meta-Analysis. Microbial Drug Resistance 24(5):648-665.
Crossref
|
|
|
|
|
Hestbjerg L, Aarestrup F, Johannes S (2002). Quantification of bioavailable chlortetracycline in pig feces using a bacterial whole-cell biosensor. Veterinary Microbiology 87:51-57.
Crossref
|
|
|
|
|
Heuer H, Schmitt H, Smalla K (2011). Antibiotic resistance gene spread due to manure application on agricultural fields. Current Opinion in Microbiology 14(3):236-243.
Crossref
|
|
|
|
|
Lhoste P (2009). L'élevage africain, source possible d'une révolution alimentaire attendue?
View
|
|
|
|
|
Magiorakos AP, Srinivasan A, Carey RB, Carmeli Y, Falagas MY, Giske CG, Harbarth S, Hindler JF, Kahlmeter G, Olsson-Liljequist B, Paterson DL, Rice LB, Stelling J, Struelens MJ, Vatopoulos A, Weber JT, Monnet DL (2012). Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clinical Microbiology and Infection 18(3):268-281.
Crossref
|
|
|
|
|
Manishimwe R, Buhire M, Uyisunze A, Turikumwenayo JB, Tukei M (2017). Caractérisation d'Escherichia coli résistant aux antibiotiques dans différents systèmes avicoles de la province de l'Est et de la ville de Kigali au Rwanda. Revue d'élevage et de Médecine Vétérinaire Des Pays Tropicaux 70(1).
Crossref
|
|
|
|
|
Mensah SEP, Koudandé OD, Sanders P, Laurentie M, Mensah GA, Abiola FA (2014). Antimicrobial residues in foods of animal origin in Africa: public health risks. Revue scientifique et technique (International Office of Epizootics) 33:3.
Crossref
|
|
|
|
|
OIE (2014). Critères utilisés pour la catégorisation: Liste OIE des agents antimicrobiens importants. Organisation mondiale de la santé animale 33(0):1-9.
|
|
|
|
|
Ozawa M, Makita K, Tamura Y, Asai T (2012). Associations of antimicrobial use with antimicrobial resistance in Campylobacter coli from grow-finish pigs in Japan. Preventive Veterinary Medicine 106(3-4):295-300.
Crossref
|
|
|
|
|
Quaik S, Embrandiri A, Ravindran B, Hossain K, Al-Dhabi NA, Arasu M V, Ignacimuthu S, Ismail N (2020). Veterinary antibiotics in animal manure and manure laden soil: Scenario and challenges in Asian countries. Journal of King Saud University-Science 32(2):1300-1305.
Crossref
|
|
|
|
|
Schar D, Sommanustweechai A, Laxminarayan R, Tangcharoensathien V (2018). Surveillance of antimicrobial consumption in animal production sectors of low- and middle-income countries: Optimizing use and addressing antimicrobial resistance. PLoS Medicine 15(3):1-9.
Crossref
|
|
|
|
|
Sidibé S, Traoré B, Sidi Y, Broulaye A, Afou D, Oumar B (2019). Antibiorésistance des souches de Salmonella gallinarum isolées en aviculture moderne en zones périurbaines au Mali. Revue Elevage et de Medecine Veterinaire Pays Tropicaux 72(4):1-6.
Crossref
|
|
|
|
|
Van Boeckel TP, Brower C, Gilbert M, Grenfell BT, Levin SA, Robinson T P, Teillant A, Laxminarayan R (2015). Global trends in antimicrobial use in food animals.
Crossref
|
|
|
|
|
Vounba P, Arsenault J, Bada-Alambédji R, Fairbrother JM (2019a). Prevalence of antimicrobial resistance and potential pathogenicity, and possible spread of third generation cephalosporin resistance, in Escherichia coli isolated from healthy chicken farms in the region of Dakar, Senegal. PLoS ONE 14(3).
Crossref
|
|
|
|
|
Vounba P, Rhouma M, Arsenault J, Bada Alambédji R, Fravalo P, Fairbrother JM (2019b). Prevalence of colistin resistance and mcr-1/mcr-2 genes in extended-spectrum β-lactamase/AmpC-producing Escherichia coli isolated from chickens in Canada, Senegal and Vietnam. Journal of Global Antimicrobial Resistance 19:222-227.
Crossref
|
|
|
|
|
Vounba P, Yaghouba K, Ndiaye C, Arsenault J (2018). Molecular Characterization of Escherichia coli Isolated from Chickens with Colibacillosis in Senegal. Foodborne Pathogens and Disease 15(8):1-9.
Crossref
|
|
|
|
|
World Health Organization (WHO) (2018). WHO list of Critically Important Antimicrobials (CIA).
View
|
|
|
|
|
Yassin AK, Gong J, Kelly PLG, Guardabassi L, Wei L, Han X, Qiu H, Price S, Cheng D, Wang C (2017). Antimicrobial resistance in clinical Escherichia coli isolates from poultry and livestock, China. PLoS ONE 12(9):1-8.
Crossref
|
|