ABSTRACT
This study evaluates the antimicrobial activities of a multi-species probiotic ingredient derived from the African opaque sorghum beer during its propagation in a starchy career model. The aim was to establish the optimum growth conditions that warrant the optimum antimicrobial activities in the product. The antimicrobial activities were tested against Gram-positive bacteria (Staphylococcus aureus ATCC 27844, S. aureus MR 825), Gram-negative bacteria (Escherichia coli ATCC 25922, E. coli O157:H7 ATCC 700728, Salmonella typhi R 30951401, Klebsiella pneumoniae ATCC 35657), as well as against yeast (Candida albicans MHMR), using agar disc diffusion method. Also, the growth of viable cells and physicochemical parameters during the propagation were monitored. The results showed that the pH and dry matter content of the probiotic ingredient decrease significantly (p˂0.05) during the propagation whereas the lactic acid, the titratable acidity, lactic acid bacteria (LAB), yeasts and moulds counts increase significantly (p˂0.05). From 0 to 12 h, the product failed to inhibit the growth of all indicator strains. From 24 h and onward, the probiotic career inhibited all indicator strains except for K. pneumoniae (ATCC 35657) which could not be inhibited. Clearly, our study showed that 36 h of the propagation were sufficient to generate a probiotic ingredient with optimum antimicrobial activities.
Key words: Probiotic, antibiotic, opaque beer, antimicrobial activity.
The use of antibiotics for diseases prevention or healing and as growth factors in animals feeding contributed to the development of breeding through health improvement and zootechnic performances of animals. The estimated
antibiotic use in animal agriculture is 7.36 to 11.18 million kilogram per year (Khachatourians, 1998; Doyle, 2001). Globally, tonnes of antibiotics have been distributed in the biosphere during an antibiotic era of only about 60 years duration (Balcázar et al., 2006). However, these amounts of antibiotics have favored a strong selection among microorganisms, which developed resistance to these antibiotic chemicals, mainly by a horizontal and promiscuous flow of resistance genes (Aarestrup et al., 2000; Randall et al., 2003; SCAN, 2003 Currently, the potential for agricultural antibiotics to contribute to the development of antibiotic-resistant bacteria of human concern is the subject of intense debate and research (Wegener et al., 1999). Resistance mechanisms can arise by two ways: Chromosomal mutation or acquisition of plasmids. Chromosomal mutations cannot be transferred to other bacteria but plasmids can transfer resistance rapidly (Lewin, 1992). There is an increasing interest in finding alternatives to the use of antibiotics in animals feeding due to the ban of subtherapeutic antibiotic usage in Europe (Fajardo et al., 2012). Thus, several alternative methods are explored. Among them, probiotics are a subject of particular attention.
The probiotics with antimicrobial properties act by producing bacteriocins such as nicin (Yateem, et al., 2008) or by lowering the pH as a result of acidic compounds production such as lactic acid (Psomas et al., 2001). The progressive reduction of the use of antibiotics in animals feeding, as growth promoters, has raised renewed interest in the incorporation of microbial strains in animals feeding, in order to maintain the beneficial effect obtained with antibiotics (Guillot, 2001).
Kpete-kpete is the starter culture used to ferment tchoukoutou, the most produced and consumed opaque sorghum beer in Benin.
Lactic acid bacteria and yeasts have been reported (Kayodé et al., 2007) to be the major microorganisms involved in the fermentation of tchoukoutou. These fermentation microorganisms have been reported to possess probiotic effects, to reduce the level of pathogenic bacteria occurring in beverages and to reduce the severity duration and morbidity of diarrhea (Mensah et al., 1991; Kimmons et al., 1999). Recent researches in Ivory Coast showed net body weight increase and increased feeding efficiency in broilers when settling of palm wine or yogurt probiotic were used as feeding supplementations (Bohoua, 2008). In Benin, an investigation by Houndonougbo et al. (2011) showed low mortality and increased body weight gain when chicken’s feed was supplemented with starter culture harvested from opaque sorghum beer. In addition, a recent survey conducted by N’tcha et al. (2015) showed that Kpete-kpete is used to cure humans and animals diseases such diarrhoea, dysentery and wounds. The aim of the present study is to optimize the antimicrobial activity of Kpete-kpete during its propagation in a sorghum-based starch used as career.
Starchy career model
Starch extracted from a red variety of sorghum (Sorghum bicolor (L) Moench) was used as starchy career. The starch was extracted following the process described by Kayodé et al. (2012). Ten kilogrammes (10 kg) of cleaned sorghum grains were dehulled using a mini-PRL dehuller (Thiès, Sénégal) and then ground into flour.
Propagation of the probiotic ingredient and sampling
The sorghum flour is mixed with distilled water to obtain a dough (45% w/w), which is inoculated with 10% (w/w) of Kpete-kpete, kneaded into dough and allowed to ferment in a plastic bucket with lid for 72 h. Samples were withdrawn at 0, 6, 12, 24, 36, 48, 60 and 72 h of propagation for analysis. At each time point, 12.5 g of sample were aseptically taken from the flask for microbiological analysis and antimicrobial activity (10 and 2.5 g, respectively). Another sample of 50 g was kept at -10°C for pH, titrable acidity and dry matter measurements within 4 h approximately after sampling. The remaining sample was frozen for further analysis. In other to check the effect of drying temperature on the functional properties of the ingredient, we dried (42°C for 24 h) the product obtained at 72 h of propagation and derived samples for analysis of antimicrobial activities.
Microorganism materials used for antimicrobial test
The assayed pathogens included Gram-positive (Staphylococcus aureus ATCC 27844, S. aureus MR 825), Gram-negative (Escherichia coli ATCC 25922, E. coli O157:H7 ATCC 700728, Salmonella typhi R 30951401, Klebsiella pneumoniae ATCC 35657) and one yeast (Candida albicans MHMR). E. coli O157:H7 ATCC 700728, S. aureus ATCC 27844, S. typhi R 30951401, K. pneumoniae ATCC 35657 were supplied by the Laboratory of Food Safety and Water Quality of Ministry of Health, whereas E. coli ATCC 25922, S. aureus MR 825 and C. albicans MHMR were obtained from the Laboratory of Biology and Molecular Typage in Microbiology. Each stock culture was maintained in the respective growth media, containing 30% of glycerol, and stored at -80°C. Before the use in experiments, the strains were transferred into fresh growth media and incubated at suitable temperature for 18 to 24 h. This was followed by two consecutive transfers in the medium and incubated under the conditions indicated. The antimicrobial activities of ingredients were evaluated by means of disc diffusion assays.
Physicochemical analysis
Water content was determined as described (AACC, 44-15 A, 1984). Titratable acidity and pH were determined as described by Nout et al. (1989). The pH was measured using a digital pH-meter (JENWAY, Model 3505, UK) calibrated with buffers at pH 4.0 and 7.0 (WTW, Weilheim, Germany).
High pressure liquid chromatography (HPLC) analysis of sugars and organic acids
Lactic acids and soluble sugars were determined following the method developed by Mestres and Rouau (1997). 50 mg of samples were extracted with 5 mM sulphuric acid in 1.5 mL centrifuge tubes under continues agitation for 30 min at room temperature. After extraction, samples were centrifuged at 3500 x g for 30 min and filtered through a 0.45 µm pore filter before quantification by HPLC using an Aminex HPX-87H+cation-exchange column (BioRad Hercules, USA) thermostated at 37°C. Detection was done at 210 nm with an IR-detector. Elution was with sulphuric acid 5 mM at a flow rate of 0.6 mL min-1. The injection volume of the sample was 20 µL. Organic acids and sugars were expressed as g/kg dry matter. Analyses were performed in duplicate.
Counts of viable microorganisms
Total counts of lactic acid bacteria (LAB), yeasts and moulds were enumerated according to the method described by Nout et al. (1987). At each sampling time, duplicate samples (10 g) were diluted in 90 mL sterile peptone physiological saline solution (5 g peptone, 8.5 g NaCl, and 1000 mL distilled water, pH = 7.0) and homogenized with a Stomacher lab-blender (type 400, London, UK). Decimal dilutions were plated. Viable counts of LAB were determined on de Man, Rogosa and Sharpe Agar (MRSA, CM 361, Oxoid, Hampshire, England) containing 0.1% (w/v) natamycin (Delvocid, DSM, The Netherlands) with incubation in anaerobic jar (Anaerocult A, Merck KGaA, Germany) at 30°C for 72 h. Yeasts and moulds were enumerated using Malt Extract Agar (MEA, CM 59 Oxoid, Basingstoke, Hampshire, England). MEA plates were incubated at 25°C for 72 to 120 h. The colonies were then counted and expressed as logarithmic colony forming units per gram (log10 CFU/g) of the sample.
Agar disc-diffusion assay
The capacity of the ingredients to inhibit a representative group of pathogens and other was determined by modifying the disc diffusion method of NCCLS (2003). Twenty milliliters (20 mL) of molten Mueller-Hinton Agar (MHA, CM 337, Oxoid, Basingstoke, Hampshire, England) were poured into sterile Petri dishes and allow to solidify. 100 μL of the overnight Mueller-Hinton broth (MHB, CM 405, Oxoid, Basingstoke, Hampshire, England) culture of each pathogen strain, which have been adjusted to 0.5 McFarland-turbidity, was spread on the plates. Once the plates were dried aseptically, five blank discs papers (6 mm in diameter) were placed onto the surface of the agar. The moist or dried sample of probiotic ingredient was reconstituted with sterile distilled water to obtain a solution of 500 mg mL-1. This solution was stirred vigorously using a magnetic stirrer for 30 min and then centrifuged at 3 500 x g for 30 min. Forty microliters (40 μL) of each supernatant were placed into the discs. The plates were left at room temperature for 1 h so that the absorbed supernatant become diffused into the agar, and then incubated at 37°C for 24 h. The tests were carried out in duplicate.
Statistical analysis
The propagation trials were carried out in triplicate. Mean values and standard deviations were calculated from the experimental data. Statistical Package for Social Science (SPSS), version 16.0 (Chicago, IL, USA) was used. Data analyses involved one-way analysis of variance (ANOVA). Significant difference was established at 5%.
Changes in pH, titratable acidity, lactic acid, maltose and glucose contents of the ingredient during the propagation
The pH value decreased significantly (p<0.05) from 5.63 to 4.03 within 24 h of propagation (Table 1). After 24 h, the pH remains relatively stable around a value of pH = 3.84. The titratable acidity increased significantly (p<0.05) from 8.95 g/kg at 0 h (calculated as lactic acid) to 40.21 g/kg at 72 h of propagation. The progressive fall in pH and increase in titratable acidity during the fermentation process is typically characteristic of lactic acid fermentation of cereal grains (Singh et al., 2003; Vieira-Dalodé et al., 2007).
According to Tharmaraj and Shah (2009), the production of organic acids such as acetic, citric and lactic acids are responsible for the decrease of pH in such product. Interestingly, Nout (1991) and Steinkraus (1996) reported that a pH of 3.5 to 4.0 is sufficient to inhibit Enterobacteriaceae and other Gram-negative bacteria. Concomitantly to these modifications, we observed a decrease in the dry matter content of the ingredient which shifted from 51.98% at 0 h to 48.89% at 72 h of propagation.
The changes in lactic acid, maltose and glucose concentrations of the ingredient during propagation are also reported in Table 1. The amount of lactic acid in the dough increased rapidly to reach a maximum at 36 h of propagation. Thereafter, a significant decrease was observed until the end of propagation. The maltose content increased from 2.13 g/kg at 0 h to 3.84 g/kg at 24 h of fermentation and then decrease to 1.85 at 36 h of propagation and no significant decrease was observed afterward. Similar trend was observed for the glucose content with the difference that the most significant change occurred at 36 h of propagation. Water and volatile compounds production during aerobic and anaerobic catabolism by yeasts and LAB might be responsible for these changes (Hounhouigan et al., 1993). Similar modifications were reported in other African fermented cereal products (Muyanja et al. 2002; Sefa-Dedeh et al., 2003; Vieira-Dalodé et al., 2007).
Changes in lactic acid bacteria, yeasts and moulds counts during propagation
The changes in microbial count of the ingredient during the propagation are shown in Figure 1. The LAB counts increased from 6.47 to 10.44 log cfu/g. The most significant (p˂ 0.05) increase in the numbers of LAB was noted during the first 36 h of propagation. No significant growth was noted between 36 and 48 h. Further incubation led to a significant decrease of LAB counts. Similar trend was observed for yeast and mould counts. The symbiotic relationship between LAB and yeasts during the fermentation process of starch-based product is well established (Nout, 1991). Indeed, the development of yeasts is favored by the acidic environment created by LAB, while the growth of bacteria is stimulated by the presence of growth factors such as vitamins and soluble nitrogen compounds provided by yeasts.
Antimicrobial activity
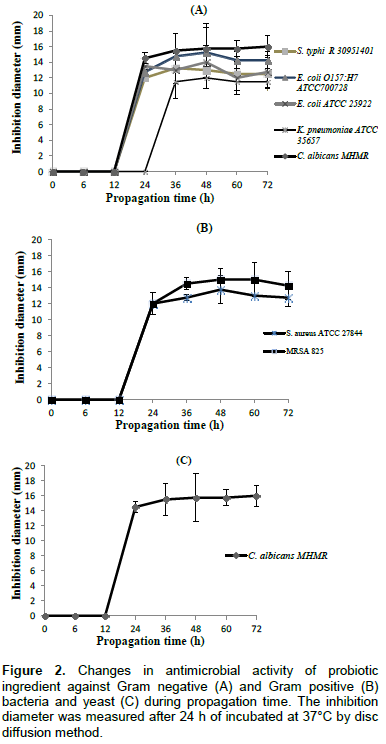
The antimicrobial activity was assessed during the probiotic ingredient propagation against indicator strains by Agar disc diffusion method. Figure 2 showed the inhibition zone diameter resulting from the antimicrobial activity of the probiotic ingredient against Gram positive, Gram negative bacteria and one yeast. A superior diameter to 1 mm around the disc was considered as a positive result. Thus from 0 to 12 h, the probiotic ingredient failed to show any inhibitory activity against all indicator strains. It is most likely that enough antimicrobial compounds were not produced by the functional microorganisms during this propagation time. Ahmad et al. (2014) reported that the antimicrobial effect of Lysinibacillus jx416856 started after 15 h of incubation in MRS broth and growth dependent bacteriocin activity was observed at early log phase (18 h). However, at 24 h of propagation, our probiotic ingredient inhibited indicator strains broadly except K. pneumoniae (ATCC 35657). After 24 h of propagation, all indicator strains were inhibited by the probiotic ingredient. The inhibition zone diameters observed at 36 h of propagation were significantly higher for all indicator strains. The antimicrobial effect increased until a stationary phase which occurs at 36 h and remained constant till 72 h. Ahmad et al. (2014) reported similar trend for Lysinibacillus jx416856 and even though indicated that its antimicrobial activity decreased eventually with a constant level. In another study, Djadouni and Kihal (2012) recorded optimal bacteriocin production in MRS after 24 h of incubation. These results supported our findings since the growth of all indicator strains tested were inhibited after 24 h of the propagation time. Interestingly, the lactic acid production, and probably bacteriocin, significantly increased after 24 h of the propagation. Between 24 and 36 h the inhibition zone diameters increase steadily and remains constant (P> 0.05) till 72 h of propagation. This suggested that 36 h of propagation is enough to inhibit all pathogen tested. Among the indicator strains, C. albicans MRMH (16±1.41 at 72 h), S. aureus RM 825 (15±1.41 at 48 h), E. coli O157: H 7 ATCC 700728 (14.75±0.35 at 36 h) were extensively inhibited by the probiotic ingredient while S. typhi R30951401 (12.5±2.12) and K. pneumoniae ATCC 35657 (11.5±2.12) were weakly inhibited. Clearly, the inhibitory activity depends on the propagation time and on the type of microorganism considered. In other to check the effect of drying temperature on the functional properties of the ingredient, we dried the product at 42°C for 24 h. Thus, after drying, the probiotic ingredient at 72 h also exhibited antimicrobial activities against all pathogen strains (Figure 3). There was no significant difference between inhibition zone diameters recorded for the dried and undried probiotic ingredient.

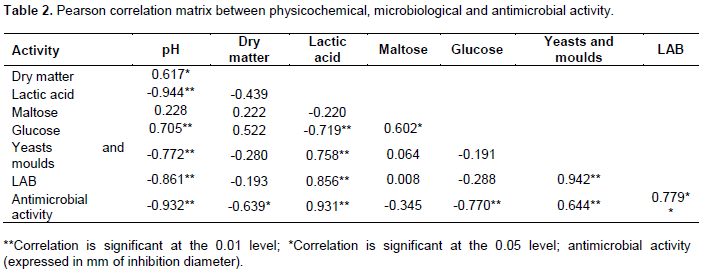
We studied the relationship between some measured parameters (Table 2). High negative correlation exists between the lower pH and the antimicrobial activity (r = - 0.932). In a similar study, Tejero-Sariñena et al. (2012) reported that the pH was inversely correlated with the diameter of inhibition against the pathogenic indicator strains. Level of lactic acid is in high positive correlation with the antimicrobial activity. The pH-lowering effect resulting from acid production is most likely responsible for the inhibitory mechanism. Indeed, the inhibitory effects of lactic acid bacteria might be due to either individual or joint production of organic acids, hydrogen peroxide, or bacteriocins (Ennahar et al., 2000; Villamil et al., 2003; Vázquez et al., 2005). Moreover, it has been reported that LAB produce a large number of antimicrobial compounds such as organic acids, H2O2, diacetyl, enzymes, bacteriocin, and biosurfactants which are effective against food spoilage and pathogenic bacteria (Sharma and Saharan, 2014).
The probiotic ingredient derived from the African opaque sorghum beer exhibited antimicrobial activity with a large spectrum being effective against Gram negative, Gram positive and the yeast microorganisms. This antibacterial activity is preserved during the propagation process of the functional microorganisms in a cereal-based starchy career. 36 h of propagation is enough to inhibit all pathogens tested. The microorganisms involved in the starter of African sorghum beers could an alternative to antibiotic chemicals. More in-deep researches are ongoing on the molecular and functional characterization of these microorganisms.
The authors have not declared any conflict of interests.
The authors are grateful to the University of Abomey-Calavi for the financial support to this research.
REFERENCES
|
AACC (1984). Approved methods of the American Association of cereal chemistry. American Association of Cereal Chemistry, St. Paul, MN.
|
|
|
|
Aarestrup FM, Kruse H, Tast E, Hammerum AM, Jensen LB (2000). "Associations between the use of antimicrobial agents for growth promotion and the occurrence of resistance among Enterococcus faecium from broilers and pigs in Denmark, Finland, and Norway." Microb. Drug Resist. 6(1):63-70.
Crossref
|
|
|
|
|
Ahmad V, Iqbal ANMZ, Haseeb M, Khan MS (2014). Antimicrobial potential of bacteriocin producing Lysinibacillus jx416856 against foodborne bacterial and fungal pathogens, isolated from fruits and vegetable waste. Anaerobe 27:87-95.
Crossref
|
|
|
|
|
Balcazar JL, de Blas I, Ruiz-Zarzuela I, Cunningham D, Vendrell D, Múzquiz JL (2006). The role of probiotics in aquaculture. Vet. Microbiol. 114:173-186.
Crossref
|
|
|
|
|
Bohoua GL (2008). Effect of palm wine yeasts and yogurt probiotics on the growth performance of broilers. Livestock Res. Rural Dev. 20:1-8.
|
|
|
|
|
Djadouni F, Kihal M (2012). Antimicrobial Activity of Lactic Acid Bacteria and the Spectrum of their Biopeptides Against Spoiling Germs in Foods. Braz. Arch. Biol. Technol. 55:435-443.
Crossref
|
|
|
|
|
Doyle ME (2001). Alternatives to Antibiotic Use for Growth Promotion in Animal Husbandry. Food Research Institute, University of Wisconsin–Madison Madison, WI 53706.
|
|
|
|
|
Ennahar S, Sashihara T, Sonomoto T, Ishizaki A (2000). Class II bacteriocins: biosynthesis, structure and activity. FEMS Microbiol. Rev. 24:85-106.
Crossref
|
|
|
|
|
Fajardo P, Pastrana L, Méndez J, Rodríguez I, Fuci-os C, Guerra NP (2012). Effects of feeding of two potentially probiotic preparations from lactic acid bacteria on the performance and faecal microflora of broiler chickens. Sci. World J. 2012:1-9.
Crossref
|
|
|
|
|
Guillot JF (2001). Consequences of probiotics release in the intestine of animals. In: Brufau J. (ed.). Feed manufacturing in the Mediterranean region. Improving safety: From feed to food. Zaragoza: CIHEAM. pp. 17-21 (Cahiers Options Méditerranéennes; n. 54).
|
|
|
|
|
Houndonougbo MF, Chrysostome CAAMZ, Amoussa LAO (2011). Tchoukoutou residue and yogurt as feed additives in broilers feed. Roavs, 1(9):597-600.
|
|
|
|
|
Hounhouigan DJ, Nout MJR, Nago CM, Houben JH, Rombouts FM (1993). Changes in the physico-chemical propertyies of maize during natural fermentation of mawè. J. Cereal Sci. 17:291-300.
Crossref
|
|
|
|
|
Kayodé APP, Deh DC, Baba-Moussa L, Kotchoni SO, Hounhouigan JD (2012). Stabilization and preservation of probiotic properties of the traditional starter of African opaque sorghum beers. Afr. J. Biotechnol. 11(30):7725 -7730.
|
|
|
|
|
Kayodé APP, Hounhouigan DJ, Nout MJR, Niehof A (2007). Household production of sorghum beer in Benin: technological and socio-economical aspects. Int. J. Cons. Stud. 3:258-264.
Crossref
|
|
|
|
|
Khachatourians GG (1998). Agricultural use of antibiotics and the evolution and transfer of antibiotic-resistant bacteria. Can. Med. Assoc. J. 159:1129-1136.
|
|
|
|
|
Kimmons JE, Brownk.H, Larteya, Collisone, Mensah PP, Dewey KG. (1999).The effects of fermentation and/or vacuum flask storage on the presence of coliforms in complementary foods prepared in Ghana. Int. J. Food Sci. Nutr. 50:195-201.
Crossref
|
|
|
|
|
Lewin CS (1992). Mechanisms of resistance development in aquatic microorganisms. In: Michel, C., Alderman, D.J. (Eds.), Chemotherapy in Aquaculture: from Theory to Reality. Office International des Epizooties, Paris, France pp. 288-301.
|
|
|
|
|
Mensah P, Tomkins AM, Drasar BS, Harrison TJ (1991). Antimicrobial effect of fermented Ghanaian maize dough. J. Appl. Bacteriol. 70:203-210.
Crossref
|
|
|
|
|
Mestres C, Rouau X (1997). Influence of natural fermentation and drying conditions on the physicochemical characteristics of cassava starch. J. Sci. Food Agric. 74:147-155.
Crossref
|
|
|
|
|
Muyanja CM, Narvhus JA, Treimo J, Langsrud T (2002). Isolation and identification of lactic acid bacteria from bushera, a Ugandan traditional fermented beverage. Int. J. Food Microbiol. 80:201-210.
Crossref
|
|
|
|
|
National Committee for Clinical Laboratory Standards (NCCLS) (2003). Method for Antifungal Disk Diffusion Susceptibility Testing of Yeast: Proposed Guideline M44-P. NCCLS, Wayne, PA, USA.
|
|
|
|
|
Nout MJR (1991). Ecology of accelerated natural lactic fermentation of sorghum-based infant food formulas. Int. J. Food Microbiol. 12:217-224.
Crossref
|
|
|
|
|
Nout MJR, Rombouts FM, Havelaar A (1989). Effect of accelerated natural lactic fermentation of infant food ingredients on some pathogenic micro-organisms. Int. J. Food Microbiol. 8:351-361.
Crossref
|
|
|
|
|
Nout MJR, Beernink G, Bonants-Van Laarhoven TMG (1987). Growth of Bacillus cereus in soyabean tempeh. Int. J. Food Microbiol. 4:293-301.
Crossref
|
|
|
|
|
N'tcha C, Adéyèmi AD, Kayodé APP, Vieira-Dalodé G, Agbobatinkpo BP, Codjia JTC, Baba-Moussa L (2015). Indigenous knowledge associated with the production of starters culture used to produce Beninese opaque sorghum beers. J. Appl. Biosci. 88:8223-8234.
Crossref
|
|
|
|
|
Psomas E, Andrighetto C, Litopoulou-Tzanetaki E, Lombardi A, Tzanetakis N (2001). Some probiotic properties of yeast isolates from infant feces and feta cheese. Int. J. Food Microbiol. 69:125-133.
Crossref
|
|
|
|
|
Randall LP, Ridley AM, Cooles SW, Sharma M, Sayers AR, Pumbwe L, Newell DG, Piddock LJV, Woodward M (2003). "Prevalence of multiple antibiotic resistance in 443 Campylobacter spp. isolated from humans and animals," J. Antimicrob. Chemother. 52(3):507-510.
Crossref
|
|
|
|
|
Scan (2003). Opinion of the Scientific Committee on Animal Nutrition on the criteria for assessing the safety of microorganisms resistant to antibiotics of human clinical and veterinary importance. European Commission Health and Consumer Protection Directorate-General.
|
|
|
|
|
Sefa-Dedeh S, Cornelius B, Afoakwa EO (2003). Effect of fermentation on the quality characteristics of nixtamalized corn. Food Res. Int. 36:57-64.
Crossref
|
|
|
|
|
Sharma D, Saharan BS (2014). Simultaneous production of biosurfactants and bacteriocins by probiotic Lactobacillus casei MRTL3. Int. J. Microbiol. 2014:1-7.
Crossref
|
|
|
|
|
Singh S, Kulkarni SD, Singh KK (2003). Handling banana chain-Management aspects for an Agri Industrial Approach. 6th All India Congress, National Academy of Agricultural Sciences, Bhopal.
|
|
|
|
|
Steinkraus KH (1996). Handbook of Indigenous Fermented Foods, 2nd edn. Marcel Dekker, New York Revised and Expanded.
|
|
|
|
|
Tharmaraj N, Shah NP (2009). Antimicrobial effects of probiotics against selected pathogenic and spoilage bacteria in cheese-based dips. Int. Food Res. J. 16:261-276.
|
|
|
|
|
Tejero-Sari-ena S, Barlow J, Costabile A, Gibson GR, Rowland I (2012). In vitro evaluation of the antimicrobial activity of a range of probiotics against pathogens: Evidence for the effects of organic acids. Anaerobe 18:530-538.
Crossref
|
|
|
|
|
Vázquez JA, González MP, Murado MA (2005). Effects of lactic acid bacteria cultures on pathogenic microbiota from fish. Aquaculture. 245(1-4):149-161.
Crossref
|
|
|
|
|
Vieira-Dalodé G, Jespersen L, Hounhouigan DJ, Lange Moller P, Nago MC, Jakobsen M (2007). Lactic acid bacteria and yeasts associated with gowé production from sorghum in Bénin. J. Appl. Microbiol.103:342-349.
Crossref
|
|
|
|
|
Villamil L, Figueras A, Planas M, Novoa B (2003). Control of Vibrio alginolyticus in Artemiaculture by treatment with bacterial probiotics. Aquaculture 219(1-4):43-45.
Crossref
|
|
|
|
|
Wegener HC, Aarestrup FM, Jensen LB, Hammerum AM, Bager F (1999). Use of antimicrobial growth promoters in food animals and Enterococcus faecium resistance to therapeutic antimicrobial drugs in Europe. Emerg. Infect. Dis. 5(3):329-335.
Crossref
|
|
|
|
|
Yateem A, Balba MT, Al-Surrayai T, Al-Mutairi B, Al-Daher R (2008). Isolation of lactic acid bacteria with probiotic potential from camel milk. Int. J. Dairy Sci. 3:194-199.
Crossref
|
|