In the present study, rhizobia partners of gum acacia (Senegalia senegal L.), shittah tree (Vachellia seyal), pigeon pea (Cajanus cajan L.) and cowpea (Vigna unguiculata L.) were characterized for their specific traits to each of the three host plants. In total 12 soil samples were collected and used to trap root-nodule rhizobia. Averagely, 24 rhizobia strains were isolated, of which 3, 4, 6 and 11 were respectively from root-nodules of S. senegal, V. seyal, C. cajan and V. unguiculata. Isolates were morphologically rod-shaped and measured 2.5-3.2 mm in diameter. They were wet (22), wet/sticky (01) and wet/creamy (01) in appearance. Isolates (23) were convex, and flat (1) in elevation. They were all Gram-negative, and none form endospores. They indicated positive reactions to ammonia and indole acetic acid tests, but showed various reactions to catalase, phosphate solubilization, starch, triple sugars ion agar and urea tests. Nodulation and nitrogen fixation capacity were confirmed by either pink or brown internal coloration of nodules. Inoculation of hosts with isolates significantly (p < 0.0001) increased both the plant and nodule weights compared to that of control. This study has revealed that all the identified isolates possess are Rhizobium, belonging to the same cross-inoculation group, thus may be useful in increasing the symbiotic nitrogen fixation in the studied legumes. Theses findings provide the basis for further research on the phylogeny of rhizobia strains nodulating legumes, as well as their use as potential inoculants to improve production in semi-arid lands such as those of the northern Cameroon.
Legumes constitute a large group of flowering plants belonging to the Fabaceae or Leguminosae family (Lewis et al., 2005). Many crop legumes have the capacity to use two different sources of nitrogen, such as uptake of mineral nitrogen present in the soil solution by root like every high plant, and biological of atmospheric nitrogen (N2) fixation by rhizobia inhabiting their rhizosphere. These features make these crop legumes particularly attractive for agricultural and natural ecosystems, and hence, easily adaptable to infertile soils (Voisin et al., 2015; Giller et al., 2016). Legumes provide a stable and safe food for human, as well as fresh fodder for animals, because they constitute a source of nitrogen and micronutrients (Snapp et al., 2018).
Bacteria associated to crop legumes are collectively known as rhizobia. The term “rhizobia” was originally used to name bacteria belonging to the genus Rhizobium. Nowadays, other genera were later identified, such as Bradyrhizobium (Jordan, 1982), Sinorhizobium (Chen et al., 1988) and Mesorhizobium (Jarvis et al., 1997). Until recently, rhizobia were thought to belong exclusively to the Alphaproteobacteria, namely the order Rhizobiales, which also includes many species that are not legume microsymbionts. However, some unexpected findings from research on wild legumes microsymbionts has resulted in the identification of rhizobia from the Betaproteobacteria class (Moulin et al., 2001). They are commonly described as aerobic, rod-shaped, non-sporulating and Gram-negative soil-inhabiting bacteria containing nodulation and atmospheric N2 fixation genes needed for the symbiosis (Giller et al., 2016). Rhizobia are also used as biofertilizers because of their ability to fix atmospheric nitrogen through their symbiotic association with crop legumes. Therefore, the mutualism between the two partners is a classic and reciprocal relationship in which, carbohydrates are provided to rhizobia by the host legume, while rhizobia in turn supply fixed-nitrogen to the host (Denison, 2000; West et al., 2002; Kiers et al., 2003). Rhizobia have also been reported to improve plant health or increase yield, and are usually referred to as plant growth-promoting rhizobacteria (PGPR) (Kloepper et al., 1980).
In order to know which root nodule bacteria are able to establish effective symbiosis with a particular legume, it is important to isolate and characterize them. To identify rhizobia inhabiting root nodules, the methods described by Vincent (1970) and Somasegaran and Hoben (1985) are often known to be easier to use in principle. Therefore, morphological, biochemical and symbiotic characterization on selective medium seem to be more adequate. Successful nodulation and N2 fixation in the Legume-Rhizobium symbiosis require a close compatibility between the two partners and appropriate soil environment (Hirsch et al., 2003; Leibovitch et al., 2001; Zhang et al., 2002; Mishra et al., 2012). Although rhizobia from four crop legumes have been characterized and have shown efficiency in increasing the host plants yield in the Guinea-Savannah zone of Cameroon (Ngakou et al., 2009), nothing is known about the similarities/differences between rhizobia symbionts of food crop legumes and those of tree legumes such as acacia species. This study is focused on legume-Rhizobium relationships, specifically the morpho-physiological and biochemical characteristics of Rhizobium strains associated to some food (Cajanus cajan, Vigna unguiculata), and tree (Senegalia senegal, Vachellia seyal) crop legumes. In the present research, the characterization of rhizobia from root nodules of the above four crop legumes, and the evaluation of their nodulation efficiency through a cross-nodulation trial are discussed. This study could serve as preliminary databases for the further study relevant for biofertilizers research in the region.
Study areas
Soils were collected from 2 different agro-ecolgical zones of Cameroon: the Sudano-Sahelian zone comprising the Far-North and North regions, characterized by a long dry season (November-June), with the annual rainfall between 500-1000 mm and annual average temperature of 28°C; and the Guinean Savannah of the Adamawa region, characterized by a long rainy season (April-October), a mixture of Woodland and grassy savannah as vegetation, an annual mean rainfall varying from 1227.9-1675.8 mm and annual average temperature of 22.93°C (Pamo, 2008).
Soil sampling
Soils were sampled at the beginning of the dry season in the rhizosphere of the four selected crop legumes (S. senegal, V. seyal, C. cajan, V. unguiculata), in order to optimize the chances of getting specific rhizobia of affiliated to these legumes. A total of 12 rhizospheric composite soil samples of 25 kg each (4 legumes × 3 regions) were collected at 20 cm depth and at 25 cm radius within each plant rhizosphere. On the site, each soil composite sample was sieved through a 2 mm pores sieve to discard stones or gravels, before they were separately filled into 20 sterile plastic bags of 1 kg each per rhizospheric soil.
Trapping experimental set up
For each soil from any of three regions (Ngaoundere, Garoua, Maroua), the trapping was carried out as pots experiment in a completely randomized block design (CRBD) system, in which the host plants (S. senegal, V. seyal, C. cajan and V. unguiculata) were considered as treatments, while the 20 plastic bags filled with 1 kg soil each were the replicates. For each host plant, seeds were surface sterilized by three successive immersions in 70% alcohol followed by three washings in sterile water. In each of the 1 kg plastic bags containing soils, rhizobia were trapped using four seeds per crop legume. Seeds of S. senegal and V. seyal were manually scarified before sowing, while C. cajan and V. unguiculata seeds were sown without any dormancy break off. After germination, plantlets were thinned to two to reduce competition between plantlets. The trial set was watered daily and maintained till the formation of effective root nodules (at 45 days after sowing).
Isolation and purification of Rhizobium isolates on Congo Red YEMA medium
At 45 days after sowing, plants were harvested by tearing the plastic bag, then the roots were delicately rinsed with tap water to clear roots from soil particles and to view root-nodules. Root-nodules were manually discarded from roots and introduced into labelled jars (name of plant and origin of soils) and transported to the laboratory. Petri dishes were cleaned with tap water, rinsed with distilled water and allowed to dry at room temperature. Cleaned dishes were sterilized in the dry oven at 105°C for 2 h. The Yeast Extract Mannitol Agar (YEMA) medium was prepared as described by Vincent (1970) with the following composition: 10 g mannitol; 0.2 g MgSO4, 7H2O; 1 g yeast extract; 0.5 g KH2PO4/K2HPO4; 0.1 g NaCl; pH = 6.8. The medium was supplemented with 0.25% Congo red to restrict possible contaminants. Rhizobia were isolated from the fresh root nodules as described by Somasegaran and Hoben (1985). Hence, 15 efficient root nodules were randomly selected for each legume species and each soil type were immersed in 70% alcohol for 30 s, and then in 0.1-0.2% HgCl2 for 5 min, before they were rinsed with distilled water to remove all traces of alcohol and HgCl2. Sterilized nodules were ground in a Petri dish using a sterile carpel. Aliquot of the macerate was taken and aseptically struck onto the YEMA solid medium. Inoculated petri dishes were incubated at 28±°2C temperature. Three days after the first streak, different colonies grown separately were sub-cultured onto the YEMA-Congo Red solid medium under the same conditions, in order to screen all the single colonies. Three days after sub-culturing, the morpho-cultural characteristics of isolated colonies (number, elevation, surface, colour, aspect, edges) were described before the study of physiological and biochemical characteristics of isolates.
Morphological characterisation of isolates
Three days after incubation at 28±2°C on YEMA growth medium in the dark, different typical colonies were identified, counted and their morphological characters (shape, size, appearance, elevation, surface, colour and edge) evaluated as presented in Table 2 (CIAT, 1988; Somasegaran and Hoben, 1985, 1994).
Screening of Rhizobium strains
The method described by Vincent (1970) and Somasegaran and Hoben (1994) was used to screen Rhizobium strains through Congo red test, Bromothymol blue test, Gram staining and cell microscopy observation, while the method of Bernaerts and Deley (1963) was performed for the ketolactose test.
Physiological and biochemical characterization of isolates
The purified Rhizobium isolates were studied for their resistance to different stresses. Hence, they were streaked on media at different NaCl (1, 2, 3, 5 and 10%), pH (4, 7, 8, 9, 11) and also at different temperatures (28, 30, 35, 38 and 42°C) (Vincent, 1970; Somasegaran and Hoben, 1994).
Catalase test
All the isolates were struck on YEMA solid media and incubated at 28±2°C for 3 days. The test was performed by adding 2-3 drops of 3% hydrogen peroxide on different cultures of isolates. After incubation, the formation of gas bubbles indicates the presence (+) or the absence (-) of catalase (Graham and Parker, 1964).
Starch hydrolysis
Starch hydrolysis test consisted of adding 0.2% starch powder to nutrient agar medium plates per isolates. Labelled plates containing nutrient agar medium were incubated at 28±2°C for 3 days. Positive test indicated the isolates with competence to solubilize starch by producing the enzyme amylase. After incubation period, culture plates were flooded with Gram’s iodine, and the presence (+) or absence (-) of halos around the bacterial colonies was recorded (De Oliveira et al., 2007).
Triple sugar ion agar test
Triple sugar iron agar medium (3 g/L beef extract, 3 g/L yeast extract, 15 g/l peptone, 5 g/L NaCl, 10 g/L lactose, 10 g/L sucrose, 1 g/m dextrose, 0.2g/l ferrous sulphate, 0.3g/l thiosulphate, 0.24 g/L phenol red, 15 g/L agar, pH 7.0) test was used to determine the ability of isolates to use varied carbohydrate sources as media for growth. The test was performed to distinguish among the different groups, which are able of fermenting glucose with the production of acid and hydrogen sulphide (Kliger, 1918).
Urea production
YEMA medium plate was prepared, consisting of a mixture of 2% urea and 0.012% Phenol Red (Jarvis et al., 1977). After sterilization by filtration and autoclaving, about 10 μl of each isolate type was struck on the prepared solid medium plates, and incubated at 28 ±2°C for 3 days. On the basis of colour shift of medium, which is initially red, the results were evaluated: as pink coloration indicating the hydrolysis (+) of urea to form ammonia and carbon dioxide in an alkaline environment. A yellowish coloration was a sign of a negative reaction (-) in an acidic environment.
Plant growth promoting traits
Ammonia production
The capacity of different isolates to synthetize ammonia in Peptone broth medium (1 g peptone, 0.5 g NaCl, 0.5 g potassium nitrate, and 1000 ml distilled water) was tested. A volume of 5 ml sterilized broth was dispersed in each labelled test tubes with a control, and incubated for 3 days. After incubation, 1ml of Nessler’s reagent was added to each of the bacterial culture tubes, and the development of orange to brown colour indicatiing the presence (+) of ammonia was recorded (Joseph et al., 2007; Mahbouba et al., 2013).
Indole 3-acetic acid production
Qualitative analysis of indole acetic acid production by different isolates was carried out. Hence, culture tubes containing nutrient broth were prepared and autoclaved. About 50 μg/ml broth of filtered tryptophan solution was added to each tube. Culture tubes with different isolates, including the negative control were incubated at 28±2°C for 3 days. After addition of 4 ml of the reagent to 1 ml of the supernatant, the solution was thoroughly mixed and incubated for 30 min for the development of pink colour which indicates IAA production (+) by isolates (Hartmann et al., 1983).
Phosphate solubilization
All the isolates were screened on Pikovaskaya agar medium (10 g glucose, 0.5 g yeast extract, 0.5 g (NH4)2SO4, 0.2 g KCl, 0.2 g NaCl, 0.1 g MgSO4 7H2O, Trace FeSO4, Trace MnSO4 7H2O, 5 g Ca3 (PO4)2, 18 g Agar, pH 7.0-7.2) in labelled sterile Petri plates as described by Sarker et al. (2014). Formation of clear zone around the growing isolated colony was the indication of phosphate solubilization (+) by the isolates, while the absence clear zone was the sign of no phosphate solubilization (-).
Cross-nodulation test and assessment of plant growth
Rhizobium colonies from each of the crop legumes (S. senegal, V. seyal, C. cajan, V. unguiculata) growing on solid medium were transferred into Erlenmeyer flasks containing 200 ml YEMA Congo Red liquid medium, where agar was reduced from 15 to 1.5 g/L. Rhizobia were allowed to grow to 106-108 cells/ml for 72 h under shaking conditions on a magnetic stirrer (Somasegaran and Hoben, 1994). A total of 18 inoculants were cultured with 1 inoculum (SGL), 3 inocula (VY1, VY2, mVY= VY1 and VY2 mixture), 5 inocula (CC1, CC2, CC3, CC4, mCC = CC1-4 mixture) and 8 inocula (VU1, VU2, VU3, VU4, VU5, VU6, VU7, mVU = VU1-7 mixture) produced from rhizobia isolated from S. senegal, V. seyal, C. cajan and V. unguiculata respectively, and 1 total mixture (mT). The Rhizobium isolates obtained were tested for their potential to induce the formation of efficient root nodules from the four studied crop legumes in a cross-nodulation study. To achieve this, sand was laid out in the autoclave at 121°C and filled in 1 kg sterile plastic bags. For each of the host plant (S. senegal, V. seyal, C. cajan and V. unguiculata), the plastic bags were disposed in a CRBD system with 19 treatments as Inocula (SGL, VY1, VY2, mVY, CC1, CC2, CC3, CC4, mCC, VU1, VU2, VU3, VU4, VU5, VU6, VU7, mVU, mT and NC = negative control), each of which was replicated in 6 plastic bags. Three surface sterilized seeds of each crop legume were sown in a plastic bag prior to inoculation with 2 ml of a specific inoculum. Plants were thinned to 1 after germination to avoid competition, and were daily watered with tap water (Somasegaran and Hoben, 1994).
Statistical analysis
The effects of different treatments were analysed by One-way ANOVA, using the plant weights and the nodule weights as variables. Means among treatments were compared with the least significant difference (LSD), using the STATGRAPHICS 5.0 programme.
Morphological and cultural traits of the isolates
Three days after incubation at 28±2°C on YEMA medium, 24 colony types were identified and were represented by 12.5% S. senegal, 16.67% V. seyal, 25% C. cajan and 45.83% V. unguiculata respectively (Table 1).
All the isolates were round in shape, and reached a mean colony diameter size of 3.2 mm. Isolates appeared, either wet (22), creamy (01) or sticky (01). They were convex (23) or flat (01) in elevation. Colonies were either milky (10), white (03), yellowish (03), milky/translucent (02), white/turbid (02), pink (01), orange (01), translucent (01), and/or orange/yellowish (01) in colour. All the colonies were smooth in surface and regular in edge. Rhizobia isolates with similar characteristics from Vigna radiata L. were reported in India (Bhatt et al., 2013). Colony morphologies from 14 rhizobia isolates from Crotolaria junceae L. root nodules were also previously observed (Singha et al., 2015). Rhizobia with similar characteristics were reported from root nodules of four food legumes namely Arachis hypogeae L., Glycine max L., Vigna subterranea L. and V. unguiculata L. (Ngakou et al., 2009).
Based on these cultural characteristics, 14 distinct isolates representing 7.14, 14.29, 28.57 and 50% for S. senegal, V. seyal, C. cajan and V. unguiculata respectively, were considered for further studies. Isolates did not absorb Congo red at 28±2°C in the dark, indicating the absence of contaminants (Somasegaran and Hoben, 1994; Giller et al., 2016). All the isolates showed negative reaction to ketolactose test after incubation at 28±2°C for 3 days, indicating that they were all Rhizobium strains, but not Agrobacteria (Table 2). Isolates were tested Gram negative and were microscopically rod-shaped and pink in colour after treatment with iodine reagent, confirming that they belonging to the genus Rhizobium. Related results were obtained respectively from Arachis hypogaea L. and Telfairia occidentalis in Nigeria (Agah et al., 2016), from Glycine max L. in Zambia (Kapembwa et al., 2016), from Mucuna pruriens L. in Nepal (Paudyal and Gupat, 2017), from V. mungo L. and V. radiata L. in India (Tyagi et al., 2017). These isolates were also recognized as acid producers, since they shifted the green colour of Bromothymol blue to yellow when incubated at 28±2°C for 3 days (Table 2).
Physiological traits of the isolates
Physiological characterization of different rhizobia isolates was carried out based on their growth on YEMA medium at various salt concentrations, pH and at different incubation temperatures (Table 3). As the results, isolates grew well at 1-2% salt concentration, 28-35°C temperature range and at pH 7-8. A previous research has pointed out an optimum growth of root nodule bacteria from Mung bean at 1-2% salt, 28-37°C temperature, and at pH 7 (Bhatt et al., 2013). Optimal growth of most Rhizobium strains has been reported to occur at 25-30°C temperature and at pH 6-7 (O’Hara et al., 2016).
Biochemical traits of the isolates
Biochemical studies has revealed 64.29% of isolates with positive reaction to catalase test, 42.86% able to solubilize starch, 71.43% positive to triple sugar iron test, 71.43% positive reaction to urease test, whereas all had very strong alpha-amylase (+++) activity (Table 4). Regarding the production of Plant Growth Promoting metabolites, 100% of isolates produced ammonia, 100% produced IAA, while only 28.57% were able to solubilize inorganic phosphate (Table 4). In a related study in Nigeria, Agah et al. (2016) reported 100% of isolates as producer of both ammonia and IAA. According to findings of Bhatt et al. (2013), none of the Rhizobium isolates from Mung Bean was revealed to produce IAA, neither was positive to urea tests, although other tests confirmed their characteristics as Rhizobia. Another result from Crotolaria junceae root nodules has reported 14 rhizobia isolates positive to catalase, starch and urea tests (Singha et al. 2015). The fact that some of the isolates from the present study were positive to TSI (sucrose, lactose and glucose) is in agreement with other results indicating that 100% of isolates from root nodules of S. senegal, V. seyal, C. cajan and V. unguiculata (Saad, 2008), or from root nodules of Hadysarum pallidum Desf. (Rayane and Oussam, 2015), and were able to use carbohydrates as sole carbon sources. Moreover, 100% of isolates from root nodules of Genista and Argyrolobium genera were also shown to hydrolyse urea in Algeria (Dekak, 2010).
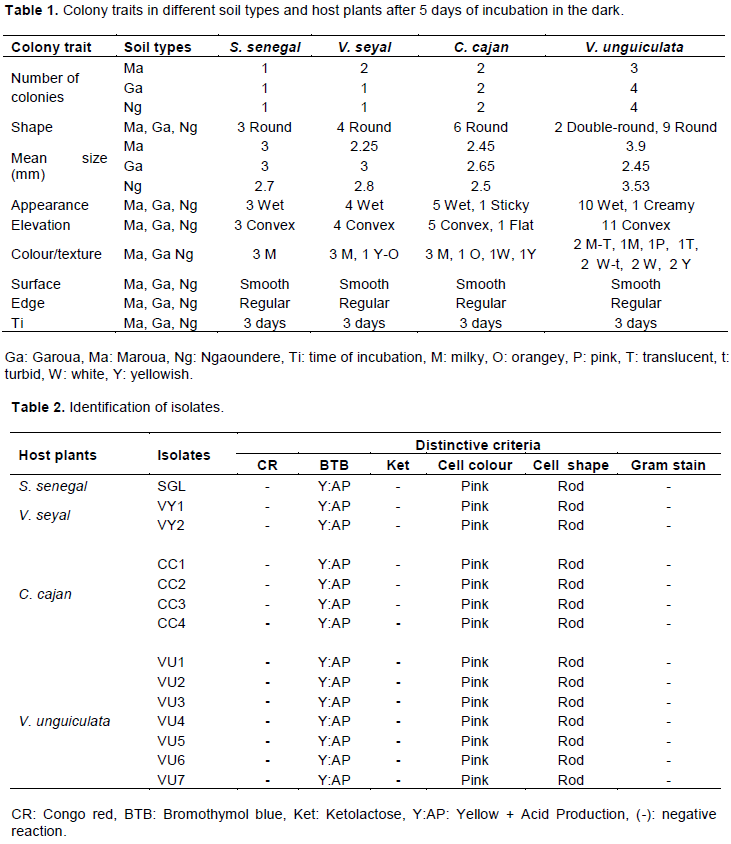
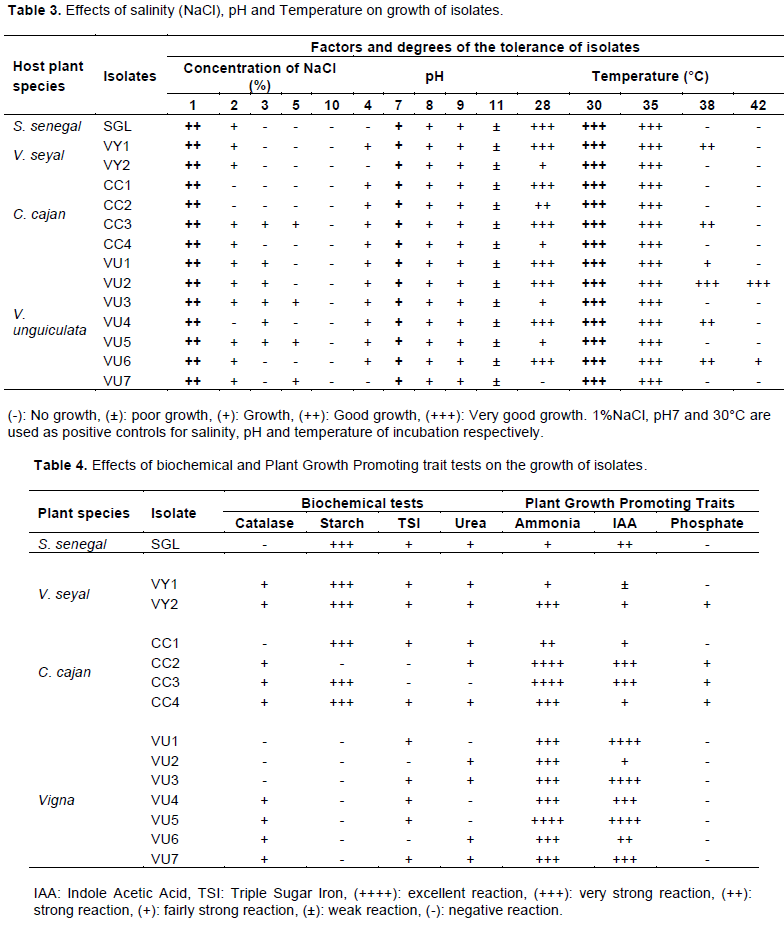
Responses of plants to cross-nodulation
The study of the effect of inoculation of one host plant with Rhizobium strains from another host has demonstrated that all rhizobia isolates respectively from S. senegal (p = 0.0031), V. seyal (p = 0.0001), C. cajan (p = 0.003) and V. unguiculata (p < 0.0001) had biomasses statistically higher than that of negative control. Inoculated plants of A. senegal, A. seyal, C. cajan and V. unguiculata with Rhizobium strains, mT, mVY1 and CC2 best contributed to improved plant biomasses (Table 5). Concerning nodulation, all Rhizobium isolates were able to form nodules on their host plant roots, except the control plants. There was a highly significant difference (p < 0.0001) between the nodule biomasses from all inoculated host plants and that of the control. Inoculation of plants with isolates SGL, mVY, mT and CC4 greatly increased the nodules biomass of S. senegal, V. seyal, C. cajan and V. unguiculata (Table 6). These results indicate that inoculating one host plant with rhizobia isolate from another under pot conditions positively and significantly impacted the treated host plant and nodule biomasses compared to values displayed by non-inoculated plants (Tables 5 and 6), suggesting that all the isolates were efficiently involved in biological nitrogen fixation with their four tested host legumes.
This is an advantage to the grower in terms of efficacy, reduce time and cost. The two tree legumes (S. senegal and V. seyal) had the plant and nodule biomasses consistently lower than those of the food crop legumes (C. cajan and V. unguiculata) at 45 DAP, which is obvious for the following reasons: i) growth of trees was slower than that of most herbaceous species; ii) even after scarification prior to sowing, S. senegal and V. seyal) seeds emerged after weeks, while the seed emergence of C. cajan and V. unguiculata occurred not more than after 4 DAP; iii) at 45 DAP, C. cajan and V. unguiculata already had huge biomasse, were even flowering and fruiting, while S. senegal and V. seyal) still had few leaves; iv) the nodule number and size of C. cajan and V. unguiculata was always higher than that of S. senegal and V. seyal), lining with the positive and significant correlation between the number, size and biomass of nodules reported by Ngakou et al. (2009).
These differences could probably be attributed to either the stage of plant development, or the plant sub-family, because, both S. senegal and V. seyal are perennial plants (Kew, 2016) which have a longer life cycle than that of C. cajan and V. unguiculata, which are semi-perennial and annual plants respectively (SY, 2001). Besides, responses of legumes to cross-inoculation with rhizobial inoculant may with legume species, a deeper root system exploring more soil mineral nitrogen contents (Yates et al., 2016). Previous research have reported successful nodulation of Vigna mungo by all the rhizobia isolated from seven tree legumes, except rhizobia from Abizia lebbeck in response to inoculation under arid environment (Mahmood and Athar, 2008), in agreement with the obtained results from this atudy. Similarly, Argaw (2012) indicated that Rhizobium leguminosarum isolated from some Ethiopian soils was able to significantly increase plant performances through nodule numbers, nodule dry biomasses, plant dry biomasses and yields, just like Waheed et al. (2014) in a related research on pea crop in comparison to the negative control in Pakistan. Once again, this cross-inoculation experiment has confirmed the promiscuous status of rhizobia associated to food/tree crop legumes, similar to the same findings between four food crops legumes as pointed out by Gomoung et al. (2017).