ABSTRACT
The frequency of occurrence and four principal kinds of aflatoxin concentration in maize seeds grown in Burkina Faso was investigated. Ten (10) samples collected, were analyzed by high performance liquid chromatography (HPLC) with post-column derivatisation after immunoaffinity column cleanup. Eight strains of Aspergillus section Flavi were previously isolated from these samples and cultivated on “Aspergillus flavus and parasiticus agar (AFPA)” to ascertain if they belong to A. flavus or A. parasiticus species. The qualitative ability of aflatoxin production was also previously performed by fluorescence emission under ultra violet light at 365 nm after four (4) days of incubation at 30 °C on Coconut Agar Medium (CAM). Results showed that 70% of samples were contaminated by aflatoxins. The levels ranged from 0.93 to 58.94 µg/kg. Samples M1 and M10 had high concentrations, 58.94 µg/kg and 70.73 µg/kg; whereas M4 and M5 had low concentrations from 1.68 to 0.93 µg/kg, respectively. In these samples, four were contaminated with aflatoxin B1 (AFB1) and aflatoxin G1 (AFG1), two with AFB1 and aflatoxin B2 (AFB2) and one (01) with AFB1 only. We notice that AFB1 was the most prevalent member of aflatoxins, and AFG2 was absent in all samples.
Key words: Maize, Aspergillus, aflatoxins, HPLC, Burkina Faso.
Cereals are a staple food for humans and animals. In Burkina Faso, their annual consumption is estimated at 62% of the food consumed by households (Waongo et al., 2013). Among food crops, maize is the most used product by over 98% of rural households (Bambara, 2021). In Burkina Faso, maize ranks second among cultivated cereals, both in area, production and consumption (Somda, 2016). It also makes a significant contribution to the country’s economies. The area devoted to maize cultivation in the country increased from 790,321 ha in 2010 to 1,019,181 ha in 2018 (GDSSS/MAHAD, 2020). National maize production reached 1,700,127 T in 2018 against 1,133,480 T in 2010 (GDSSS/MAHAD, 2020). Maize is grown widely around the tropical world owing to its good adaptation to climate and its popularity. Besides being distributed widely, maize can be used for many purposes, such as animal feed, industrial uses, and is even the staple food in many developing countries. It also makes a large contribution to the economies of developed and developing countries. Nevertheless, the aflatoxin phenomenon is undermining the sector in Africa (Chauhan et al., 2016). Thus, maize is food crop that is easily contaminated with mycotoxins such as aflatoxins that are cancer-causing, immuno-suppressive mycotoxins (Makhlouf, 2019). According to Bambara (2021), 40% of maize production is affected by aflatoxins in developing countries. Contamination of crops with aflatoxin is a global food safety issue (Compaoré et al., 2021). The most important members of AFs are AFB1, AFB2, AFG1 and AFG2. The International Agency for Research on Cancer (IARC) has classified AFB1, AFB2, AFG1 and AFG2 as Group I human carcinogens (IARC, 2002). Among the many known toxins in the world, aflatoxins are highly toxic and carcinogenic compounds that can cause diseases in livestock and humans (Ouattara-Sourabié, 2018). AFs are a group of mycotoxins produced as secondary metabolites by species in Aspergillus section Flavi. The species most notorious for aflatoxin production are Aspergillus flavus (produces only aflatoxins B) and Aspergillus parasiticus (produces both B and G aflatoxins) (Kachapulula et al., 2017).
Previous studies in Africa have found that the occurrence of aflatoxins in food products is mainly influenced by favorable conditions such as high moisture content and temperature (Waré et al., 2017). In fact, in the Sahelian zone dry, post-harvest conservation is the only means of ensuring the link between the harvest occurring once in the year and consumption that is permanent and obligatory. The harvests, kept in general under inappropriate conditions, are attacked by insects, rodents and molds (Waongo et al., 2013). Also, in Burkina Faso the improper storage or preservation methods used such as maize bad drying would also lead to attacks by micro-organisms including molds of the genus Aspergillus, Penicillium, Fusarium, and Alternaria (Sanou, 2000). Aflatoxins contamination of maize has always remained a topic of debate in terms of international market as well as economic development of country which are part of trade market (Chauhan et al., 2016). In view of the huge economic losses and health problems caused by mycotoxins; a great deal of interest is currently accorded to them throughout the world. Thus to guarantee the health of consumers, each country is obliged to adopt specific legislation for the main mycotoxins in foods liable to harbor toxigenic molds (Waré et al., 2017). Several countries in the world have established or proposed regulatory limits for mycotoxins in foods. European Union countries edited regulations that have been revised periodically to limit their presence in the foods in Europe (EU Regulation 1881, 2006). In Africa certain countries also have regulations on mycotoxins and produce significant research, especially on aflatoxins and fumonisins that affect health (Ezekiel et al., 2014). Unfortunately, Burkina Faso, a country of West Africa has not set a mycotoxin regulation and uses those of Codex alimentarius which fixes the maximum levels of AFB1 and total aflatoxins respectively at 2 µg/Kg and 5 µg/Kg (EU Regulation 1881, 2006){Warth, 2012 #683;FAO, 2004 #690}. Indeed, there are few studies and surveys about the contamination of mycotoxins in maize in Burkina Faso and this does not really reflect the problem in our country (Sanou, 2000). Our farmers therefore often find it difficult to export their products to countries whose mycotoxin regulations are in force. Indeed, because crops in tropical and subtropical regions are more susceptible to contamination due to favorable climatic and their inadequate storage conditions which facilitate the proliferation of molds and their secondary metabolites (Waré et al., 2017). Therefore, the aim of the proposed work is to determine the fungal load of maize samples from Ouagadougou City in Burkina Faso and to quantify the concentration of all kinds of aflatoxins using high performance liquid chromatography. This will confirm the results of the qualitative demonstration of aflatoxins production capacity of molds previously isolated from these maize samples, such as fluorescence emission under ultra violet light at 365 nm and those in “A. flavus and parasiticus agar (AFPA)”.
Physicochemical analyses
The maize samples were first subjected to physicochemical analyses such as humidity and pH using standards methods. The tests were repeated three times.
Sampling and fungi isolation from maize seeds
A total of ten (10) maize samples in commercially available were collected during the period from December to February 2021 at Ouagadougou markets. Fungi were isolated and purified on Potato Dextrose Agar (PDA) and subculture in “Aspergillus flavus and parasiticus agar (AFPA)” to identify A. flavus and A. parasiticus according to Pitt et al. (1983)and Cotty (1994)protocols. Systematic determination and the strains identification were made on Potato Dextrose Agar (PDA) at 25 and 37 °C depending on the methods used by Christensen (1981), Hocking (1982)and Cooney and Emerson (1964). Inoculation was done in three points equidistant.
Reference strains
For comparison of cultural and microscopic characters between strains isolated from maize seeds and those of reference strains, three references belonging to Aspergillus section Flavi were used. They were UBOCC-A-106031 (A. flavus aflatoxinogenic) of French origin, UBOCC-A-111042 (A. parasiticus var. globosus aflatoxinogenic) of Japanese origin and S2 (A. flavus aflatoxinogenic) previously isolated from groundnuts and identified in Burkina Faso using molecular method by two (2) PCR based on 28S ribosomal sub unit (D1-D2 region) and the hyper variable ITS1-5.8S-ITS2 region, (Compaoré et al., 2016). This comparison was performed on Potato Dextrose Agar (PDA) medium at 30 °C for seven days.
Aflatoxins quantification
Sample extraction (AOAC, 2005)
Aflatoxins production is confirmed by HPLC using aflatoxins B and G Standard, Romer Biopure, and a blank consisting of the extraction solution. HPLC analysis was performed at the Toxicological Department of the National Public Health Laboratory (LNSP) in Ouagadougou. Ten (10) maize samples were subjected to this analysis.
The principle was used to extract aflatoxin from the samples using suitable organic solvents, to purify this aflatoxin on an immunoaffinity column and then to identify and quantify it. To do this, 25 ± 0.2 g of mix ground maize was weighed and added approximately 3 g of sodium chloride and placed in blender cup. 125 ml of extraction solution methanol-distilled water (70:30; v/v) was added to the sample and the whole was stirred for 20 min. The solution was then filtered through Whatman No.4 filter paper and 15 ml of the filtrate was transferred into a beaker and diluted with 30 ml of distilled water. The diluted homogenized sample solution was filtered through glass microfiber membrane.
Sample cleanup
The immunoaffinity columns were previously conditioned by passing 10 ml phosphate buffered saline (PBS) through the column by gravity and placed on the variant cuvette; the silica gel was allowed to flow. 15 ml of the diluted filtrate (1g sample equivalent) was taken and poured into the immunoaffinity column where it retained the desired molecules. The molecules were then washed with 10 ml of distilled water which was poured twice into the immunoaffinity column. Under gravity, the bound aflatoxins were eluted with 1 ml of pure methanol and air was pushed through the column to collect the last drops of eluate. 1 ml of distilled water was added to the eluate. The eluate was filtered through 0.45 µm Methanol-compatible membrane filter (PTFE or Nylon) and collected into a micro sample vial. Then this solution containing the aflatoxin molecules was sent to the HPLC for the detection and quantification of these aflatoxin molecules. Each experiment was conducted in triplicate and aflatoxins contents were determined according to their corresponding standard curves.
HPLC analyses
Spiked samples were prepared on relevant matrix for each batch of sample to obtain the % recovery. 0.5 ml of the working solution was added (100 µg/kg B1, G1 and 25 µg/kg B2, G2) per 25 g of matrix, mixed well and proceeded to sample extraction. The chromatographic system consisted of an automatic Agilent 1200, with Immunoaffinity column cleanup and post-column derivatisation manufactured by Shim-pack VP-ODS, 4.6 mm (ID) x 150 mm (L). The post-column derivatisation was achieved using a Kobra Cell to obtain electrochemically generated bromine (ISO 16050 CEN/TC-34, 2006); Romer Biopure). It is equipped with an auto-sampler (10 μl, injector vol), a Shim-pack VP-ODS column with a Reverse-Phase C18 (4.6 (ID) x 150 mm (L)) and a fluorescence detector. The detector was set at EX= 350 nm, EM = 450 nm. The mobile phase was isocratic and composed of methanol: water (45:55)-KBr-HNO3 mixture with 450 ml of methanol, 119 mg of potassium bromide and 87.5 μl of 16 M nitric acid per liter of mobile phase. The flow rate was set at 1 ml/min.
The quality control standard (40 µg/kg calibration solution) was injected 3 times. The % CV of the peak area corresponding to the consecutive injections shall be within ± 10%. 10 μl of each of the Calibration Standards Solutions (2.5 µg/kg, 5 µg/kg, 10 µg/kg, 20 µg/kg, 40 µg/kg and 80 µg/kg) was injected into the HPLC system, followed by sample.
The concentration of Aflatoxins in the sample is calculated using the following formula:
Statistical analysis (XLSTAT. 2016)
The differences in aflatoxins concentration in maize samples between the Ouagadougou zones Burkina Faso and those of physicochemical analyzes were compared by Analysis of variance (ANOVA) using XLSTAT-Pro 7.5.2 software. Interpretation of values was performed using Newman-Keuls test at probability level p = 5%. The results were expressed as mean ± SD and the measures were repeated three times (n=3).
Physicochemical analyses of maize sample
Humidity rate analysis carried out on the ten (10) samples of maize seeds revealed that our samples are not very humid with an average value between 3.91 and 4.66% (Table 1).
These values ??are lower than the average moisture content of mold growth. In fact, the minimum humidity for certain molds to start growing is 10% (Compaoré, 2017). Our data could be explained by contamination of samples in the field or during storage. Mold strains are able to survive unfavorable conditions for their growth by producing large numbers of spores (Tabuc, 2007). Thanks to its great adaptability to environmental conditions, A. flavus can grow both on crops in the field, during harvest as well as later, during storage (Makhlouf, 2019). For the different samples, statistical analyses showed significant differences between the different humidity values. From this analysis it appears that the humidity is very variable from one sample to another. The pH results of the various maize samples analyzed indicate that all of the samples are slightly acidic, with pH values ??ranging from 5.83 to 5.99 (Table 2).
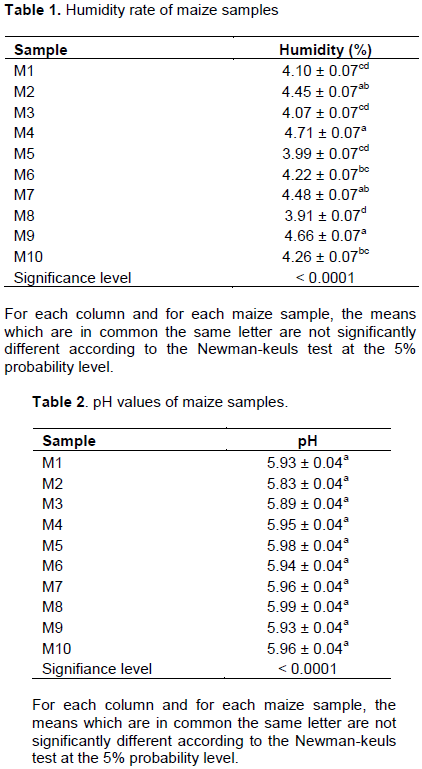
The pH values ??are very favorable to the growth of molds because according to Gauthier (2016), molds can grow in a pH range from 3 to 8, with optimal growth being rather between 5 and 6. The production of mycotoxins takes place for pH close to optimal growth pH (Makhlouf, 2019). Due to their acidity, many foods are much more prone to fungal than bacterial spoilage (Tabuc, 2007). Statistical analyses showed no significant difference between the different pH values.
Fungi isolation from maize seeds
In our study, ten (10) maize samples were used as a matrix for the collection of strains of Aspergillus section Flavi. From the consortium of fungi grown on maize, twenty-three (23) isolates were collected. Macroscopic observation of the Petri dishes made it possible to retain only the isolates forming colonies of yellowish and greenish color and have powder aspect. In optic microscopy, we were interested in those with non-septate and hyaline conidiophores. Eight (08) local Aspergillus section Flavi strains were therefore isolated from the consortium of fungi grown on maize seeds (Table 3).
Aflatoxins level in maize samples
Results of the quantitative aflatoxin analysis are reported in Table 4. 70% of the samples were found to be contaminated with total aflatoxins. These were M1, M2, M4, M5, M6, M9 and M10. The total aflatoxin contents in the maize samples ranged from 0.93 to 70.78 µg/kg. M1 and M10 had high concentrations (58.86 and 70.78 µg/kg respectively) and M4 and M5 had low concentrations with respective values ??of 1.67 and 0.93 µg/kg. In all the contaminated samples, aflatoxin B1 was present and the most concentrated with however the absence of aflatoxin G2 as it can be seen from the chromatograms in Figure 1. Out of seven (7) samples, four (04) were contaminated with aflatoxin B1 (AFB1) and aflatoxin G1 (AFG1), two (02) with AFB1 and aflatoxin B2 (AFB2) and one (01) with AFB1 only.
The contamination of our samples with aflatoxins could be explained by the presence of toxigenic strains Aspergillus section Flavi. Indeed, the latter are the fungal contaminants most frequently encountered in cereals and which secrete aflatoxins there when environmental conditions (temperature, humidity, pH, etc.) are met, as is the case with the pH of our maize samples which are slightly acidic with a pH around 5.83 to 5.99 (Table 2). Maize is considered among the fragile crops at high risk of contamination by toxigenic molds unlike other cereals, particularly barley and wheat (Brahmi and Zahi, 2016).
According to the Codex alimentarius, the maximum levels of AFB1 and total aflatoxins (B1, B2, G1 and G2) are respectively 2 and 5 µg/Kg {IARC, 2002 #674}(EU Regulation 1881, 2006). Our results revealed that the contamination rate of the different samples varied from sample to sample. Four (04) samples had total aflatoxin levels between 13 and 71 µg/Kg and therefore well above the Codex alimentarius standard. Only the M2, M3, M4, M5, M7 and M8 samples complied with the standard. The high concentration of aflatoxins in our samples could be explained by climatic conditions in Africa, poor agricultural practices and poor crop storage conditions. According to Zinedine (2004), crops grown in hot, humid climates and exposed to toxigenic molds provide optimal conditions for mold growth and can be contaminated with mycotoxins. According to Makhlouf (2019), the infestation of maize, by Aspergillus flavus (producer of AFB) before harvest, is often linked to the aggression of the plant by insects and rodents in the field. Cereals can be contaminated either by the spores that are initially found in the cereals or later during storage mainly if it is bad (Waré et al., 2017). Indeed, overripe crops and grain damaged during threshing are postharvest conditions that promote fungal growth in crops (Bhat et al., 2015). In addition, crops, usually stored in inadequate conditions, are attacked by insects, rodents and molds (Waongo et al., 2013). In Burkina Faso, the ears of maize are kept either in bundles or sheaves and hung from branches or above the hearths, or in straw or mud granaries. These storage systems do not protect the food from direct attack by insects and many other pests such as birds and rodents which carry microorganisms (Somda, 2016).
Aflatoxin B1 (AFB1) was predominantly present in all seven (7) contaminated samples with concentrations ranging from 0.54 ± 0.08 to 63.24 ± 0.07 µg/Kg. The maximum values ??of AFB1 that we obtained are much lower than the results obtained by Vargas et al. (2001)in Brazil, where the level of contamination of maize by aflatoxins reached 129 µg/kg. Nevertheless, our results are superior to those obtained in Ethiopia by Chauhan et al. (2016)which was 53 µg/Kg.
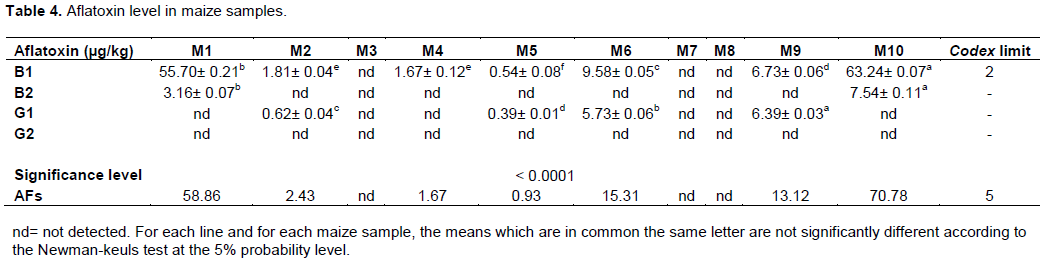
Aflatoxins are produced mainly by Aspergillus section Flavi species, mainly A. flavus and A. parasiticus. These two species are both producers of type B aflatoxins, but A. parasiticus also produces type G aflatoxins (Vargas et al., 2001). Out of seven (07) samples, three (03) were only contaminated with AFB. Aspergillus spp. isolated from these maize samples would therefore belong either to A. flavus or to A. parasiticus. Four (04) on the other hand were contaminated with AFB and AFG, indicating the presence in these samples of strains of mold belonging to the species A. parasiticus. Samples M1, M4 and M10 would therefore be contaminated by Aspergillus flavus strains while M2, M5, M6 and M9 would be contaminated by A. parasiticus.
The presence of the different types of aflatoxins in our maize samples would confirm the morphological characterization of our different isolates carried out previously. Thus, the A1, A2 and A7 isolates from sample M10 would be strains of A. flavus. A5 isolated from M2; A4, A6, A8 isolated from M5 and A3 isolated from M9, would all be strains of A. parasiticus. Indeed, in a previous study by Compaoré et al. (2021), we determined by qualitative methods the ability of A2 and A3 isolates to produce aflatoxins through blue fluorescence emission when cultured on Coconut Agar Medium (CAM). Both isolates were also subcultured on “Aspergillus flavus and parasiticus Agar” (AFPA) medium a four days incubation at 30 °C to study their ability to produce aspergillic acid. The results showed that both isolates are capable of producing aflatoxin. Reference strains UBOCC-A-111042 and S2 which are aflatoxinogenic were also tested in order to compare the obtained results with those of our two maize isolates. The results showed that both isolates are capable of producing aflatoxin. In the present study, the quantitative analysis of the different types of aflatoxins came to confirm the morphological identification carried out previously.
Aflatoxins production and their determination by fluorescence HPLC
Results of the qualitative aflatoxin analysis are reported in Figure 2. Aflatoxin production abilities tested previously by fluorescence under UV light of strains by cultivating them on Coconut Agar Medium (CAM) and AFPA medium were in concordance with those obtained by HPLC determination (Table 5). We have found that both A2 and A3 isolates showing fluorescence under UV light produced aflatoxins in CAM.
The present study included ten (10) samples of maize seeds grown in Burkina Faso. The results of the aflatoxin analysis showed that the majority of these are of unsatisfactory sanitary quality and have a fairly high average mold load predominantly dominated by genus Aspergillus. A total of eight (8) fungal strains belonging to Aspergillus section Flavi were isolated and characterized. The quantitative aflatoxin analysis method such as HPLC performed in the present study attests to the results of aflatoxin-producing ability previously performed and confirms that isolates A2 and A3 belong respectively to A. flavus and A. parasiticus. Nevertheless, this identification should be confirmed by Biology molecular methods. In view of the high levels of aflatoxins in cereals in Burkina Faso and the danger represented by the ingestion of contaminated food. The players in the maize sector must observe the Good Practice pre and post-harvest as well as decontamination methods such as biopreservation by lactic acid bacteria and Bacillus in order to preserve the health of consumers.
The authors have not declared any conflict of interests.
The authors thank the ministry in charge of trade for having funded this work through the project “Reduction of Contamination of Maize and Maize by-products by Aflatoxins in Burkina-Faso, West Africa (ReCMA-BF)”
REFERENCES
|
Association of Official Agricultural Chemists (AOAC) (2005). AOAC International; Aflatoxins in corn, raw peanuts and peanut butter: immunoaffinity column (aflatest) method.
|
|
Bambara A (2021). Characterization of Bacillus sp. strains with antifungal activities against Aspergillus section Flavi contaminating maize. Master memory, University of Ouagadougou, Ouagadougou 96 p.
|
|
Bhat RV, Shetty HPK, Vasanthi S (2015). Human and animal health significance of mycotoxins in sorghum with special reference to fumonisins natural occurrence of fumonisins in sorghum 11 p.
|
|
Brahmi N, Zahi M (2016). Search for aflatoxinogenic fungi of the genus Aspergillus in poultry feed and detection of aflatoxins. Master II graduation document, Saad Dahlab-Blida University, Algeria 97 p.
|
|
Chauhan NM, Washe AP, Minota T (2016). Fungal infection and aflatoxin contamination in maize collected from Gedeo zone, Ethiopia. SpringerPlus 5(1):1-8.
Crossref
|
|
Christensen M (1981). A synoptic Key and evaluation of species in the Aspergillus flavus group. Mycologia 73:1056-1084.
Crossref
|
|
Compaoré H (2017). Search for substances with antibacterial activities produced by strains of mold isolated from certain foods in Burkina Faso. Doctorate thesis, University of Ouagadougou, Ouagadougou, 185 p.
|
|
Compaoré H, Sawadogo/Lingani H, Waré LY, Guira F, Samandoulougou S, Savadogo A, Traore AS (2016). Morphological and molecular characterization of three fungi strains isolated from locals foods in Burkina Faso. International Journal of Biosciences, 9(6):374-382.
Crossref
|
|
Compaoré H, Samandoulougou S, Waré LY, Bambara A, Ratongue H, Sawadogo I, Sawadogo-Lingani H (2021). Identification of Aspergillus section Flavi and Fumigati in maize grown in Burkina Faso. International Journal of Biosciences 18(6):25-36.
|
|
Cooney DG, Emerson R (1964). Thermophilic fungi: An account of their Biology, Activities and classification. ed. W.H. Freeman and Company. San Francisco, Calif. 188 p.
|
|
Cotty PJ (1994). Comparison of four media for the isolation of Aspergillus flavus group fungi. Mycopathologia 125:157-162.
Crossref
|
|
EU Regulation 1881 (2006). EU Regulation 1881/2006; setting maximum levels for certain contaminants in foodstuffs. Official Journal of the European Communities pp. 20-25.
|
|
Ezekiel CN, Udom IE, Frisvad JC, Adetunji MC, Houbraken J, Fapohunda SO (2014). Assessment of aflatoxigenic Aspergillus and other fungi in millet and sesame from Plateau State, Nigeria. Mycology 5:16-22.
Crossref
|
|
Gauthier A (2016). Mycotoxins in food and their impact on health. Doctorate thesis, University of Bordeaux, Bordeaux, 132 p.
|
|
GDSSS/MAHAD (2020). Yearbook of agricultural statistics 2018, General Directorate of Studies and Sectoral Statistics, Ministry of
|
|
Agriculture and Hydro-Agricultural Development, Burkina Faso 290 p.
|
|
Hocking AD (1982). Aflatoxigenic fungi and their detection. Food Technology in Australia 34:1-3.
|
|
IARC (2002). Monograph on the Evaluation of Carcinogenic Risk to Humans. In: Some Traditional Herbal Medicines, Some Mycotoxins, Naphtalene and Styrene, volume. 82. Lyon: IARC 171-300.
|
|
Kachapulula PW, Akello J, Bandyopadhyay R, Cotty PJ (2017). Aspergillus section Flavi community structure in Zambia influences aflatoxin contamination of maize and groundnut. International Journal of Food Microbiology 261:49-56.
Crossref
|
|
Makhlouf J (2019). Characterization of Aspergillus section Flavi biodiversity isolated from foods marketed in Lebanon: molecular, metabolic and morphological approach. Doctorate thesis, University of Toulouse, Toulouse 142 p.
|
|
Ouattara-Sourabié PB (2018). Inhibition de la croissance et de la production d'aflatoxines chez Aspergillus spp. par des extraits de plantes (Cymbopogon citratus, Cymbopogon giganteus et Hyptis spicigera). Thèse unique de Doctorat, Université Ouaga I Pr Joseph Ki-Zerbo, Burkina Faso 154 p.
|
|
Pitt JI, Hocking AD, Glenn DR (1983). An improved medium for detection of Aspergillus flavus and Aspergillus. parasiticus. Journal of Applied Bacteriology 54:109-114.
Crossref
|
|
Sanou D (2000). Study of the prevalence of mycotoxins in agricultural products from Burkina Faso: Case of contamination of maize (zea mays L.) by aflatoxins and fumonisins in western Burkina Faso. Advanced Studies Diploma Thesis, University of Ouagadougou 59 p.
|
|
Somda KBM (2016). Problems of post-harvest conservation of maize in the upper basins. Engineer thesis, Polytechnic University of Bobo-Dioulasso, Burkina Faso 77 p.
|
|
Tabuc C (2007). Fungal flora of different substrates and optimal conditions for the mycotoxins production. Doctorate thesis, University of Toulouse, Toulouse 190 p.
|
|
Vargas EA, Preis RA, Castro L, Silva CMG (2001). Co-occurrence of aflatoxins B1, B2, G1, G2, zearalenone and fumonisin B1 in Brazilian corn. Food Additives and Contaminants 18(11):981-986.
Crossref
|
|
Waongo A, Yamkoulga M, Dabiré-Binso LC, Ba NM, Sanon A (2013). Post-harvest conservation of cereals in the South Sudanese zone of Burkina Faso: Farmer perception and stock assessment. International Journal of Biological and Chemical Science 7(3):1157-1167.
Crossref
|
|
Waré LY, Durand N, Nikiema PA, Alter P, Fontana A, Montet D, Barro N (2017). Occurrence of mycotoxins in commercial infant formulas locally produced in Ouagadougou (Burkina Faso). Food Control 73:518-523.
Crossref
|
|
Zinedine A (2004). Determination of mycotoxins in food and study of the reduction of aflatoxins by lactic acid bacteria isolated from traditional bread ferments. Doctorate thesis, Sidi Mohammed Ben Abdellah University, Morocco 162 p.
|