Full Length Research Paper
ABSTRACT
Bovine brucellosis is a major zoonosis caused by Brucella abortus. It is a disease with a high hygienic and economic impact that mainly affects breeders, handlers and consumers of dairy products and animal health professionals. It is therefore a real public health problem. The objective of this study was to assess seroprevalence and risk behaviour for zoonotic transmission of bovine brucellosis in Namentenga Province in Burkina Faso. To do this, a 2-degree random sampling was conducted. Thus, a blood sample was taken from 600 randomly selected cattle. The individual serological status of these 600 cattle in 60 farms was determined by two tests: Tamponed Antigen Test and indirect Enzyme Linked Immunosorbent Assay for confirmation. The frequency of risk behaviours towards this zoonotic disease was determined through two epidemiological questionnaires that identified known risk factors for the transmission of brucellosis between animals and humans. Individual seroprevalence was estimated at 6.8% CI 95% [5.4-8.2]. At least one animal was infected in 30% (18/60) of herds. Positivity in the indirect Enzyme Linked Immunosorbent Assay test was significantly associated with age, breed, livestock conditions and vaccination status in cattle. The most frequently observed risk behaviours in humans in Namentenga Province are assisted calving and abortions, handling of the runt without a glove, consumption of raw milk or unpasteurized curd milk and fresh cheese. In view of this result, Brucella abortus circulates in cattle farms in Namentenga Province. Since animal products such as milk and meat from these farms are consumed by the population, adequate measures must be taken to better protect and guide the means of prevention against this zoonosis among the inhabitants.
Key words: Bovine brucellosis, Burkina Faso, Namentenga Province, Public health, Seroprevalence, Zoonosis.
INTRODUCTION
Brucellosis is a zoonotic disease that is both a severe human disease that affects public health and an animal disease whose economic consequences are far from negligible (Calvet et al., 2010; Tialla et al., 2014; Tialla et al., 2018). Humans most often contaminate themselves through the dermal mucocutaneous pathway through contact with diseased animals and/or their products and through the digestive pathway with the consumption of infected animal products (Dao et al., 2009; Calvet et al., 2010; Tialla et al., 2014). Brucellosis slows the growth of livestock, compromises any attempt to improve animal productivity, and reduces the supply of meat to populations (Boussini et al., 2012; Tialla, 2016). It also poses a serious threat to human health (Dao et al., 2009; Tialla, 2016). Brucellosis is the most common zoonotic infection in the world, with more than 500,000 new cases reported each year (Calvet et al., 2010). In Kyrgyzstan, brucellosis is a public health priority as the annual incidence is greater than 50 cases per 100,000 population with a seroprevalence of 8.8% in humans and 2.8% in cattle (Bonfoh et al., 2011). It posted 1,014 people in Bosnia and Herzegovina in 2008 and 458 (officially reported cases) in 2009 (Calvet et al., 2010). In Senegal, the prevalence of human brucellosis has been estimated at 60.9% among dairy cattle farmers in the peripheral area of Dakar (Tialla, 2012). Still prevalent cases among humans were found in rural Mali (23.3%) (Tasei et al., 1982), Mopti in Mali (58%) (Dao et al., 2009), Chad (2%) (Schelling et al., 2004), Ethiopia (2.6%) (Animut et al., 2009), Egypt (3%) (Afifi et al., 2005) and Tanzania (6.2%) (Kunda et al., 2007). Brucellosis can cause sterility and abortion in both animals and humans, making it a very serious problem for the health and well-being of populations (WHO, 2006; OIE, 2007; Adesokan et al., 2016). It also hinders the marketing of animals and their products (Boussini et al., 2012; Douangngeun et al., 2016; Hernandez-Mora et al., 2017). Despite recent progress in controlling this zoonotic disease, it remains common in urban, peri-urban and rural areas of developing countries (Traoré et al., 2004; Tialla et al., 2014).
Bovine brucellosis is a major zoonotic disease that can have a significant impact on public health, with transmission generally occurring through the consumption of contaminated raw milk (Dao et al., 2009; Calvet et al., 2010; Tialla et al., 2014). Females of dairy species excrete tweezers such as Brucella melitensis, Brucella abortus in their milk (Calvet et al., 2010; Makita et al., 2011). However, due to a lack of pasteurisation and cold chain, milk is often consumed in its fermented, curdled or fresh form. The consumption of raw milk appears to be a societal norm for some African populations who are convinced that in this form, milk is of good quality and cannot make them sick (Fokou et al., 2010). The consumption of raw milk and derived products is not without consequences for the health of populations. The overall objective of our study was therefore to assess seroprevalence and risk behaviour for zoonotic transmission of bovine brucellosis in the Namentenga Province of Burkina Faso.
MATERIALS AND METHODS
Study area
The study took place from 1 February 2021 to 20 July 2021 in the Namentenga Province of Burkina Faso. Located in the Centre-North region, the Namentenga Province covers an area of 6 158 km². It is under the influence of a North Sudanese climate. This climate is characterized by the alternation of two distinct types of season: a dry season from mid-November to mid-May. The dry season is subdivided into two major periods: from mid-November to the end of February, the period is relatively fresh and dry with absolute minimum temperatures of the order of 16°C. It is during this period that the cool and dry winds of North-East and South-West direction dominate widely; these are the warm continental trade winds. From March to mid-May it is the warmest period of the year with average temperatures of 40°C. Absolute highs can reach 42°C in the shade and a rainy season from mid-May to mid-November with precipitation ranging from 644.5 and 849 mm for the last 03 years. Indeed, the province is located between the isohyetes 700 and 900 mm. The average precipitation is of the order of 697.45 mm. The heaviest rains were recorded in July and August.
Study population and sampling method
The population studied consisted, on the one hand, of herds of cattle with more than ten heads (blood samples) and, on the other hand, of people in direct contact with these herds located in the Namentenga Province. The two-stage random sampling method was used (Toma et al., 2010). The first stage involved the random draw of cattle farms in our study area. As no exhaustive lists of successive sampling units were available, a preliminary survey was carried out. This survey identified 121 farms, 78 of which met the inclusion criteria. Of the 78 farms, 60 were randomly selected. The second degree involved a random draw of 10 cattle from each selected flock, or a total of 600 cattle. In each farm, two visits were carried out: the first for the awareness and written consent of each farmer for the two studies (animals and humans), and the second for collection of blood samples from animals. Two epidemiological questionnaires, one for humans and one for animals, each containing mainly closed-type questions, were developed to establish risk behaviours for this zoonotic disease. The interviews lasted an average of 20 min per person and were conducted in Mooré, Dioula or, in some cases, French. In animals, the animal health status, age, sex, breed, vaccination against brucellosis and some known symptoms of bovine brucellosis such as history of abortion and the presence of hygroma were identified. To compare young cattle with older cattle, two age classes were defined. This is Class 1 which includes animals aged 0 to 2 years and Class 2 for animals aged over 2 years. The cattle collected were divided into sex and two breed categories, the local breed and the exotic breed. The questions on farmers focused on the ethnicity, habitat and at-risk practices of the farmers surveyed, such as seasonal movements, the mode of rearing, handling of an underage without wearing a glove, assistance of the pregnant cows during stockings-low or abortions, the mode of food (consumption of raw milk and unpasteurized dairy products), and the sale and circuit of this sale.
Diagnostic methods
Blood samples were collected from the jugular vein into a dry tube identified by the farm code and the animal number. The sera were collected after centrifugation and placed in cryo-tubes using sterile disposable pipettes. For the serological diagnosis for brucellosis, two serological tests were used in parallel: the Tamponed Antigen Test (TAT) and the indirect Enzyme Linked Immunosorbent Assay (iELISA). The iELISA test allows to make the confirmation. TAT is a fast, simple, cost-effective test that is considered sensitive (90%) and relatively non-specific (75%) (Mai et al., 2012). The iELISA test is considered to be very sensitive (≥95%) and very specific (≥95%) (Nielsen, 2002; Lesceu and Pourquier, 2016). The iELISA Kit (ID.vet Innovative Diagnostics) has made it possible to search for anti-Brucella antibodies in our serums by plate micro-method according to the recommendations of the World Organisation for Animal Health (OIE). The plates were read at 450 nm using a plate reader (Thermo SCIENTIFIC Multiskan GO Version 1.00.38). This made it possible to detect recent and old infections by highlighting IgM and IgG. The results of the analyses were interpreted according to the manufacturer’s recommendations. The questionable cases were retested in order to be better determined on their serological status.
Statistical analysis
The data was entered into Epidata® and processed using Epidata Analysis® software. The variables of interest, coded in presence/absence, were positivity to the laboratory diagnostic test. The explanatory variables were individual and collective characteristics. Risk factors in cattle and risk behaviours in humans were identified using a multivariate model. A logistic regression model (proc logistic, SAS 9.3) was used to analyse positivity on the diagnostic test based on explanatory variables considered as risk factor or risk behaviour. The significance threshold was set at 5%.
Ethical consideration
This study received approval clearance from Centre Muraz ethical committee (number 2016-15/MS/SG/CM/IEC).
RESULTS
Individual and collective characteristics of cattle surveyed in Namentenga Province, Burkina Faso
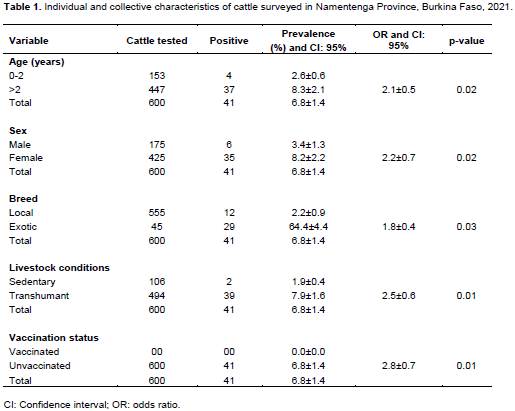
The individual and collective characteristics of the cattle surveyed in the Namentenga Province of Burkina Faso in 2021 are recorded in Table 1.
Test positivity by iELISA was significantly associated with age, sex, breed, livestock conditions and vaccination status of cattle. These explanatory variables were considered to be identified risk factors in animals.
Seroprevalence of bovine brucellosis in Namentenga Province
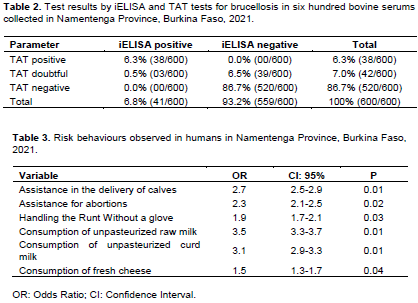
Of 600 serums, 38 (6.3%) and 42 (7%) gave a positive and questionable response to TAT, respectively. Following the analysis of these 80 samples using the iELISA test, the 38 TAT positive samples and 3 of the 42 TAT suspicious samples provided a positive response to the iELISA test. A total of 41 (6.8% CI 95% [5.4-8.2]) reported a positive response to the iELISA test and 30% (18/60) of the farms reported at least one positive response to the TAT and iELISA tests. The results of brucellosis testing on 600 bovine serums collected in the Namentenga Province are recorded in Table 2.
Identified risk behaviours in humans
The most common risk behaviours observed in humans are assisting with births and abortions, handling the runt without a glove, and consuming unpasteurized raw or curdled milk and fresh cheese. The results are presented in Table 3.
DISCUSSION
The individual seroprevalence of bovine brucellosis in this study was estimated at 6.8%. This result confirms the presence of brucellosis in this area of Burkina Faso, Namentenga Province. This value is almost similar to 6.6% obtained by Kubafor et al. (2000) in Ghana. It is higher than 3.61% obtained by Boussini et al. (2012) in the intra and peri-urban area of Ouagadougou in Burkina Faso. This value is also higher than those observed by some authors in Senegal (1.52%) (Kouamo et al., 2010), Chad (2.6%) (Delafosse et al., 2002), Central African Republic (3.3%) (Nakouné et al., 2004), Eritrea (5.6%) (Omer et al., 2000), Ethiopia (3.1%) (Ibrahim et al., 2010) and Zimbabwe (5.6%) (Matope et al., 2010). These differences could be related to livestock conditions. In addition, in extensive or traditional breeding, the seroprevalence of bovine brucellosis remains relatively low compared to intensive breeding (Koutinhouin et al., 2003; Kouamo et al., 2010). According to work of Terefe et al. (2017), herds reared in intensive livestock showed the highest seroprevalence. On the other hand, it should be noted that our prevalence of 6.8% is less than 13.2% obtained by Traoré et al. (2004) and 18.3% obtained by Tialla et al. (2018) in Burkina Faso, to 8.8% obtained by Sanogo et al. (2008) in Côte d'Ivoire, to 9.2% obtained by Dean et al. (2013) in Togo, 8.4% obtained by Bayemi et al. (2009) in Cameroon, 10% obtained by Arimi et al. (2005) in Kenya, 15% obtained by Bonfoh (2002) in Mali, 18.7% obtained by Chimana et al. (2010) and 23.9% obtained by Muma et al. (2007a) in Zambia, 15.8% obtained by Faye et al. (2005) and 34% obtained by Magona et al. (2009) in Uganda and 36.36% obtained by Tialla et al. (2014) in Senegal. These differences may be due to the climates, farming methods, sensitivity and specificity of the different tests used. Indeed, the hot and humid climate is favorable to the survival of the pathogen. Some breeders in our study area control the entry of sick animals into their herds by carrying out the Bengal rose test which could explain our low prevalence. Indeed, the Rose Bengale test is by far the most widely used test in sub-Saharan Africa due in particular to its simplicity, its relative good sensitivity and its low cost (Muma et al., 2009). This test allows a rapid assessment of individual serological status at the local or regional level (OIE, 2007). However, the specificity of this test is quite low due in particular to the cross-reactions of the Brucella antigen with antibodies linked to other Gram-negative related bacteria such as Yersinia enterocolitica O:9, Francisella tularensis, Vibrio cholerae, Escherichia coli O:157, Salmonella species, and Sternotrophomonas maltophilia (Nielsen, 2002; Saegerman et al., 2004; Sanogo et al., 2008). This would lead to false positive serological responses that tend to overestimate the individual prevalence of brucellosis in some regions of sub-Saharan Africa (Bankole et al., 2010; Makita et al., 2011; Sanogo et al., 2012). In addition, Saegerman et al. (2004) showed that the specificity of the indirect ELISA test for the detection of brucellosis varies according to the nature of the conjugate used. The same authors reported that the specificity of the indirect ELISA test also depends on the microbism of the study area.
Our herd prevalence is well below 96.6% obtained by Tialla et al. (2014) in Senegal and 95% obtained by Tialla et al. (2018) in Burkina Faso. This could be explained by the fact that almost all the herds in these study areas were sedentary, unlike the herds in this current study which were mostly transhumant (82.3%). Indeed, according to Kouamo et al. (2010), herd prevalence remains relatively low in extensive and traditional transhumance farms. An epidemiological survey conducted by Omer et al. (2002) in Eritrea showed the influence of the livestock system, with a higher seroprevalence in dairy farming linked to higher animal density compared to a nomadic agro-pastoral system.
Test positivity was significantly associated with age, sex, breed, livestock conditions and vaccination status. Intrinsic factors such as race, sex and age can play a major role in the transmission of brucellosis. Indeed, our study found that older cattle were the most affected. The risk of infection appears to increase with age, contrary to what was described in Chad by Delafosse et al. (2002). According to Akapko and Bonarel (1987), the prevalence of brucellosis generally increases with age. This trend seems logical because with time the animal is more likely to have been infected, to remain infected and to be dangerous to other animals (Koutinhouin et al., 2003). Serological prevalence was higher in females than males. This is certainly due to the low impact of males in the epidemiology of the disease. However, our results are consistent with those of Akakpo (1987), who observed that serological prevalence in females was significantly higher than in males during the study in Burkina, Rwanda and Togo. On the other hand, this observation is reversed in Niger and appears identical for both sexes in Benin and Cameroon (Akakpo, 1987). As for the breed, the results show that exotic animals were the most affected. This could be explained by their low resistance to the harsh climatic conditions prevailing in our study area. Furthermore, the exotic breed remains particularly sensitive to pathogens (Akakpo, 1987). Extrinsic factors can also have an impact on disease transmission. Sedentary animals were the most affected. According to Delafosse et al. (2002), conditions for extensive livestock rearing limit the spread of brucellosis in contaminated herds. The mode of rearing can be considered as a risk factor for brucellosis in that contact with animals varies with the latter. Thus, in intensive farming the risk is higher, which corresponds to the findings of Akakpo (1987). No animals in this study were vaccinated against brucellosis. The antibodies detected therefore stem from contact of cattle with the pathogen. Indeed, vaccination against brucellosis is not practiced in Burkina Faso.
The most common risk behaviours observed in humans have been assisting with births and abortions, handling the runt without a glove, and consuming unpasteurized raw or curdled milk and fresh cheese. These results are consistent with those of Al-Shamahy et al. (2000) in Yemen, Dao et al. (2009) in Mali, Dean et al. (2013) in Togo and Tialla et al. (2014) in Senegal. Assisting with births and abortions and handling runts without wearing gloves are important risk factors because they are potentially very dangerous contacts. This observation was described by several authors (Bikas et al., 2003; Toma et al., 2010; Sanogo et al., 2012). The consumption of milk and non-pasteurized derived products is also a very important risk factor. This remark was also noted by Mailles and Vaillant (2007), Muma et al. (2007b) and Bonfoh et al. (2011).
CONCLUSION
The overall objective of this study was to assess seroprevalence and behaviour at risk for zoonotic transmission of bovine brucellosis in Namentenga Province, Burkina Faso. It was confirmed that brucellosis is present in cattle farms in the Namentenga Province in Burkina Faso, with an individual prevalence estimated at 6.8%. The most common risk behaviours observed in humans were assisting with calving and abortions, handling the runt without a glove, drinking raw milk or unpasteurized curd milk and fresh cheese. As the consumption of products from these farms is not without public health consequences, adequate measures must be taken to protect the population against this zoonosis. The implementation of an integrated approach, which takes into account the complex relationships between humans, animals and the environment within the different production systems; and the establishment of a multi-sectoral framework involving physicians, veterinarians and all public health stakeholders in the context of a one health approach should be considered.
CONFLICT OF INTERESTS
The author has not declared any conflict of interests.
ACKNOWLEDGEMENTS
The author thanks Mr. BEMBAMBA Patoinné-wendé Léandre, Mr. SEBOU DAH Jean-Baptiste and the whole team of the laboratory of animal husbandry, animal health and zoonosis of the National School of Livestock and Animal Health of Burkina Faso for their collaboration, their participation and contribution to the study. The work was carried out thanks to the financial support of the National School of Livestock and Animal Health of Burkina Faso.
REFERENCES
|
Adesokan HK, Alabi PI, Ogundipe MA (2016). Prevalence and predictors of risk factors for Brucellosis transmission by meat handlers and traditional healers' risk practices in Ibadan, Nigeria. Journal Prevention Medicine and Hygiene 57(3):164-171. |
|
|
Afifi S, Earhart K, Azab MA (2005). Hospital- Based surveillance for acute febrile illness in Egypt: a focus on community-acquired bloodstream infections. American Journal Tropical Medicine and Hygiene 73(2):392-399. |
|
|
Akakpo AJ (1987). Brucellose animale en Afrique tropicale. Particularité épidémiologique, clinique et bactériologique. Revue Elevage et Médecine Vétérinaire des Pays tropicaux 40(4):307-320. |
|
|
Akakpo AJ, Bonarel P (1987). Epidémiologie des brucelloses animales en Afrique tropicale: enquêtes cliniques, sérologique et bactériologique. Revue Science Technique Office International Epizootie 6(4):981-1027. |
|
|
Al-Shamahy HA, Whitty CJM, Wright SG (2000). Risk factors for human brucellosis in Yemen: a case control study. Epidemiology and Infectiology 125:309-313. |
|
|
Animut A, Mekonnen Y, Shimelis D, Ephraim E (2009). Febrile Illnesses of Different Etiology among Outpatients in Four Health Centers In Northwestern Ethiopia. Journal Infectious Diseases 62(2):107-110. |
|
|
Arimi SM, Koroti E, Kang'Ethe EK, Omore AO, Mcdermott JJ (2005). Risk of infection with Brucella abortus and Escherichia coli O157:H7 associated with marketing of unpasteurized milk in Kenya. Acta Tropical 96(1):1-8. |
|
|
Bankole AA, Saegerman C, Berkvens D, Fretin D, Geerts S, Ieven G, Walravens K (2010). Phenotypic and genotypic characterisation of Brucella strains isolated from cattle in the Gambia. Veterinary Record 166(24):753-756. |
|
|
Bayemi PH, Webb EC, Nsongka MV, Unger H, Njakoi H (2009). Prevalence of Brucella abortus antibodies in serum of Holstein cattle in Cameroon. Tropical Animal Health Production 41(2):141-144. |
|
|
Bikas C, Jelastopulu E, Leotsinidis M, Kondakis X (2003). Epidemiology of human brucellosis in a rural area of north-western peloponese in Greece. European Journal of Epidemiology 18(3):267-274. |
|
|
Bonfoh B (2002). Hygiène et qualité du lait et des produits laitiers au Mali : Implication en production laitière et santé publique. In : Rapport d'étude : lait sain pour le sahel, Bamako, Mali pp. 25-35. |
|
|
Bonfoh B, Kasymbekov J, Durr S, Toktobaev N, Doherr MG, Schueth T, Zinsstag J, Schelling E (2011). Representative Seroprevalences of Brucellosis in Humans and Livestock in Kyrgyzstan. EcoHealth 9(2):132-138. |
|
|
Boussini H, Traoré A, Tamboura HH, Bessin R, Boly H, Ouédraogo A (2012). Prévalence de la tuberculose et de la brucellose dans les élevages bovins laitiers intra-urbains et périurbains de la ville d'Ouagadougou au Burkina Faso. Revue Science Technique Office International Epizootie 31(3):943-951. |
|
|
Calvet F, Heaulme M, Michel R, Demoncheaux JP, Boué S, Girardet C (2010). Brucellose et contexte opérationnel. Médecine et armées 38(5):429-434. |
|
|
Chimana HM, Muma JB, Samui KL, Hangombe BM, Munyeme M, Matope G, Phiri AM, Godfroid J, Skjerve E, Tryland MA (2010). Comparative study of seroprevalence of brucellosis in commercial and small-scale mixed dairy-beef cattle enterprises of Lusaka province and Chibombo district, Zambia. Tropical Animal Health Production 42(7):1541-1545. |
|
|
Dao S, Traoré M, Sangho A, Dantoume K, Oumar AA, Maiga M, Bougoudogo F (2009). Séroprévalence de la brucellose humaine à Mopti, Mali. Revue Tunisienne d'Infectiologie 2:24-26. |
|
|
Dean AS, Bonfoh B, Kulo AE, Boukaya GA, Amidou M, Hattendorf J, Pilo P, Schelling E (2013). Epidemiology of brucellosis and Q fever in linked human and animal populations in Northern Togo. PLoS ONE 8:e71501. |
|
|
Delafosse A, Goutard F, Thébaud E (2002). Epidémiologie de la tuberculose et de la brucellose des bovins en zone périurbaine d'Abéché, Tchad. Revue Élevage et Médecine Vétérinaire des Pays Tropicaux 55(1):5-13. |
|
|
Douangngeun B, Theppangna W, Soukvilay V, Senaphanh C, Phithacthep K, Phomhaksa S, Yingst S, Lombardini E, Hansson E, Selleck PW, Blacksell SD (2016). Seroprevalence of Q fever, Brucellosis, and Bluetongue in Selected Provinces in Lao People's Democratic Republic. American Journal Tropical Medicine and Hygiene 95(3):558-561. |
|
|
Faye B, Castel V, Lesnoff M, Rutabinda D, Dhalwa J (2005). Tuberculosis and brucellosis prevalence survey on dairy cattle in Mbarara milk basin (Uganda). Preventive Veterinary Medecine 67:267-281. |
|
|
Fokou G, Koné BV, Bonfoh B (2010). « Mon lait est pur et ne peut pas rendre malade » : motivations des acteurs du secteur informel et qualité du lait local au Mali. Revue Africaine de Santé et de Productions Animales 8(5):75-86. |
|
|
Hernandez-Mora G, Ruiz-Villalobos N, Bonilla-Montoya R, Romero-Zuniga JJ, Jimenez-Arias J, Gonzalez-Barrientos R, Barquero-Calvo E, Chacon-Diaz C, Rojas N, Chaves-Olarte E, Guzman-Verri C, Moreno E (2017). Epidemiology of bovine brucellosis in Costa Rica: Lessons learned from failures in the control of the disease. PLoS One 12(8):e0182380. |
|
|
Ibrahim N, Belihu K, Lobago F, Bekena M (2010). Sero-prevalence of bovine brucellosis and risk factors in Jimma zone of Oromia Region, South-western Ethiopia. Tropical Animal Health Production 42(1):35-40. |
|
|
Kouamo J, Habimana S, Alambedji Bada R, Sawadogo GJ, Ouédraogo GA (2010). Séroprévalences de la brucellose, de la BVD et de l'IBR et impact sur la reproduction des femelles zébus Gobra et croisements inséminées en milieu traditionnel dans la région de Thiès au Sénégal. Revue de Médecine Vétérinaire 161(7):314-321. |
|
|
Koutinhouin B, Youssao AKI, Houehou AE, Agbadje PM (2003). Prévalence de la brucellose bovine dans les élevages traditionnels encadrés par le Projet pour le Développement de l'Elevage (PDE) au Bénin. Revue de Médecine Vétérinaire 154(4):271-276. |
|
|
Kubafor DK, Awumbila B, Akanmori BD (2000). Seroprevalence of brucellosis in cattle and humans in the Akwapim-south district of Ghana : public health implications. Acta Tropical 76:45-48. |
|
|
Kunda J, Fitzpatrick J, Kazwala R (2007). Health-Seeking Behavior of Human brucellosis cases in Rural Tanzania. BMC Public Health 7:315-323. |
|
|
Lesceu S, Pourquier P (2016). Contrôle de qualité du Kit ELISA ID Screen® Brucellosis Serum Indirect Multi-species: sensibilité et spécificité. ID.vet Innovative Diagnostics 1 p. |
|
|
Magona JW, Walubengo J, Galiwango T, Etoori A (2009). Seroprevalence and potential risk of bovine brucellosis in zeograzing and pastoral dairy systems in Uganda. Tropical Animal Health Production 41:1765-1771. |
|
|
Mai HM, Irons PC, Kabir J, Thompson PN (2012). A large seroprevalence survey of brucellosis in cattle herds under diverse production systems in northern Nigeria. BMC Veterinary Research 8(1):1-14. |
|
|
Mailles A, Vaillant V (2007). Etude sur les brucelloses humaines en France métropolitaine, 2002 - 2004. Saint-Maurice : Institut national de Veille Sanitaire (Rapport), Paris, France 57 p. |
|
|
Makita K, Fèvre EM, Waiswa C, Eisler MC, Thrusfield M, Welburn SC (2011). Herd prevalence of bovine brucellosis and analysis of risk factors in cattle in urban and peri-urban areas of the Kampala economic zone, Uganda. BMC Veterinary Research 7(1):1-8. |
|
|
Matope G, Bhebhe E, Muma JB, Lund A, Skjerve E (2010). Herd-level factors for Brucella seropositivity in cattle reared in smallholder dairy farms of Zimbabwe. Preventive Veterinary Medicine 94(3-4):213-221. |
|
|
Muma J, Godfroid J, Samui K, Skjerve E (2007a). The role of Brucella infection in abortions among traditional cattle reared in proximity to wildlife on the Kafue flats of Zambia. Revue Science Technique Office International Epizootie 26(3):721-730. |
|
|
Muma J, Samui K, Oloya J, Munyeme M, Skjerve E (2007b). Risk factors for brucellosis in indigenous cattle reared in livestock-wildlife interface areas of Zambia. Preventive Veterinary Medicine 80(4):306-317. |
|
|
Muma JB, Lund A, Nielsen K, Matope G, Munyeme M, Mwacalimba K, Skjerve E (2009). Effectiveness of Rose Bengal test and fluorescence polarization assy in the diagnosis of Brucella spp. infections in free range cattle reared in endemic areas in Zambia. Tropical Animal Health Production 41(5):723-729. |
|
|
Nakouné E, Debaere O, Koumanda-Kotogne B, Selekon B, Samory F, Talarmin A (2004). Serological surveillance of brucellosis and Q fever in cattle in the Central African Republic. Acta Tropical 92(2):147-151. |
|
|
Nielsen K (2002). Diagnosis of brucellosis by serology. Veterinary Microbiology 90(1-4):447-459. |
|
|
OIE (2007). Santé animale mondiale en 2007. Organisation mondiale de la santé animale (OIE), Paris, France 619 p. |
|
|
Omer MK, Skjerve E, Holstad G, Woldehiwet Z, Macmillan AP (2000). Prevalence of antibodies to Brucella spp. in cattle, sheep, goats, horses and camels in the State of Eritrea; influence of husbandry systems. Epidemiology and Infection 125(2):447-453. |
|
|
Saegerman C, De Waele L, Gilson D, Godfroid J, Thiang P, Michel P, Limbourg B, Vo TKO, Limet J, Letesson JJ, Berkven S (2004). Evaluation of three serum i-ELISAs using monoclonal antibodies and protein G as peroxidase conjugate for the diagnosis of bovine brucellosis. Veterinary Microbiology 100(1-2):91-105. |
|
|
Sanogo M, Abatih E, Thys E, Fretin D, Berkvens D, Saegerman C (2012). Risk factors associated with brucellosis seropositivity among cattle in the central savannah-forest area of Ivory Coast. Preventive Veterinary Medicine 107(1-2):51-56. |
|
|
Sanogo M, Cissé B, Ouattara M, Walvarens K, Praet N, Brekvens D, Thys E (2008). Prévalence réelle de la brucellose bovine dans le Centre de la Côte d'Ivoire. Revue Elevage et Médecine Vétérinaire des Pays tropicaux 61(3-4):147-151. |
|
|
Schelling E, Diguimbaye C, Daoud S, Nicolet J, Zinsstag J (2004). Séroprévalences des maladies zoonotiques chez les pasteurs nomades et leurs animaux dans le Chari-Baguirmi du Tchad. Médecine Tropicale 64(5):474-477. |
|
|
Tasei JP, Ranque P, Balique H, Traoré A, Quilici M (1982). Human brucellosis in Mali, Results of a seroepidemiological study. Acta Tropical 39(8):253-264. |
|
|
Terefe Y, Girma S, Mekonnen N, Asrade B (2017). Brucellosis and associated risk factors in dairy cattle of eastern Ethiopia. Tropical Animal Health Production 49(3):599-606. |
|
|
Tialla D (2012). Brucellose humaine et bovine dans les élevages bovins laitiers en périphérie de Dakar (Sénégal). Rapport de fin de stage du mémoire de Master SEMHA 52 p. |
|
|
Tialla D (2016). Brucellose: zoonose majeure et problème de santé publique. Editions Universitaires Européennes 60 p. |
|
|
Tialla D, Koné P, Kadja MC, Kamga-Waladjo A, Dieng CB, Ndoye N, Kouamé KGG, Bakou S, Akakpo AJ (2014). Prévalence de la brucellose bovine et comportements à risque associés à cette zoonose dans la zone périurbaine de Dakar au Sénégal. Revue Elevage et Médecine Vétérinaire des Pays tropicaux 67(2):67-72. |
|
|
Tialla D, Zio AC, Yaméogo IG, Cissé A, Sagna T, Ilboudo AK, Sanou MA, Kouanda S, Ouedraogo GA, Tarnagda Z (2018). Séro-épidémiologie de la brucellose bovine et porcine à Bobo-Dioulasso, Burkina Faso. Épidémiologie et santé animale 73:175-179. |
|
|
Toma B, Dufour B, Bénet JJ, Sanaa M, Shaw A, Moutou F (2010). Épidémiologie appliquée à la lutte collective contre les maladies animales transmissibles majeures. AEEMA, 2010; 3ème édition 600 p. |
|
|
Traoré A, Tamboura HH, Bayala B, David W, Rouamba DW, Yaméogo N, Sanou M (2004). Prévalence globale des pathologies majeures liées à la production laitière bovine en système d'élevage intraurbain à Hamdallaye (Ouagadougou). Biotechnology Agronomic Society and Environnement 8(1):3-8. |
|
|
World Health Organisation (WHO) (2006). Brucellosis in humans and animals. World Health Organisation. WHO/CDS/EPR/2006, Geneve, Suisse 86 p. |
|
Copyright © 2024 Author(s) retain the copyright of this article.
This article is published under the terms of the Creative Commons Attribution License 4.0