ABSTRACT
There is presently no information on the occurrence, abundance and diversity of airborne bacteria in Botswana hospitals. There is also growing concern in the global spread of antibiotic resistant bacteria that continue to emerge and pose serious challenge to human health. This study was aimed at determining the occurrence, relative abundance and diversity of airborne bacterial species and their antibiotic resistance patterns. Correlation between the meteorological conditions and the bacterial concentrations was also determined. Air impaction method was applied for the collection of airborne bacteria on selective media, antibiotic resistance was determined by screening isolates for resistance phenotypes and polymerase chain reaction (PCR) was applied for detection of resistance genes to clinically relevant antibiotics. The assessment of total airborne bacteria in hospital units revealed high abundance of airborne bacteria in paediatric wards than in the operating theatres. The highest bacterial concentrations were observed at the paediatric wards in Palapye (PPH; 7.9 × 102 CFU/m3), Maun (LMH; 6.4 ×102 CFU/m3) and Francistown (NRH; 5.8 ×102 CFU/m3). Diverse airborne bacterial species were observed with high concentration recorded for Pseudomonas species in all three hospitals’ units. The frequency of antibiotic resistance genes detected in bacterial isolates were; dfr1 (36%), mph(A) (26%), ermC (12%), strB (10%) and intI1 (16%). The study provide evidence suggesting air in hospitals’ units as a hotspot for potentially pathogenic bacteria and antibiotic resistance genes, hence the need to develop surveillance tools for monitoring the movement of airborne bacteria in hospitals in order to mitigate possible spread of nosocomial infections.
Key words: Airborne bacteria, antibiotic resistance genes, operating theatre, paediatric, human health.
Airborne microorganisms are widespread in the atmosphere, contributing to various health aspect in
human and the ecosystem (Zhai et al., 2018). The natural sources of airborne microorganisms such as bacteria, viruses, fungi include water, soil and vegetation (plants), anthropogenic sources such wastewater and faecal material from human and animal activities also contribute to the concentration of airborne microorganisms in the atmosphere (Zhao et al., 2014, Smets et al., 2016). Built environments (houses and community spaces) are major sources of airborne bacteria associated with adverse human health risks, outdoor bacteria frequently enter indoors of buildings through open doors and windows (Prussin et al., 2015; So et al., 2017). Microbial contamination of indoor hospital environments like operating theatre and paediatric wards may pose as a health risk to patients because of confined spaces. Indoor spaces may harbour aerosols containing microbes and allow to build to infection levels (Augustowska and Dutkiewicz, 2006). Persistent exposure to pathogenic airborne microorganisms leads to severe respiratory disorders, allergic reactions, hypersensitivity pneumonitis and sick building syndromes (Górny et al., 2002; Fracchia et al., 2006; Griffin et al., 2003; Yassin and Almouqatea, 2010). The most susceptible population to airborne nosocomial infections are children, the elderly and immunocompromised patients.
The atmospheric microbial contamination in hospital environment, mainly in operating theatre wards continue to increase prevalence of nosocomial infections which lead to high mortality rates among hospitalized patients going through post-operative surgery (Weigelt et al., 2010; Hailemariam et al., 2016; Spagnolo et al., 2013). Findings from these studies indicated that pathogenic microorganisms in operating theatres are acquired from exogenous sources such as the operating theatre surrounding environment, surgical personnel and equipment brought to the sterile field during operation. In previous studies, the predominant bacterial species isolated from operating theatre wards were Staphylococcus species (the leading cause of surgical site infections, SSI), Enterococcus, Escherichia coli and Pseudomonas aeruginosa (Wolcott et al., 2009; Hailemariam et al., 2016). In addition to the operating theatre, the paediatric wards have also been widely studied for their role as source of bacterial infections. The most prevalent bacterial genera isolated in paediatric wards was also Staphylococcus aureus (Okten and Asan, 2012; Qudiesat et al., 2009). Another similar study conducted in a paediatric ward was by Coetzee et al. (2013), which indicated the highest isolation of P. aeruginosa. Serratia marcescens, Acinetobacter and Klebsiella species in paediatric wards (Behzadnia et al., 2014).
Antibiotic resistance has caused concern in hospitals where bacterial infections are the most frequent cause of diseases in children and the immunocompromised individuals. Hospital environments can act as reservoirs of multi-drug resistant pathogens, with the most contaminated site being the labour/delivery room followed by the dressing room and the operation theatre (Mathias et al., 2000; Solomon et al., 2017). Centre for Disease Control and Prevention (CDC, 2013) has documented increases in the number of nosocomial infections caused by antibiotic-resistant bacteria (ARB). Studies have also highlighted antibiotic resistance genes (ARGs), which can spread by adhering to airborne particles and their abundance in the indoor compared to outdoor sources such as soil water and sediments (Li and Yao, 2018). The bacterial contamination in operation theatres and paediatric wards has a major impact in terms of infection control in the hospital, health risk to the patients and medical staff, hence the need for this study. Airborne ARB and ARGs in hospital settings is poorly understood, with many studies focusing on other fomites particularly hospital equipment. Therefore, Airborne ARB and ARGs could pose serious human health issues especially in developing countries with poor infrastructure, poor infection control and lack of surveillance data on infectious agents.
An operating theatre is one of the hospital’s supposedly sterile facilities normally without windows, characterized by feature controlled temperature and humidity, where surgical operations are performed. It is usually separated from other hospital facilities, and accessed only by authorized personnel. The paediatric ward is the hospital’s facility assigned to provide health care for children under the age of 12. The operating theatre and paediatric wards were both selected in determination of the risk and possible transmission of opportunistic bacterial pathogens in highly immunocompromised surgery patients and children. This study was primarily focused on determining the occurrence, abundance, and relative diversity of bacteria in the indoor air of hospitals (paediatric and operating wards) in Botswana. The second objective was to further identify the antibiotic resistance patterns of airborne bacterial isolates by detecting the antibiotic resistance phenotypes and genotypes in selected bacteria. Furthermore, this research was extended to find correlation between the meteorological conditions (temperature and relative humidity) and the bacterial concentrations in the paediatric and operating wards.
Description of study sites
The study was conducted at Palapye Primary Hospital (PPH), Letsholathebe II Memorial Hospital (LMH) and Nyangabwe Referral Hospital (NRH). PPH is located in Palapye town in the Central district, built in the 1970s and caters for the surrounding villages as it receives referrals from local and nearby clinics as well as rural health posts. LMH is one of the recently (opened in 2008) constructed hospitals in the town of Maun, Ngamiland district. LMH is located along the edges of the Okavango Delta in the region that has been previously hit by major southern African epidemics and
pandemics; influenza, smallpox, bubonic plague, sleeping sickness, malaria, and bilharzia (Molefi, 2001, 2003; Mosothwane, 2015). NRH is located in the City of Francistown, North East district, built around 1989 is by far the largest hospital in the north eastern Botswana, and thereby receives most referrals from various regions across the country.
Sample collection and isolation of airborne bacteria
Bacteria suspended in the indoor air of operating theatre wards and paediatric wards of the three hospitals were assessed. The air samples were collected between March 2016 and April 2017. In 2016, samples were collected in 2 seasons (summer and winter), and the other samples in 2017 (autumn). Botswana’s climate is characterized by four seasons; hot summers (November, December and January), wet autumns (February, March and April), cold and dry winters (May, June and July) and arid windy springs (August, September and October). Air samples were collected in duplicates once in a month, in the morning (9 am to 12 pm) and afternoon (2 to 5 pm) sessions.
The principle of air impaction was applied for the collection of airborne bacterial samples. Air was directed against the culture media plates using a portable Microbial Air Sampler MAS-100 NT® device (Merkmillipore, Merck KGaA Darmstadt, Germany), which is considered the gold standard among air samplers, at a flow rate of 100 L/min. The air sampler was placed 1.5 m above ground level within the hospital wards. Airborne bacteria were collected by impaction onto various selective and differential agar media for presumptive selective isolation of bacterial species; Staphylococcus species, Brucella species, Pseudomonas species, Campylobacter species, Listeria species, Salmonella species, and E. coli (Table 1). Cycloheximide (1 μg/μl, Sigma-Aldrich Co., St. Louis, MO, USA), previously shown not to affect bacterial counts was added to each media to inhibit growth of fungi (particularly saprophytic molds). Aerosolized bacteria were collected for 10 min on each of the 7-selective/differential media and non-selective nutrient agar media (for total airborne bacteria). Different selective media were used to target various potentially pathogenic bacterial species commonly associated with multi-drug resistance (Tapela and Rahube, 2019). After each sampling session, the culture media plates were incubated at appropriate aerobic/anaerobic and temperature conditions as shown in Table 1.
Quantification and identification of airborne bacteria
To estimate airborne bacterial concentrations, the number of colony forming units growing in the respective media were counted and related to the volume of the air sampled. The MAS-100 NT® air sampler was set at an inflow rate of 100 L/min, the volume of air collected by air sampler per plate was estimated to 1000 L for 10 min. Bacteria growing on the selective/differential plates were counted and expressed as the average values of colony-forming units per cubic meter (CFU/ m3). The actual microbial count per m3 of air was corrected and calculated using the integrated Feller conversion table (or FELLER’s statistical correction table) provided by MAS-100 NT® air sampler operating manual.
Bacteria were then identified by morphological procedures according to Bergeys Manual of Determinative Bacteriology (Holt et al., 1994). Morphological characterization of the airborne bacteria was carried out by examining colony size, shape, and cellular arrangement. Several colonies were randomly picked and further confirmed by Gram stain and biochemical tests. A total of 300 isolates were randomly selected, purified and stored at -80°C in nutrient broth with 50% glycerol solution prior to DNA extraction and antibiotic resistance testing.
Antibiotic resistance testing
A total of 300 pure culture isolates were assayed for resistance against various antibiotics at clinical breakpoint concentrations; ampicillin (32 μg/ml), cephalosporin (32 μg/ml), penicillin (16 μg/ml), erythromycin (8 μg/ml), Sulfonamide (512 μg/ml), meropenem (4 μg/ml), tetracycline (16 μg/ml), ciprofloxacin (4 μg/ml), streptomycin (30 μg/ml) and trimethoprim (16 μg/ml), similar to those reported as minimum inhibitory concentrations (MIC) for standard antibiotic susceptibility testing (Rahube et al., 2014a). These clinically relevant antibiotics were selected for identification of resistance phenotypes and genotypes in both Gram positive and Gram negative bacteria. Isolates were aseptically transferred using sterile toothpicks or sterile disposable inoculation loops into respective 50 grid squared nutrient agar plates containing the respective antibiotic concentrations. The antibiotic resistance testing method used was previously described in a recent publication by Tapela and Rahube (2019).
DNA extraction and PCR detection of antibiotic resistance genes
DNA extraction was carried out using the method described by Neela et al. (2015) with some modifications. Briefly, 1.5 ml of the overnight-cultured bacterial cells were harvested by centrifugation at 13,000 rpm for 10 min; the pellet was resuspended in 600 μl of lysis buffer (50 mM Tris hydrochloride -50 mM EDTA, pH 8.0, 50 mM NaCl and 5% SDS). To re-suspend the pellet, and avoiding formation of foam, the tube was inverted 25 times and gentle pipetting was done. Followed by incubation at 37°C for 30 min. Nucleic acid purification was done by adding 200 μl of phenol and chloroform/isoamyl alcohol, followed by addition of 200 μl of chloroform and vortexing for 20 s. The sample was then centrifuged at 13, 000 rpm for 3 min to pellet the protein, and the supernatant was transferred into a new tube for nucleic acid precipitation. This was followed by adding 600 μl of isopropanol and precipitation of the DNA by centrifugation at 13,000 rpm for 30 min to obtain the DNA pellet. The DNA pellet was washed in 500 μl ice-cold ethanol (70%), and dried for 5 min.
The DNA was resuspended in 100 μl TE buffer and stored at -20°C prior to PCR analysis. The antibiotic resistance genes were detected by PCR method using specific primers targeting resistance genes to different clinically important antibiotics; Sulfonamide (sul1 and sul2), tetracycline (tetA and tetB), erythromycin (ermA, ermB and ermC), macrolides (mphA), streptomycin (strA and strB), quinolone (qnrA), trimethoprim (dfr1) and class 1 integron mobile element (intI1) were used to amplify the respective antibiotic resistance gene targets (Table 2).
The PCR amplification was performed with a PCR Thermal Cycler (Proflex PCR system, Applied Biosystems). The PCR mixture contained: 2 μl of template DNA, 12.5 μl premix (EmeraldAmp® PCR Master Mix, TAKARA BIO INC), 1.5 μl of each of the primers (forward and reverse) and 7.5 μl molecular grade water. The following thermocycler parameters were used: initial denaturation at 95°C for 5 min followed by 30 cycles at 98°C, annealing for 40 s to 1 min (the annealing temperature varied among the primers, as indicated on Table 2), this was followed by extension at 72°C for 1 min with a final elongation at 72°C for 1 min. DNA of previously confirmed target antibiotic resistant isolates were used as PCR positive controls and a none-template reaction included as negative control. The PCR products were analyzed using gel electrophoresis on a 1.0% (w/v) agarose gel stained with ethidium bromide. Electrophoresis was run at a constant voltage of 60 V for about 1 h 30 min in a horizontal tank with 0.5X concentration of Tris-Borate-EDTA (TBE) buffer. After electrophoresis, the gels were visualized on a UV transilluminator.
Meteorological data and statistical analysis
Temperature (Tm) and relative humidity (RH) were recorded at each sampling site with a handheld Thermocron iButtons (Dallas Semiconductors, Model DS1920). Tm and RH were then reported as an average of 1 h 30 minutes sampling period at each sampling location. The means and standard deviations were analysed using Graphpad Prism software (version 7.0). Differences between different hospitals, sites (operating theatre and paediatric wards) were assessed using the analysis of variance (ANOVA) test. Results with a p-value less than or equal to 0.05 (p ≤ 0.05) were considered to be statistically significant.
Occurrence and abundance of airborne bacteria in operating theatre and paediatric wards
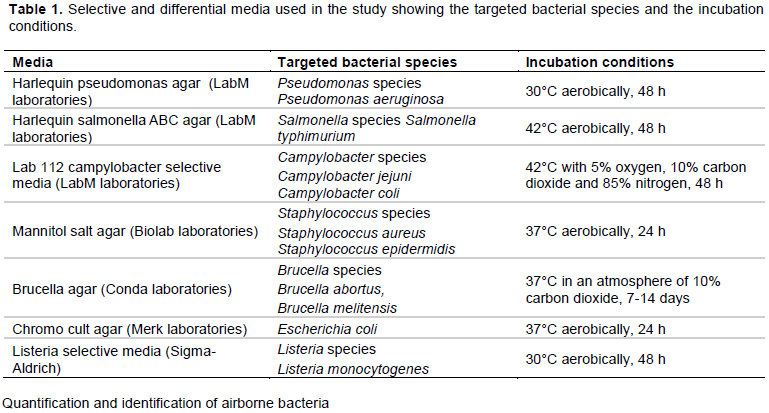
The assessment of the total airborne bacteria in hospitals revealed higher abundance of bacteria in paediatric wards than in the operating theatres (p < 0.05). The highest bacterial concentration was observed at PPH paediatric ward; 7.9 ×102 CFU/m3, followed by LMH paediatric ward (6.4 ×102 CFU/m3), followed by NRH paediatric ward (5.8 ×102 CFU/m3). The highest bacterial concentration in operating theatre was also observed at PPH (3.7 ×102 CFU/m3), which was statistically significant compared to NRH (2.7 ×102 CFU/m3) and LMH (2.6 ×102 CFU/m3) as shown in Figure 1.
There is a consistent difference in bacterial concentrations between operating theatre and paediatric wards among all the hospitals. The results are expected, and the higher bacterial concentrations in paediatric wards could have been greatly influenced by the nature and usage, including higher population of patients at the ward compared to those of the operating rooms. Several other factors such as lack of ventilation systems and depending on the outdoor air may have attributed to the vast difference in bacterial concentration between the two different hospital units. Qudiesat et al. (2009), reported that microbial concentrations in the operation rooms were dependent on the hospital setup; operating theatre wards nearer to other hospital units had higher bacterial concentrations compared to those located away from the rest of the hospital units. The level of hygiene and practice of aseptic techniques, the dirty areas separate from the operating theatre rooms, the patient flow from arrival to discharge may also contribute to airborne bacterial concentrations. The high bacterial concentrations in PPH operating theatre ward could be attributed by the washrooms located adjacent to the entrance of the operating room as well as uncontrolled traffic and activities by health care workers around the areas in close proximity to the operating theatre. The PPH operating theatre room had the highest airborne bacterial concentration compared to other operating theatres, PPH operating theatre is the hospital’s only major operating room, and therefore it experiences large volumes of activities on a daily basis. The PPH hospital is more than 30 years old, the design, layouts, furnishings, fittings, floor coverings and ventilation systems may have a significant impact on the cleaning of the unit consequently giving rise to the airborne bacterial contamination. In addition, PPH autoclave room that receives dirty materials and equipment from the operating room and the rest of the hospital is closer to the operating room thereby increasing the transfer of airborne bacteria to the operating room. Some studies have also reported that the sink drains are frequently colonized by large numbers of bacteria therefore serve as potential reservoir for aerosolized pathogens or opportunistic microorganisms (Kotay et al., 2018).
NRH operating theatre ward also had concerning abundance of airborne bacteria. This facility is also poorly located; the main entrance to the ward is along one of the busy corridors of the hospital and also opposite the Intensive Care Unit (ICU). Compared to PPH, NRH has a relatively better infrastructure however, the ventilation system of most of the hospital units at NRH are malfunctioning, there have been reports from the NRH operating theatre wards that the air conditioning systems were for a long time in bad conditions (personal communication). The poor air ventilation and unstable indoor temperatures were experienced during sampling. The findings at LMH operating theatre ward were as expected, the bacterial concentration was at the lowest compared to the other hospitals’ operating theatre wards indicating a much cleaner indoor air, which could have been attributed to the cleanliness observed at the ward. PPH bacterial loads for both operating theatre and paediatric wards remains a major concern as revealed by a statistical difference when compared with the other two hospitals. Similar studies on the assessment of bacterial concentrations in hospital operating rooms such as at State Railway Hospital (Lublin, Poland) revealed that the airborne bacterial concentrations varied from 10 to 102 CFU/m3 (Augustowska and Dutkiewicz, 2006), which is in agreement with the findings of this study. The results are also consistent with other study findings elsewhere (McCarthy et al., 2000; Li and Hou, 2003), where microbial concentrations in operating rooms were notably lower compared to other hospital units.
The paediatric wards care for children less than 12 years. Those under the age of 7 years are usually accompanied by parents during hospitalization hence the higher population of the occupants, consequently this setup leads to increased inflow of airborne bacteria. In addition, products such as food, fruits and inanimate objects like toys from the external environment are frequently brought in by visitors into the paediatric wards and may contribute to the increase in airborne bacteria; these were documented as important source of hospital contamination (Qudiesat et al., 2009). Similar to operating theatre wards results described previously, poor and deficient hygienic conditions due to minimal disinfection procedures across the three hospital’s paediatric wards might have given rise to the higher concentrations of observed airborne bacteria. The high abundance of airborne bacteria within the PPH paediatric ward might also be attributed to the age of the building. PPH paediatric ward is dimensionally too small to accommodate the inpatients, the ward is made up of a single room that is usually congested resulting in poor air circulation and also making it hard for cleaning. NRH paediatric ward also had high concentration of airborne bacteria, the ward has multiple rooms that are incompletely partitioned and separated from each other, hence allowing for free airflow between the rooms. There are no air conditioning systems at the NRH paediatric wards, therefore the ward depends on windows and doors for ventilation and this could lead to more inflow of airborne bacteria from the surrounding outdoor air. The paediatric ward of the LMH is ideally constructed, the rooms are well spaced making it easy for appropriate cleaning, the air conditioning system is in good condition, and also the ward is not congested. However, the results still reveal relatively high abundance of airborne bacteria compared to other paediatric wards. A study on airborne transmitted infections in hospitals revealed mean bacterial contamination values ranging between 3.0 -7.0 ×102 CFU/m3 during hours with less human activities and 6.0 ×103 CFU/m3 during bed making hours (Woldu et al., 2013). Roberts et al. (2006) ascertains that influence of anthropogenic activities have an effect on the rise of airborne microbial concentrations in hospital rooms.
Diversity and abundance of airborne bacterial species in operating theatre and paediatric wards
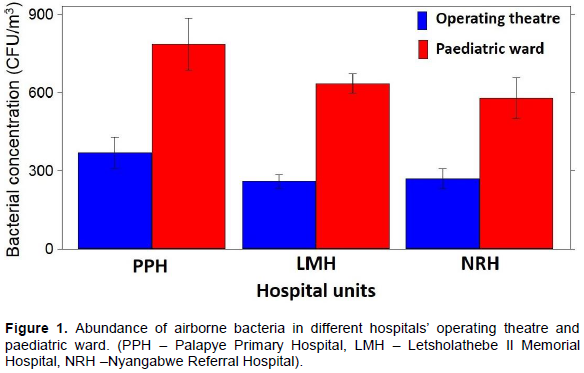
The occurrence of diverse airborne bacterial species was observed in all three hospitals’ operating theatre and paediatric wards. In operating theatre wards, high concentration was recorded for Pseudomonas species; 1.3 ×102 CFU/m3 (PPH), 1.2 ×102 CFU/m3 (LMH and NRH), Brucella species; 7.4 ×101 CFU/m3(NRH), 4.4 ×101 CFU/m3 (PPH), 2.7 ×101 CFU/m3 (LMH), ), Listeria species; 4.9 ×101 CFU/m3(PPH), 3.6 ×101 CFU/m3(LMH) and 3.3 ×101 CFU/m3 (NRH) and Staphylococcus species; 3.3 ×101 CFU/m3 (PPH), 2.4 ×101 CFU/m3 (NRH), 2.0 ×101 CFU/m3 (LMH),). The least abundant bacteria were E. coli; 0.2 x101 CFU/m3 (LMH), 0.1 ×101 CFU/m3 (PPH and NRH), and Campylobacter species 0.2 ×102 CFU/m3 (NRH), 0.1 ×101 CFU/m3 (LMH and PPH). Salmonella species were not detected in all three hospital operating theatre wards (Figure 2a).
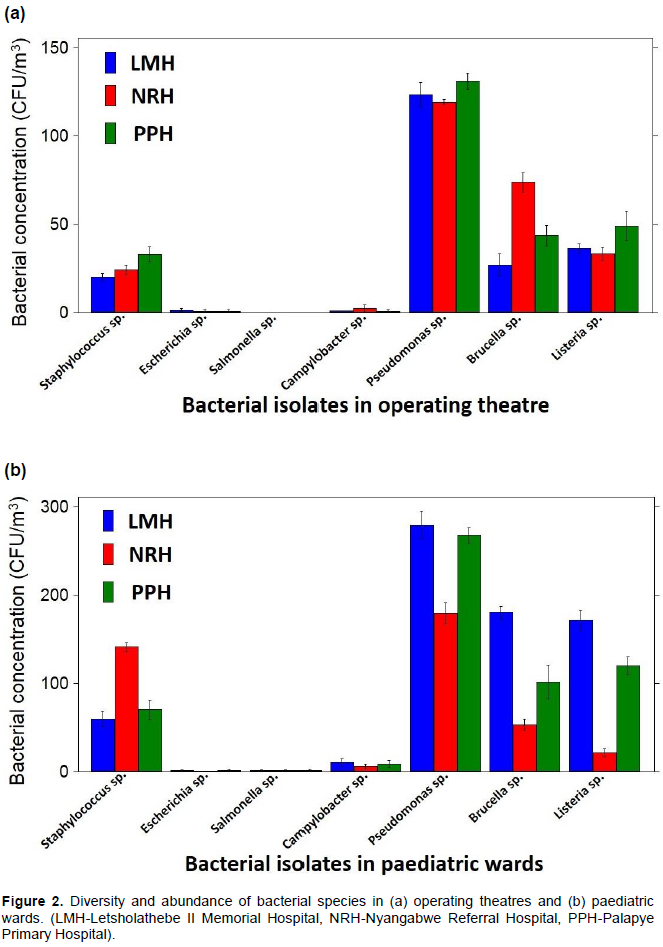
Similarly in paediatric wards, the highest concentrations were recorded for Pseudomonas species; 2.8 ×102 CFU/m3 (LMH), 2.7 ×102 CFU/m3 (PPH), 1.8 ×102 CFU/m3 (NRH), Listeria species; 1.7 ×102 CFU/m3 (LMH),1.2 ×102 CFU/m3 (PPH), 2.1 ×101 CFU/m3 (NRH), Brucella species; 1.8 x102 CFU/m3 (LMH), 1.0 ×102 CFU/m3 (PPH), 5.3 ×101 CFU/m3 (NRH) and Staphylococcus species; 1.4 ×102 CFU/m3 (NRH), 7.1 ×101 CFU/m3 (PPH), 5.9 x101 CFU/m3 (LMH). The least abundant bacteria were Campylobacter species; 1.1 ×101 CFU/m3 (LMH), 0.9 ×101 CFU/m3 (PPH), 0.6 ×101 CFU/m3 (NRH). E. coli and Salmonella species at the lowest concentrations (0.1 ×101 CFU/m3) in all paediatric wards (Figure 2b)
Pseudomonas species were the most abundant bacteria from all the operating theatre and paediatric wards. Brucella, Listeria and Staphylococcus species were also predominant. Findings from other studies where airborne bacteria were isolated in hospitals are in accordance with our results (Borrego et al., 2010; Qudiesat et al., 2009; Gilbert et al., 2010; Solomon et al., 2017). These studies also revealed higher prevalence of potentially pathogenic bacteria in the hospital air such as Staphylococcus species and Streptococcus species. Pseudomonas species, especially P. aeruginosa was also found to be prolific in the hospital atmosphere. P. aeruginosa is of particular importance due to its immense association with fatal nosocomial outbreaks (Hota et al., 2009; Gilbert et al., 2010). It is amongst the leading causes of nosocomial pneumonia associated with high mortality rates in healthcare settings (Krzowska-Firych et al., 2014). In hospitals P. aeruginosa has been previously isolated from various environments especially around water source plumping systems such as sinks and drains (Prussin et al., 2015; Solomon et al., 2017). Hota et al. (2009) support these findings and further suggest that P. aeruginosa is aerosolized during hand washing thereby easily transmitted into the atmosphere. Other abundant bacteria genera in our study were Listeria species, these were also observed in similar studies conducted in hospitals (Sarica et al., 2002), where airborne Listeria among others were reported to be predominant in various areas of the hospital under study. Listeria species such as Listeria monocytogenes have been reported to be a health concern contributing to fatality rates of 20-30% among hospitalized patients (Swaminathan and Gerner-Smidt, 2007). These bacteria although they are foodborne, they are likely to enter the atmosphere from the hospitalized patients and/or their food. This is supported by the relatively higher concentration of the Listeria species in paediatric wards where food is allowed compared to operating theatre wards.
Another rather unusual bacterial occurrence to highlight is the Brucella species in all the three hospitals’ operating theatre and paediatric wards. Brucella is a genus of Gram negative bacteria of zoonotic origin. It’s occurrence in hospital units certainly warrants further investigation. The gastrointestinal bacteria which is also classified among foodborne pathogens, Salmonella species were only isolated from the paediatric wards in LMH and PPH at low concentrations. Similar to Listeria, Salmonella species isolated may also have entered the air from the contaminated food from the hospital kitchen or the food brought in by visitors, or aerosolized upon release during baby nappy changing of an infected child. The airborne Salmonella species, although at significantly low concentrations are likely to settle on uncovered drinking water and food resulting in contamination. Salmonella outbreaks have been reported in hospitals (mostly paediatric wards and nurseries) and nursing homes (CDC, 2013).
Distribution of antibiotic resistance genes in bacterial species
Bacterial species (n=50) that exhibited resistance to more than 7 tested antibiotics were selected and isolated. The DNA of the isolates was screened for presence of targeted ARGs. The frequency (% occurrence) of ARGs detected in the selected isolates were; dfr1 (36%), mphA (26%), ermC (12%), strB (10%), In addition, genes encoding the class 1 integron mobile element intI1 (16%) were also detected. Antibiotic resistance genes; tetA, tetB, qnrA, strA, ermA, ermB, sul1 and sul2 were not detected in any of the bacterial isolates tested (Table 3).
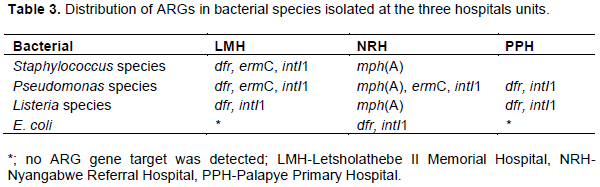
The gene that confer resistance to trimethoprim (dfr1) was detected in most of the bacterial isolates (35%). dfr genes were widespread amongst bacterial species; these were identified from Staphylococcus species (isolated from LMH operating theatre and paediatric ward) and E. coli (isolated from NRH paediatric ward), Listeria and Pseudomonas species (isolated from PPH, LMH operating theatres and paediatric wards). The high frequency of dfr genes in these environments is likely due to the wide application of trimethoprim in the treatment of urinary tract infections that are common in hospitalized patients. According to Jury et al. (2010), dfr genes are mostly associated with Class 1 integron mobile elements (conserved with sulfonamide, sul1 resistance gene) that can be horizontally co-transferred by plasmids, contributing to rapid spread of multiple resistance genes amongst bacterial communities.
In the current study, the presence of gene encoding macrolide 2'-phosphotransferase I, mphA gene was identified in 26% of the airborne bacterial species; Staphylococcus, Pseudomonas and Listeria. The bacterial isolates from which mphA gene was isolated were from NRH paediatric ward, LMH operating theatre ward and the paediatric ward. The findings of this study are in agreement with those from Nakamura et al. (2000) where mphA gene was identified in P. aeruginosa clinical isolates. mphA gene has also been detected in clinical isolates belonging to Enterobacteriaceae and Staphylococcaceae (Sutcliffe et al., 1996; Faccone et al., 2018). Other macrolide resistance genes, ermA and ermB, that confer resistance to erythromycin were not identified in any of the bacterial isolates; however the percentage of the airborne bacterial isolates harboring the ermC gene was 12%. The bacterial strains carrying the ermC belonged to the Staphylococcus and Pseudomonas species, isolated from LMH and NRH paediatric wards. This study detected genes conferring resistance to macrolides in most of the Pseudomonas species. Similar results were found in a study conducted by Türkyilmaz et al. (2010) where genes conferring resistance to macrolides like ermC were also detected in the majority of Pseudomonas species.
Class 1 integrons (intI1) were detected in 16% of the bacterial isolates; Staphylococcus, Pseudomonas, Listeria
species and E. coli from all the three hospitals. The intI1 gene is highly associated with multiple antibiotic resistances and this gene also regulates the expression of some exogenous resistance genes such as sulfonamide resistance gene (sul1) that is commonly located adjacent to Class 1 integrons (Gillings et al., 2015). The findings of this study are similar to those by Shin et al. (2015), in which the Class 1 integron carrying multiple resistance genes was highly prevalent among Gram-negative bacteria. Class 1 integrons are commonly associated with genes conferring resistance to certain antibiotics including ampicillin, tetracycline, trimethoprim, chloramphenicol, kanamycin, gentamicin, and streptomycin (Rahube et al., 2014b) (Table 3).
Temporal variation of airborne bacteria
Facilities like hospitals commonly have air-conditioning systems that facilitate temperature and humidity regulation thus the temperature and humidity recorded in hospital facilities are independent of the season. Figure 3 shows the findings of the effects of temperature and humidity on airborne bacterial concentration in the three hospitals under study.
Temperature and relative humidity (RH) are reported as an average of a 1 h 30 min sampling period for each sampling. During the study, temperature ranged between 20 and 29°C, RH ranged between 30 and 72%. The bacterial concentrations at PPH were relatively high in May 2016 (4.6 ×102 CFU/m3) and March 2017 (4.2 ×102 CFU/m3) compared to August (2.1 ×102 CFU/m3). LMH recorded high bacterial concentrations in August (6.3 ×102 CFU/m3) compared to May 2016 (5.5 ×10 CFU/m3) and April 2017 (8.8 ×10 CFU/m3). NRH also recorded the highest bacterial concentration of all in April (3.9 ×102 CFU/m3, and low bacteria concentrations observed in June (9.9 ×10 CFU/m3) and August (1.6 ×102 CFU/m3) (Figure 3).
The results indicate that in addition to human activities discussed previously, the atmospheric bacterial concentration is directly affected by temperature. When temperatures are low (below 22°C) the bacterial concentration in the atmosphere is low then rises with the increase in temperatures (above 24°C). According to Mouli et al. (2005) temperature is a significant factor for airborne bacteria, which governs the rate of change of water vapor and the rate of change of heat between the surface and environment. Other studies have also shown that the source of ventilation air in buildings; airflow rates, relative humidity and temperature correlates with the diversity and composition of indoor bacterial communities (So et al., 2017). The findings of this study also indicate a distinct trend between the effects of humidity and bacteria concentration observed at LMH and NRH. These results also reveal the distinctive spatial variation due to differences in the locations of the hospital units.
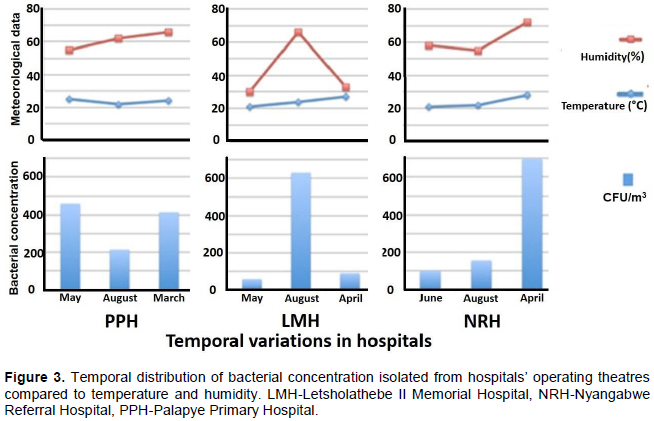
In hospital facilities, through the use of the air conditioning systems, the temperatures are usually maintained around 24°C. However, in instances where the air conditioning systems are malfunctional, the temperature and relative humidity in the outdoors may have an impact on the indoor air. In some occasions during this study, some of the hospitals’ air conditioning systems were malfunctioning, thus affecting the desired temperature and humidity, consequently affecting the bacterial abundance. Guiamet et al. (2011) reported that air movements caused by ventilation could avoid high microbial concentrations in the air. Qudiesat et al. (2009) further supports the effects of ventilation on airborne bacteria by reporting that old and poor ventilation systems might serve as potential source of airborne bacteria in hospital units. Augustowska and Dutkiewicz (2006) studied the diurnal fluctuations of the airborne bacteria levels in pneumonological department ward of a hospital over a period of a year. Their study recorded the highest airborne microbial concentration during autumn whereas the lowest was observed during winter.
Occurrence and high abundance of airborne, potentially pathogenic and antibiotic resistant bacteria in hospitals’ operating theatres and paediatric wards indoor air poses a major concern. The results of this study provide evidence that indoor air of the hospitals’ units is a hotspot for antibiotic resistance determinants (ARB, ARGs, mobile genetic elements), which could be due to prolonged exposure to antibiotics and cross contamination caused by free movement of airborne bacteria from one source to another. The risk of antibiotic resistance dissemination is very high in both hospital units and can lead to acquired infections. Mobile genetic elements such as Class 1 integrons are important in accumulation of clinically relevant ARGs, their occurrence in opportunistic human pathogens such as Pseudomonas species may facilitate transmission of ARGs to other pathogenic bacteria such as Staphylococcus species and E. coli. There is urgent need to develop surveillance tools for monitoring the movement of airborne bacteria particularly in operating theatres and paediatric wards in order to mitigate possible spread of nosocomial infections. Improvement of infrastructures (for example new designs for hospital units, installation of new air conditioning systems) and good hygiene practices are very critical for effective control of airborne bacterial concentrations in the units.
The authors have not declared any conflict of interests.
The authors like to thank Botswana International University of Science and Technology (BIUST) for the financial support to Teddie O. Rahube (Research Initiation Grant R0004) and Lindiwe Tamocha (Postgraduate Research grant). Mothomang Onyinloye assisted with the initial design of the study and participated in the writing of the manuscript, Botswana Ministry of Health and Wellness and PPH, NRH and LMH for research permit and access to the hospital units.
REFERENCES
|
Augustowska M, Dutkiewicz J (2006). Variability of airborne microflora in a hospital ward within a period of one year. Annals of Agricultural and Environmental Medicine 13(1):99-106.
|
|
|
|
Behzadnia S, Davoudi A, Rezai MS, Ahangarkani F (2014). Nosocomial infections in paediatric population and antibiotic resistance of the causative organisms in north of Iran. Iranian Red Crescent Medical Journal 16(2):1-6.
Crossref
|
|
|
|
|
Boerlin P, Travis R, Gyles CL, Reid-Smith R, Lim NJH, Nicholson V, McEwen SA, Friendship R, Archambault M (2005). Antimicrobial resistance and virulence genes of Escherichia coli isolates from swine in Ontario. Applied and Environmental Microbiology 71(11):6753-6761.
Crossref
|
|
|
|
|
Borrego S, Guiamet P, de Saravia SG, Batistini P, Garcia M, Lavin P, Perdomo I (2010). The quality of air at archives and the biodeterioration of photographs. International Biodeterioration and Biodegradation 64(2):139-145.
Crossref
|
|
|
|
|
Castro-Alarcon N, Ribas-Aparicio RM, Silva-Sánchez J, Calderón-Navarro A, Sánchez-Pérez A, Parra-Rojas I, Aparicio-Ozores G (2011). Molecular typing and characterization of macrolide, lincosamide and streptogramin resistance in Staphylococcus epidermidis strains isolated in a Mexican hospital. Journal of Medical Microbiology 60(6):730-736.
Crossref
|
|
|
|
|
Centers for Disease Control and Prevention (CDC) (2013). Antibiotic Resistance Threats in the United States. Atlanta, GA.
View.
|
|
|
|
|
Coetzee E, Rode H, Kahn D (2013). Pseudomonas aeruginosa burn wound infection in a dedicated paediatric burns unit. South African Journal of Surgery 51(2):50-53.
Crossref
|
|
|
|
|
Faccone D, Lucero C, Albornoz E, Petroni A, Ceriana P, Campos J, Viñas MR, Francis G; AZM-R-Group, Melano RG, Corso A (2018). Emergence of azithromycin resistance mediated by mph(A) gene in Salmonella Typhimurium clinical isolates in Latin America. Journal of Global Antimicrobial Resistance 13:237-239.
Crossref
|
|
|
|
|
Fracchia L, Pietronave S, Rinaldi M, Martinotti MG (2006). The assessment of airborne bacterial contamination in three composting plants revealed siteâ€related biological hazard and seasonal variations. Journal of Applied Microbiology 100(5):973-984.
Crossref
|
|
|
|
|
Gilbert JA, Field D, Swift P, Thomas S, Cummings D, Temperton B, Weynberg K, Huse S, Hughes M, Joint I, Somerfield PJ (2010). The taxonomic and functional diversity of microbes at a temperate coastal site: a 'multi-omic' study of seasonal and temporal variation. PloS one 5(11):13-16.
Crossref
|
|
|
|
|
Gillings MR, Gaze WH, Pruden A, Smalla K, Tiedje JM, Zhu YG (2015). Using the class 1 integron-integrase gene as a proxy for anthropogenic pollution. International Society for Microbial Ecology 9(6):1269.
Crossref
|
|
|
|
|
Górny RL, Reponen T, Willeke K, Schmechel D, Robine E, Boissier M, Grinshpun SA (2002). Fungal fragments as indoor air biocontaminants. Applied and Environmental Microbiology 68(7):3522-3531.
Crossref
|
|
|
|
|
Griffin DW, Kellogg CA, Garrison VH, Lisle JT, Borden TC, Shinn EA (2003). Atmospheric microbiology in the northern Caribbean during African dust events. Aerobiologia 19:143-157.
Crossref
|
|
|
|
|
Guiamet P, Borrego S, Lavin P, Perdomo I, GoÌmez de Saravia S (2011). Biofouling and biodeterioration in materials stored at the Historical Archive of the Museum of La Plata, Argentine and at the National Archive of the Republic of Cuba. Colloids and Surfaces B: Biointerfaces 85:229-234.
Crossref
|
|
|
|
|
Hailemariam M, Birhaneselase M, Azerefege E (2016). Bacterial load and antibiotic susceptibility pattern of isolates in operating rooms at Hawassa University Referral Hospital, southern Ethiopia. Journal of Microbiology and Antimicrobials 8(1):1-6.
Crossref
|
|
|
|
|
Holt JGK, Sneath NR, Staley PH, Williams JT, Stanley T (1994). Bergey's manual of determinative bacteriology. (No. QR81 S6 1994).
|
|
|
|
|
Hota S, Hirji Z, Stockton K, Lemieux C, Dedier H, Wolfaardt G, Gardam MA (2009). Outbreak of multidrug-resistant Pseudomonas aeruginosa colonization and infection secondary to imperfect intensive care unit room design. Infection Control and Hospital Epidemiology 30(01):25-33.
Crossref
|
|
|
|
|
Jury KL, Vancov T, Stuetz RM, Khan SJ (2010). Antimicrobial resistance dissemination and sewage treatment plants. In: MeÌndez-Vilas AF, Ed, Current Re- search, Technology and Education Topics in Applied Microbiology and Microbial Biotechnology 2:509-519.
|
|
|
|
|
Kotay SM, Donlan RM, Ganim C, Barry K, Christensen BE, Mathers AJ (2019). Droplet- rather than aerosol-mediated dispersion is the primary mechanism of bacterial transmission from contaminated hand-washing sink traps Applied and Environmental Microbiology 85(2):e01997-18.
Crossref
|
|
|
|
|
Krzowska-Firych J, Kozłowska A, Sukhadia T, Al-Mosawi LK (2014). Hospital-acquired infections caused by antibiotic resistant bacteria. Postępy Nauk Medycznych 16:19-21.
|
|
|
|
|
Lanz R, Kuhnert P, Boerlin P (2003). Antimicrobial resistance and resistance gene determinants in clinical Escherichia coli from different animal species in Switzerland. Veterinary Microbiology 91(1):73-84.
Crossref
|
|
|
|
|
Li CS, Hou PA (2003). Bioaerosol characteristics in hospital clean rooms. Science of the Total Environment 305(1):169-176.
Crossref
|
|
|
|
|
Li J, Yao MS (2018). State-of-the-art status on airborne antibiotic resistant bacteria and antibiotic resistance genes. Chinese Journal of Preventive Medicine 52:440-445.
|
|
|
|
|
Madsen L, Aarestrup FM, Olsen JE (2000). Characterisation of streptomycin resistance determinants in Danish isolates of Salmonella Typhimurium. Veterinary Microbiology 75(1):73-82.
Crossref
|
|
|
|
|
Mathias AJ, Somashekar RK, Sumithra S, Subramanya S (2000). An assessment of reservoirs of multi-resistant nosocomial pathogens in a secondary care hospital. Indian Journal of Microbiology 40(3):183-190.
|
|
|
|
|
McCarthy J, Luscuere P, Streifel A, Kalliokoski P (2000). Indoor air quality in hospitals and other health care facilities. Proceeding of Healthy Buildings, Espoo, Finland 1:65
|
|
|
|
|
Memon J, Kashif J, Hussain N, Yaqoob M, Ali A, Buriro R, Soomro J, Hassan MF, Sahito B, Hongjie F (2016). Serotypes, Genotypes, Virulence Factors and Antimicrobial Resistance Genes of Escherichia coli Isolated in Bovine Clinical Mastitis from Eastern China. Pakistan Veterinary Journal 36(4):3-7.
|
|
|
|
|
Molefi RKK (2001). Of rats, fleas, and peoples: towards a history of bubonic plague in southern Africa, 1890-1950. Pula: Botswana Journal of African Studies 15:259-267.
|
|
|
|
|
Molefi RKK (2003). Smallpox and history: the example of Botswana, 1930-1964. Pula: Botswana Journal of African Studies 17:20-36.
|
|
|
|
|
Mosothwane MN (2015). A Note on the Demographic and Health Pattern of a Historical Sleeping Sickness Cemetery at Letsholathebe Memorial Hospital, Maun, Botswana. International Journal of Osteoarchaeology 6:5-8.
Crossref
|
|
|
|
|
Mouli P, Mohan S, Reddy S (2005). Assessment of microbial (bacteria) concentrations of ambient air at semi-arid urban region: Influence of meteorological factors. Applied Ecology and Environmental Research 3(2):139-149.
Crossref
|
|
|
|
|
Nakamura A, Miyakozawa I, Nakazawa K, O'Hara K, Sawai T (2000). Detection and characterization of a macrolide 2′-phosphotransferase from a Pseudomonas aeruginosa clinical isolate. Antimicrobial Agents and Chemotherapy 44(11):3241-3242.
Crossref
|
|
|
|
|
Neela FA, Nasrin Akhter Banu MST, Rahman A, Habibur Rahman M, Firoz Alam M, (2015). Occurrence of antibiotic resistant bacteria in pond water associated with integrated poultry-fish farming in Bangladesh. Sains Malaysiana 44(3):371-377.
Crossref
|
|
|
|
|
Okten S, Asan A (2012). Airborne fungi and bacteria in indoor and outdoor environment of the Pediatric Unit of Edirne Government Hospital. Environmental Monitoring and Assessment 184(3):1739-1751.
Crossref
|
|
|
|
|
Prussin AJ, Garcia EB, Marr LC (2015). Total concentrations of virus and bacteria in indoor and outdoor air. Environmental Science and Technology Letters 2(4):84-88.
Crossref
|
|
|
|
|
Qudiesat K, Abu-Elteen K, Elkarmi A, Hamad M, Abussaud M (2009). Assessment of airborne pathogens in healthcare settings. African Journal of Microbiology Research 3(2):66-76.
|
|
|
|
|
Rahube TO, Marti R, Scott A, Tien Yuan-Ching, Murray R, Sabourin L, Zhang Y, Duenk P, Lapen DR, Topp E (2014a). The impact of fertilizing with raw or anaerobically digested sewage sludge on the abundance of pathogenic and antibiotic resistant bacteria and antibiotic resistance genes in soil, and vegetables at harvest. Applied and Environmental Microbiology 80(22):6898-6907.
Crossref
|
|
|
|
|
Rahube TO, Viana LS, Koraimann G, Yost CK (2014b). Characterization and comparative analysis of antibiotic resistance plasmids isolated from a wastewater treatment plant. Frontiers in Microbiology 5:558.
Crossref
|
|
|
|
|
Roberts SA, Shore KP, Paviour SD, Holland D, Morris AJ (2006). Antimicrobial susceptibility of anaerobic bacteria in New Zealand: 1999-2003. Journal of Antimicrobial Chemotherapy 57(5):992-998.
Crossref
|
|
|
|
|
Robicsek A, Strahilevitz J, Jacoby GA, Macielag M, Abbanat D, Park CH, Bush K, Hooper DC (2006). Fluoroquinolone-modifying enzyme: a new adaptation of a common aminoglycoside acetyltransferase. Nature Medicine 12(1):83.
Crossref
|
|
|
|
|
Sarica S, Asan A, Otkun MT, Ture M (2002). Monitoring indoor airborne fungi and bacteria in the different areas of Trakya University Hospital, Edirne, Turkey. Indoor and Built Environment 11(5):285-292.
Crossref
|
|
|
|
|
Shin HW, Lim J, Kim S, Kim J, Kwon GC, Koo SH (2015). Characterization of trimethoprim-sulfamethoxazole resistance genes and their relatedness to class 1 integron and insertion sequence common region in Gram-negative bacilli. Journal of Microbiology and Biotechnology 25(1):137-142.
Crossref
|
|
|
|
|
Smets W, Moretti S, Denys S, Lebeer S, (2016). Airborne bacteria in the atmosphere: Presence, purpose, and potential. Atmospheric Environment 139:214-221.
Crossref
|
|
|
|
|
So F, Daisuke T, Fumito M (2017). Transmission of Airborne Bacteria across Built Environments and Its Measurement Standards: A Review. Frontiers in Microbiology 8:2336.
Crossref
|
|
|
|
|
Solomon FB, Wadilo FW, Arota AA, Abraham YL (2017). Antibiotic resistant airborne bacteria and their multidrug resistance pattern at University teaching referral Hospital in South Ethiopia. Annals of Clinical Microbiology and Antimicrobials 16(1):29.
Crossref
|
|
|
|
|
Spagnolo AM, Ottria G, Amicizia D, Perdelli F, Cristina ML (2013). Operating theatre quality and prevention of surgical site infections. Journal of Preventive Medicine and Hygiene 54(3):77-80.
|
|
|
|
|
Sunde M (2005). Prevalence and characterization of class 1 and class 2 integrons in Escherichia coli isolated from meat and meat products of Norwegian origin. Journal of Antimicrobial Chemotherapy 56(6):1019-1024.
Crossref
|
|
|
|
|
Sutcliffe J, Grebe T, Tait-Kamradt A, Wondrack L (1996). Detection of erythromycin-resistant determinants by PCR. Antimicrobial Agents and Chemotherapy 40(11):2562-2566.
Crossref
|
|
|
|
|
Swaminathan B, Gerner-Smidt P (2007). The epidemiology of human listeriosis. Microbes and Infection 9:1236-1243.
Crossref
|
|
|
|
|
Tapela K, Rahube T (2019). Isolation and antibiotic resistance profiles of bacteria from influent, effluent and downstream: A study in Botswana. African Journal of Microbiology Research 13(15): 279-289.
Crossref
|
|
|
|
|
Türkyılmaz S, Tekbıyık S, Oryasin E, Bozdogan B (2010). Molecular epidemiology and antimicrobial resistance mechanisms of methicillinâ€resistant Staphylococcus aureus isolated from bovine milk. Zoonoses and Public Health 57(3):197-203.
Crossref
|
|
|
|
|
Weigelt JA, Lipsky BA, Tabak YP, Derby KG, Kim M, Gupta V (2010). Surgical site infections: causative pathogens and associated outcomes. American Journal of Infection Control 38(2):112-120.
Crossref
|
|
|
|
|
Wolcott RD, Gontcharova V, Sun Y, Zischakau A, Dowd SE (2009). Bacterial diversity in surgical site infections: not just aerobic cocci anymore. Journal of Wound Care 18(8):317-323.
Crossref
|
|
|
|
|
Woldu MA, Suleman S, Workneh N, Berhane H (2013). Retrospective study of the pattern of antibiotic use in Hawassa University Referral Hospital Pediatric Ward, Southern Ethiopia. Journal of Applied Pharmaceutical Science 3(2):93.
|
|
|
|
|
Yassin MF, Almouqatea S (2010) Assessment of airborne bacteria and fungi in an indoor and outdoor environment. International Journal of Environmental Science Technology 7(3):535-544.
Crossref
|
|
|
|
|
Yousefi S, Nahaei MR, Farajnia S, Ghojazadeh M, Akhi MT, Sharifi Y, Milani M, Ghotaslou R (2010). Class 1 integron and imipenem resistance in clinical isolates of Pseudomonas aeruginosa: prevalence and antibiotic susceptibility. Iranian Journal of Microbiology 2(3):115.
|
|
|
|
|
Zhai Y, Li X, Wang T, Wang B, Li C, Zeng G (2018). A review on airborne microorganisms in particulate matters: Composition, characteristics and influence factors. Environment International 113:74-90.
Crossref
|
|
|
|
|
Zhao Y, Aarnink AJ, De Jong MC, Groot Koerkamp PW (2014). Airborne microorganisms from livestock production systems and their relation to dust. Critical Review Environment Science and Technololgy 44(10):1071-1128.
Crossref
|
|