Full Length Research Paper
ABSTRACT
Thyme is one of the most important medicinal plants in wild rangeland in Iran that has lots of benefits. Pseudomonas flourescence is one of the effective plant growth promoting bacteria (PGPR) as a bioinoculant for medicinal plants. This study aims to survey three inoculation techniques with PGPR on growth and oil content in Thymus kotschyanus for organic cultivation. For this research, an experiment was conducted in Randomized Complete Block Design at Research Institute Forest and Rangelands, Tehran, Iran, with four treatments and three replications. In the first method, thyme seed was treated in liquid bacterial suspension for 3 h, in the second method bacterial suspension injected around the root before cultivation in farm and in the third method both seed and root have been treated by the bacterial suspension. In all three methods of inoculation of P. fluorescence, an increase in growth and the amount of essential oil was determined. The highest amount of root volume (30 mL) compared to the control (15 mL) was significant at the level of P=0.05 and the highest amount of essential oil (1.74%) was obtained by using the third inoculation method. It could be concluded that the microbial inoculation method has a great influence on the yield of T. kotschyanus and bacterial inoculation of seeds and roots had a greater effect, rather than inoculated separately for organic cultivation.
Keywords: Inoculation method, biofertilizer, thymus, plant growth promoting.
INTRODUCTION
The genus Thymus belongs to the family Lamiaceae and 18 species of this genus have been identified in Iran. There are approximately 350 different species of thymus worldwide. Thymus kotschyanus is one of the most important medicinal plants found in wild rangelands in Iran. Secretory hairs are the site of the accumulation of thyme essential oil (Salehnia et al., 2021).
All thymus species are rich in volatile compounds and mainly contain thymol and carvacrol, which are potent disinfectants. The essential oil of this plant has a special place in world trade. The best way to prepare essential oil from thyme is to distill it with water, which produces the highest yield of essential oil. Thyme contains 0.8 to 2.6% of essential oil, most of which are phenols, monoterpene hydrocarbons, and alcohols. Thyme contains compounds such as flavonoids, saponins, and bitter substances (Hedden et al., 2002).
Thyme aerial parts contain essential oils, tannins, saponins, and herbal disinfectants. Thyme leaves are used in food products, as well as plant essential oils in beverage, pharmaceutical and cosmetic industries.
Application of microorganisms as biofertilizers for improve crops and production has been used and become a common practice in the last years. Plant growth-promoting rhizobacteria (PGPR) (Kloepper, 1993) has been known as biofertilizer because these microorganisms adapt and grow rapidly around plant rizosphers (Hernández-Montiel et al., 2017; Azimova et al., 2012). However, some reports indicate that rhizobacteria inhibit the maximum growth of some plants by producing hydrogen cyanide. Research has shown that PGPR can increase plant growth and mineral uptake. They facilitate plants growth even in stressful situations (Salehnia et al., 2020).
The use of PGPR reduces the need for chemical fertilizers and pesticides for medicinal and aromatic plant species (Elavarasi et al., 2020; Amalan et al., 2017).
There have been numerous reports of stimulant effects of these bacteria in the production of more valuable plant chemicals and medicinal metabolites (Strigul et al., 2006; Salehnia et al., 2020).
Pseudomonas fluorescens can cause increase in the plant's access to absorbable iron in the rhizosphere and subsequently play an important role in improving plant growth in terms of quantity and quality (Ghorbanpour et al., 2014). They are famous biofertilizers and also through various mechanisms such as stimulating the production of plant hormones such as auxin, cytokine and gibberellin and also preventing the production of ethylene, increasing the solubility of inorganic and organic phosphate, producing microbial siderophores to increase plant access to absorbable iron, nitrogen fixation in symbiotic or non-symbiotic relationship. Jaleel et al. (2007) indicated a significant increase on the amount of ajmalicin by the application of non-native P. fluorescens on Vinca seedlings (del Rosario et al., 2017).
The beneficial effects of PGPR and the mechanism of joint adaptation of plants exposed to water deficit stress (WDS) are always related to the interactions of plants and microorganisms, which have exceptional effects on morphological and anatomical traits of roots, such as root networks and their biomass (Shahin et al., 2010; Shrivastava et al., 2014).
Phosphate solubilizing bacteria expand plant growth by absorbing essential minerals and increasing the solubility of phosphorus in low-soluble mineral phosphates such as phosphate rock, and many of them also release phosphorus from organic compounds by producing phosphatase enzymes (Messele, 2012).
Utilizing biofertilizers under the organic agricultural system is an approach to harvest high-quality and safe products from medicinal plants (Dawa et al., 2014).
This study aimed to increase the quantity as well as the quality of T. kotschaynus yield using P. fluorescens without chemical fertilizers. Evaluation of three microbial inoculation methods was carried out as well. This study was conducted at the Alborz Research Complex, Research Institute of Forests and Rangelands, in Randomized Complete Block Design (RCBD) with four treatments and three replications, on T. kotschyanus inoculated with P. fluorescens.
MATERIALS AND METHODS
In this study, to investigate the efficiency of three inoculation methods of P. fluorescens on growth, characteristics and percentage of essential oil of T. kotschyanus, an experiment was conducted in Randomized Complete Block Design with four treatments and three replications at Alborz Research Complex, Research Institute of Forests and Rangelands.
Farm soil characteristics
Phosphorus (ppm), 51/9; Potassium (ppm), 788/475; Organic matter (percentage), 1/878; Texture, Lumi Sandy; Acidity, 8/1.
Bacterial inoculum origin
The standard bacterial strain of P. fluorescens (169) was obtained from the Soil and Water Research Institute, Tehran, Iran.
Bacterial inoculation methods in thymus
Method1
Thymus seeds were placed in a sterile plate after determining the germination potential. For each seed, 5 mL of bacterial liquid suspension of P. fluorescence standard strain (169) prepared by the Soil and Water Research Institute with a population of 108 cfu/mL was added. For better effectiveness, Arabic gum as a carrier was added for seed adhesive. Bacterial suspension was inoculated at 108 cfu/mL at room temperature (25°C) on a shaker at 120 rpm. Then, after 48 h of incubation, constant turbidity with absorption of 560 nm was read by spectrophotometry.
Method 2
After rooting and seedlings emergence, 50 mL of the bacterial suspension was added with 108 cfu/mL using sterile syringe, in the zone around the root, in fact in the rhizosphere area of the plants. The suspension was prepared and the turbidity was fixed as in the first method.
Method 3
Both site inoculation, bacterial suspension with a population of 108 cfu/mL grown in B-King liquid medium was added by half McFarland method in Erlenmeyer 100 mL with physiological serum in a ratio of 1 to 9 to reach a population of 107 cfu/mL, then as in the first method (5 mL per seed) and the as the second method (50 mL of the bacterial suspension in contact with plant roots) was added.
Thymus growth characteristics
For plants harvested after 3 months from transplanting, the branch height, number of branches, plant dry weight, root volume, fresh and dry weight of roots were measured. After the growth period of the plants was completed, eight complete plants were removed from the middle of each cultivation line and transferred to the laboratory. After thorough washing and complete removal of sludge and dewatering with paper towels, first, the fresh weight of roots and shoots was read by a digital scale with an accuracy of 0.001 g and then by an oven at 70°C for 96 h. It was dried and the dry weight of roots and shoots was determined (Jones et al., 1993).
After irrigation, the roots were carefully removed from the culture medium and washed. A graduated cylinder with a specified volume of water was used to determine the root volume (mL). Root volume was measured by the amount of water displaced in the graduated cylinder.
Oil content
The branches were dried for 10 days in shade, then ground and oil content was estimated after the steam distillation using Clevenger’s apparatus for 4 h.
Statistical analysis
Statistical analysis of the data was done by one-way (ANOVA) with Tukey’s post-hoc test.
RESULTS AND DISCUSSION
In all three methods of inoculation of P. fluorescence, an increase in growth and the amount of essential oil was determined (Table 1). The effect of inoculation by mixed method (roots and seeds) had a greater effect on morphological traits, plants growth and percentage of thyme essential oil. The highest amount of root volume with 30 mL compared to the control (15 mL) was significant at the level of P≤0.5. The highest dry weight of the plant (24.75 g) and the highest number of branches (25), respectively, compared to the control (20 g and 18 g) showed no significant differences. The highest amount of essential oil (1.74%) was obtained in inoculation of P. fluorescens in the third method (seeds and roots) (Figure 1). In this study, inoculation of P. fluorescence by liquid suspension method with seeds, roots and rhizosphere of the plant had a positive effect on morphological characteristics and percentage of T. kotschyanus essential oil, which had a significant effect on some characteristics. The increased fresh and dry weight and root volume of roots in comparison with control was observed.
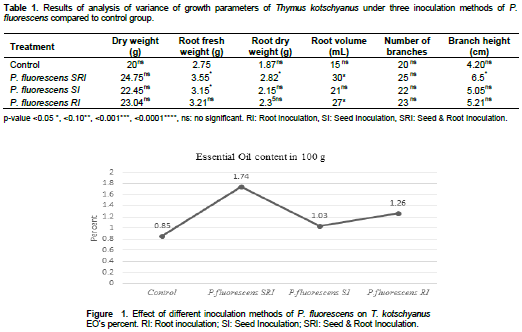
In general, seed and root inoculation in comparison with seed and root inoculation was significantly significant at p <0.05 root dry weight and branch height.
The study results are in agreement with those of Eltayeb (2017), which indicated that dipping method was more effective in inoculating plants with biofertilizers, than soil application technique.
Also, the study proved that inoculating bacterial biofertilizers could increase oil content which is in an agreement with the study of Hamed et al. (2017) who used Azotobacter chroococcum, Bacillus megaterium and Saccharomyces cerevisiae as biofertiliers to enhance the yield of lemon grass and its essential oil content.
CONCLUSION
It could be concluded that the microbial inoculation methods had a great influence on the yield of T. kotschyanus for both the blossoms and the essential oil content. All three inoculation methods increased the growth of thyme, but combination methods had the greater effect which means that inoculation on seeds and roots can be more effective and utilizing for organic cultivation of thyme. Although the effect of inoculation of P. fluorescens has a positive effect on plants growth and as a biofertilizer it can be combined with arbuscular mycorrhiza fungi (AMF). But the most effective techniques or methods of inoculation of these bacteria must be considered for best results and it will be helpful for organic farming and achieving best production.
CONFLICT OF INTERESTS
The authors have not declared any conflict of interests.
ACKNOWLEDGEMENT
The authors thank the Research Institute Forest and Rangelands of Iran for the necessary laboratory and field experiment facilities provided.
REFERENCES
|
Amalan RG, Kalaikandhan R, Chidambaram Al (2017). Impact of PGPR inoculation on photosynthetic pigment and protein contents in Arachis hypogaea L. Journal of Scientific Agriculture 1:29. |
|
|
Azimova SS, Glushenkova AI (2012). Thymus kotschyanus var. kotschyanus. Lipids, Lipophilic Components and Essential Oils from Plant Sources pp. 537-537. |
|
|
Dawa K, Farid S, El-Bauomy A (2014). Effect of biofertilizers inoculation methods and some foliar application treatments on yield and quality of pea plants. Journal of Plant Production 5(11):1759-1775. |
|
|
del Rosario CL, Chiappero J, Santoro MV, GiordanoW, Banchio E (2017). Inducing phenolic production and volatile organic compounds emission by inoculating Mentha piperita with plant growth-promoting rhizobacteria. Scientia Horticulturae 220:193-198. |
|
|
Elavarasi P, Yuvaraj M, Gayathri P (2020). Application of Bacteria as a Prominent Source of Biofertilizers. Biostimulants in Plant Science. IntechOpen. |
|
|
Eltayeb FME (2017). Biological Control of root knot disease of tomato caused by Meloidogyne javanica using Pseudomonas fluorescens bacteria. International Journal of Current Microbiology and Applied Sciences 6(6):1176-1182. |
|
|
Ghorbanpour M, Hatami M, Kariman K, Khavazi K (2014). Enhanced Efficiency of Medicinal and Aromatic Plants by PGPRs. Plant-Growth-Promoting Rhizobacteria (PGPR) and Medicinal Plants pp. 43-70. |
|
|
Hamed ES, Toaima WIM, El-Shazly M (2017). Effect of planting density and biofertilization on growth and productivity of Cymbopogon citratus (DC.) Stapf. (Lemongrass) plant under Siwa Oasis conditions. Journal of medicinal plants studies 5(2):195-203. |
|
|
Hedden P, Harrewijn P, van Oosten AM, Piron PGM (2002). Natural terpenoids as messengers. A multidisciplinary study of their production, biological functions and practical applications. Annals of Botany 90(2):299-300. |
|
|
Hernández-Montiel LG, Chiquito Contreras CJ, Murillo Amador B, Vidal Hernández L, Quiñones Aguilar EE, Chiquito Contreras RG (2017). Efficiency of two inoculation methods of Pseudomonas putida on growth and yield of tomato plants. Journal of soil science and plant nutrition 17(4):1003-1012. |
|
|
Kloepper JW (1993). Plant Growth Promoting Rhizobacteria as Biological Control Agents. In: F.B. Meeting, Jr. (Ed.) Soil Microbial Ecology, Applications in Agricultural and Environmental Management. New York: Marcel Dekker Inc pp. 255-274. |
|
|
Jaleel CA, Manivannan P, Sankar B, Kishorekumar A, Gopi R, Somasundaram R, Panneerselvam R (2007). Pseudomonas fluorescens enhances biomass yield and ajmalicine production in Catharanthus roseus under water deficit stress. Colloids and Surfaces B: Biointerfaces 60(1):7-11. |
|
|
Jones DL, Darrah PR (1993). Re-sorption of organic compounds by roots of Zea mays L. and its consequences in the rhizosphere. II. Experimental and model evidence for simultaneous exudation and re-sorption of soluble C compounds. Plant and Soil 153:47-59. |
|
|
Messele B (2012). Effects of Inoculation of Sinorhizobium ciceri and Phosphate Solubilizing Bacteria on Nodulation, Yield and Nitrogen and Phosphorus Uptake of Chickpea (Cicer arietinum L.) in Shoa Robit Area. Journal of Biofertilizers and Biopesticides 3(5). |
|
|
Salehnia SA, Anvari M, Matinizadeh M, Mirza M (2021). Evaluation of Inoculation Pseudomonas fluorescens and Arbuscular Mycorrhizal Fungus on Growth, Morphological Characteristics and Essential Oil Percentage of Thymus kotschyanus. Journal of Medicinal plants and By-product. |
|
|
Salehnia SA, Anvari M, Matinizadeh M, Mirza M (2020). The Synergistic Effect of Arbuscular Mycorrhizal Fungi and Pseudomonas fluorescens on Growth and Qualitative and Quantitative Yield of Thymus kotschyanus Essential Oil. Journal of Essential Oil Bearing Plants 23(3):532-547. |
|
|
Shahin S, El Taweel A, Omar M (2010). Effect of Inoculation by Some Plant Growth Promoting Rhizobacteria (Pgpr) on Production of Olive Trees (Manzanillo cultivar). Journal of Plant Production 1(12):1577-1592. |
|
|
Shrivastava S, Egamberdieva D, Varma A (2014). Plant Growth-Promoting Rhizobacteria (PGPR) and Medicinal Plants: The State of the Art. Plant-Growth-Promoting Rhizobacteria (PGPR) and Medicinal Plants pp. 1-16. |
|
|
Strigul NS, Kravchenko LV (2006). Mathematical modeling of PGPR inoculation into the rhizosphere. Environmental Modelling and Software 21(8):1158-1171. |
|
Copyright © 2024 Author(s) retain the copyright of this article.
This article is published under the terms of the Creative Commons Attribution License 4.0