ABSTRACT
Candida albicans and Staphylococcus aureus can cause many diseases which are considered in many cases life threatening infections. Their biofilm formation on the surface of medical devices is difficult to be treated. Our study evaluates the effect of some anti-inflammatory drugs on the growth, biofilm formation and the expression of some adhesion-related genes. Antimicrobial activity of the tested drugs was determined by microbroth dilution method, their effect on biofilm formation was determined by crystal violet assay method, the mechanism of action was assessed by scanning electron microscope and ethidium bromide uptake assay. Their effects on the expression of icaA, ALS1 and HWP1 genes were determined by RT-PCR. Diclofenac Sodium had the highest antimicrobial activity followed by Meloxicam. Ethidium bromide uptake increased in the presence of diclofenac sodium in both S. aureus and C. albicans strains. All tested drugs showed significant inhibitory effect on both biofilm formation and the preformed biofilm. NSAIDs, dexamethazone and ketoconazole down-regulated C. albicans tested genes except for ketoprofen that up-regulated HWP1 gene. NSAIDs and levofloxacin down-regulated the expression icaA gene but dexamethazone showed no effect on icaA gene expression. Although, dexamethazone had no antimicrobial activity, it had good anti-biofilm activity against S. aureus and C. albicans.
Key words: Candida albicans, Staphylococcus aureus, biofilm, HWP1, ALS1, icaA, Dexamethazone, NSAIDS.
Candida spp. can cause opportunistic diseases especially in patients who are immune compromised, aged, receiving prolonged antibacterial and aggressive cancer chemotherapy or undergoing invasive surgical
procedures and organ transplantation (Pfaller and Diekma, 2007). C. albicans readily forms biofilms on a wide variety of polymers used to make indwelling medical devices, such as dental materials, stents, prostheses,
implants, endotracheal tubes, pacemakers, and catheters (Douglas, 2003).
S. aureus is found to be common in polymicrobial biofilm infections especially with C. albicans. It is estimated that 27% of nosocomial C. albicans bloodstream infections are polymicrobial, with Staphylococcus aureus (Klotz and Chasin, 2007). Interestingly, the combined effect of C. albicans and S. aureus results in synergism and increased mortality in mice (Viale and Stefani, 2006).
Different studies have already described changes in gene expression levels during biofilm development (O’Gara, 2007). Adherence of microorganisms to an implanted device is a crucial step in the development of biofilm and biomaterial-entered infection (Seo et al., 2008). Adhesion of C. albicans to host cells depends on many adherence molecules such as the agglutinin-like sequence (ALS) family, hyphal wall protein (HWP) and cell wall glycoproteins. Also, the intercellular adhesin (ICA) operon is a virulence factor identified in staphylococci related to biofilm production. As S. aureus formation of biofilms requires the synthesis of polymeric N-acetylglucosamine and the enzymes responsible for its synthesis are encoded by the ica operon (Gotz, 2002).
The biofilm-associated microorganisms are refractory to both antimicrobial agents and the host immune response. The resistance of biofilm forming microorga-nisms to antimicrobials represents a major challenge facing most of therapeutic and prophylactic strategies. Re-evaluation of the existing drugs of known mecha-nisms of action may result in identifying new mechanisms and targets that can affect bacterial metabolism (Golia et al., 2011).
Non-steroidal anti-inflammatory drugs (NSAIDs) are usually prescribed with or without antibiotics for patients had fever or pain due to an infectious condition, musculoskeletal condition (e.g. rheumatoid arthritis, gout), painful condition (e.g. metastatic bone pain, trauma, migraine headache), or the prophylaxis of ischemic heart disease. NSAIDs inhibit the cyclooxyge-nase enzymes COX-1 and COX-2, which are involved in the biosynthesis of mammalian prostaglandins (Pina-vaz et al., 2000).
Glucocorticoids (GC) have both immunosuppressive and anti-inflammatory actions so that they are used in the treatment of various immune-mediated inflammatory disorders, such as asthma, psoriasis, rheumatism, and multiple sclerosis (MS). GC also inhibits some transcript-tion factors, such as nuclear factor kappa B and AP-1 (Auphan et al., 1995). As a result, the transcription of several cytokines that are involved in inflammation is decreased and the production of lipid mediators such as prostaglandins is inhibited and the expression of ICAM-1 and E-selectin on endothelial cells is reduced (Scheinman et al., 1995).
It was found that C. albicans can produce prostaglandins but their role in fungal biology is not yet known (Alem and Douglas, 2005). Prostaglandins can act as regulators of C. albicans eicosanoids pathway which have a vital role in controlling both morphogenesis and biofilm formation. Also the release of drugs that can target fungal prostaglandins pathways may combat fungal colonization and infection (Erb-Downward and Noverr, 2007). Glucocorticoids are used in many preparations in combination with antifungals and antibacterials (topical skin preparation, eye drops and ear drops). So, we thought to test the in-vitro activity of NSAIDs and dexamethasone on the growth, adherence, biofilm formation and the expression of some genes affect adhesion of C. albicans and S. aureus to know if the tested drugs have any additional effect on the microbial infection or not.
Microbial strains
Two C. albicans and two S. aureus clinical strains obtained from the Department Of Microbiology And Immunology, Faculty of Pharmacy, Minia University were used in this study. S. aureus (ATCC 6538) and C. albicans ATCC 10231 were obtained from MIRCIN culture collection of the Faculty of Agriculture, Ain Shams University, Egypt.
Drugs
Stock solutions of Diclofenac sodium (150.4 mM), Ketoprofen (78.4 mM), Meloxicam (112 mM), Piroxicam (120 mM), Dexamethasone (4 mg/ml), Levofloxacin (122.8mM) and Ketoconazole (74.24) (Sigma) were prepared in Dimethyl sulfoxide (DMSO).
Determination of minimum inhibitory concentration (MIC) and minimum bactericidal concentrations (MBC) by microbroth dilution method
According to the guidelines of Clinical and Laboratory Standards Institute (CLSI, 2008), the microbial suspensions of S. aureus and C. albicans cultures were made in sterile normal saline (0.89% NaCl wt/vol; Himedia, India) and the turbidity were adjusted to 0.5 McFarland standards (equivalent to 1.5 × 108 colony forming units (CFU) /ml). Two-fold serial dilutions of tested drugs were prepared in Mueller Hinton Broth (MHB; Difco Laboratories) and dispensed in 96-well microtiter plates (Tarson, India). The microbial suspensions was added to each well of the plate resulting in the final inoculum of 5 × 105 CFU/ml. Levofloxacin was used as standard antibacterial agent while ketoconazole was used as standard antifungal agent. DMSO was used as negative control (show no antibacterial or antifungal activity). The plates were incubated at 37°C for 18 h and were visually read for the absence or presence of turbidity. The minimum concentration of the tested agents showing no turbidity was recorded as MIC. The MBC was determined by spreading 100 μl on tryptic soy agar (Himedia, India) from the wells showing no visible growth. The plates were incubated at 37°C for overnight. Concentrations showed no growth on the surface of TSA was considered as MBC (CLSI, 2008).
Scanning electron microscopy (SEM)
To study the effect of the tested agents on the cell morphology of S.aureus and C. albicans, SEM was used.
After one hour contact with the MIC concentrations of the tested drugs, the cells were prefixed in 2% glutaraldehyde for one hour at 4°C. Post–fixation was done using a 2% osmium tetroxyd solution. After each fixation, the cells were washed twice with PBS. The samples were gold covered by cathodic spraying (Edwards S 150 B). Finally, the samples were examined as described with the scanning electron microscope (Stereoscann 360, Cambridge) (Benyahya et al., 1992).
Biofilm susceptibility assay
The biofilms of S. aureus strains (ATCC 6538 and two clinical strains) and C. albicans strains (ATCC 10231 and 2 clinical strains) were prepared in 96-well polystyrene microtiter plates (Tarson, India), using a method of Wei et al. (2006) with a few modifications. The microbial suspensions were prepared from the overnight grown culture and the turbidity of the suspension was adjusted to 0.7 OD610 (1 × 109 CFU/ml). Two fold serial dilutions for NSAIDs, dexamethasone, levofloxacin and Ketoconazole were prepared in tryptone soya broth (Difco laboratories) supplemented with 0.5% glucose. Forty microliters of fresh TSB with 0.5% glucose was added to each well, followed by the addition of 60 μl of above microbial suspension. Then, different dilutions of the tested products were added and the plates were incubated for 18 h at 37°C. The plates were washed by phosphate buffer saline (PBS) and biofilms were fixed with methanol for 15 to 30 min, stained with Crystal Violet (Sigma, USA). Biofilm formation was quantified by the addition of 95% ethanol to the crystal violet stained wells and recording the absorbance at 595 nm using a microplate reader (Multiskan spectrum, Finland) (Wei et al., 2006).
The effect of the tested drugs also examined on preformed biofilms. The biofilms were prepared by inoculating the suspension of S. aureus and C. albicans strains into the wells of a polystyrene microtiter plate as mentioned above. After incubation at 37°C for 18 h, the culture supernatant from each well was decanted and planktonic cells were removed by washing the wells with PBS (pH 7.2). Two fold serial dilutions of NSAIDs, dexamethasone, levofloxacin and ketoconazole were prepared in trypticase soy broth (TSB) and 200 μl of each dilution was added to the biofilm in the wells. The plate was further incubated at 37°C for 18 h. The biofilm was fixed, stained and quantified as described above (Wei et al., 2006).
Ethidium bromide accumulation assay
The action of NSAIDs, dexamethasone, levofloxacin and ketoconazole on cell membrane permeability of S. aureus ATCC 29213 and C. albicans ATCC 10231 cells were evaluated by the method as described by Cox et al. (2000). The microbial cells were grown overnight in Muller Hinton broth (MHB), resuspended in 50-mmol/l sodium phosphate buffer. The turbidity of the suspension was adjusted to 0.7 OD610 (1 × 109 CFU/ml) and 1 ml of it was added to flask containing 19 ml buffer and the MIC of the tested levofloxacin, ketoconazole, NSAIDs and dexamethasone at 4 mg/ml. Following 60 and 120 min incubation at room temperature, 200 μl aliquots were transferred into tubes containing 3.8 ml phosphate buffer. These tubes were stored on ice and 20 μl of staining solution, consisting of ethidium bromide (Sigma) (10 μg/ml) dissolved in milliQ water. Then, the fluorescence was measured using A Perkin Elmer 45 luminescence spectrometer connected to the FLWINLAB software (United Kingdom) (Cox et al., 2000).
Germ Tube Formation
C. albicans ATCC 10231 colonies were suspended in sterile saline and adjusted to density of 0.5 Mcfarland. Human serum was added to 1 ml of cell suspensions of C. albicans ATCC 10231. Drugs were added to the suspension at MIC concentration and saline was added to the control tube. The cell suspensions were incubated with gentle shaking at 37°C for 2 h and were examined for the presence of germ tubes by using a light microscope. Photomicrographs of colonies and invasive growth were taken with a DMRXA microscope (Leica, Germany) (Liu et al., 1994).
RT-PCR analysis of C. albicans adhesion-related genes
Total RNA was extracted from C. albicans biofilms using QIAGEN RNeasy Mini kit (cat no.74524, Germany) according to the guidelines of manufacturer. RNA concentrations and RNA purity were determined using a spectrophotometer (GeneQuart 1300, Germany). An equal amount of RNA was subjected to cDNA synthesis using the cDNA Reverse Transcriptase reagent kit (Applied Biosystems, cat no. 1311190, USA). Real-time PCR primers were designed for the target genes ALS1, HWP1 using Real Time Equipment (Strategene MXP 3000, Germany). The β-actin gene (ACT1) was used as an endogenous reference gene. The sequences of the primers are shown in Table 1. QuantiTect®SYBR® Green PCR Kit (Applied Biosystems, cat no. 204141, USA) was used. All PCR reaction mixtures contained: 10 μl QuantiTect®SYBR® Green PCR Master Mix (2X), 2 μl first strand cDNA, 0.5 μl each primer, 0.4 μl ROX Reference Dye (50X) and RNase-H2O to the final volume of 20 μl. The program for amplification was 95°C for 15min as an initial denaturation step to activate the HotStartTaq® DNA polymerase, followed by 40 cycles of PCR consisting of 94°C for 15 s, 60°C for 30 s and 72°C for 30 s.

Negative controls (water as template) were included in each run. After amplification, a melting curve was analyzed to confirm the specificity of the primers. Expression of each investigated gene was normalized to the housekeeping ACT1 gene and analyzed using comparative Ct method (ΔΔCt). Expression of ALS1 and HWP1, genes from cells grown under drug treatment was indicated. Each experimental condition was performed in duplicate and each experiment was repeated twice (Ding et al., 2014).
RT-PCR analysis of S. aureus adhesion-related gene
TaqMan®Fast Universal quantitative Real-time PCR for icaA gene. Taqman primers and probe are summarized in Table 1. Probe was labeled with the reporter dye 6-carboxyfluorescein (6’-FAM) at the 5’end and with the quencher dye 6-carboxy-tetramethylrodamine (TAMRA) at the 3’end. TaqMan®Fast Universal PCR Kit (Applied Biosystems, cat no.4351891, USA) was used. Thermal cycling conditions were as follows: 10 min at 95°C followed by 45 repeats of 15 s at 95°C, and 1 min at 60°C. Data collection was performed during each annealing phase. Each experimental condition was performed in duplicate and each experiment was repeated twice (Paniagua-Contreras et al., 2012).
Statistical analysis
Data were described as mean ± SD. All statistical analyses were performed by statistical analysis computer software package SPSS 17.0 (SPSS Inc., IL, USA). Student’s t-test or one-way ANOVA were used to compare the biofilm formation, planktonic growth, and the gene expression of C. albicans and S. aureus strains in the presence or absence of drugs. Results with a p-value less than 0.05 were considered statistically significant.
The attachment of microbial cells to surfaces and accumulation of these cells in forming multilayered cell clusters are key steps in any infection (Ji et al., 1995). In this regard, adhesion formation is considered as one of the major virulence factors in C. albicans and S. aureus.
C. albicans is the fourth leading cause of bloodstream infections and the third most commonly isolated organism from intravascular catheters and is associated with the highest incidence of mortality (Crump and Colligan, 2000). C. albicans and S. aureus readily form biofilms on a wide variety of polymers used to make indwelling medical devices (Kojic and Darouiche, 2004). There is some evidence to suggest that a large proportion of device-related C. albicans infections involve biofilms (Dominic et al., 2007). In addition, severe catheter-related S. aureus infections have been reported in many studies to be important causes of morbidity, mortality, and a source of concern in the primary and emergency care context over the past decade (Parker and Doebbeling, 2012).
In recent years, due to the increased resistance of many bacteria to the commonly used antimicrobial agents, attention has shifted to drugs belonging to different pharmacological classes for possible antimicrobial activity. Many studies showed that NSAIDs have antibacterial activity, antifungal activity and decrease adherence and biofilm formation by bacteria and fungi (Ashraf et al., 2015; Mohsen et al., 2015). Our results showed that Meloxicam and Diclofenac sodium had the highest activity against the tested strains. Diclofenac sodium exhibited higher anti-candidal activity than that showed by Ketoconazole while Levofloxacin had the highest antibacterial activity against S. aureus followed by Meloxicam and Diclofenac sodium. Dexamethasone showed no activity against the tested strains (Table 2). Also, we tested the synergistic activity among NSAIDs, dexamethasone and the tested standard antimicrobials; it was found that the tested NSAIDs increased the activity of the tested antimicrobials but dexamethasone showed no increase in their activity (data not shown). The combined effect of antifungal or antibacterial agents and COX inhibitors in Candida infections was reported in many studies (Pina-vaz et al., 2000; Abdelmegeed and Shaaban, 2013).
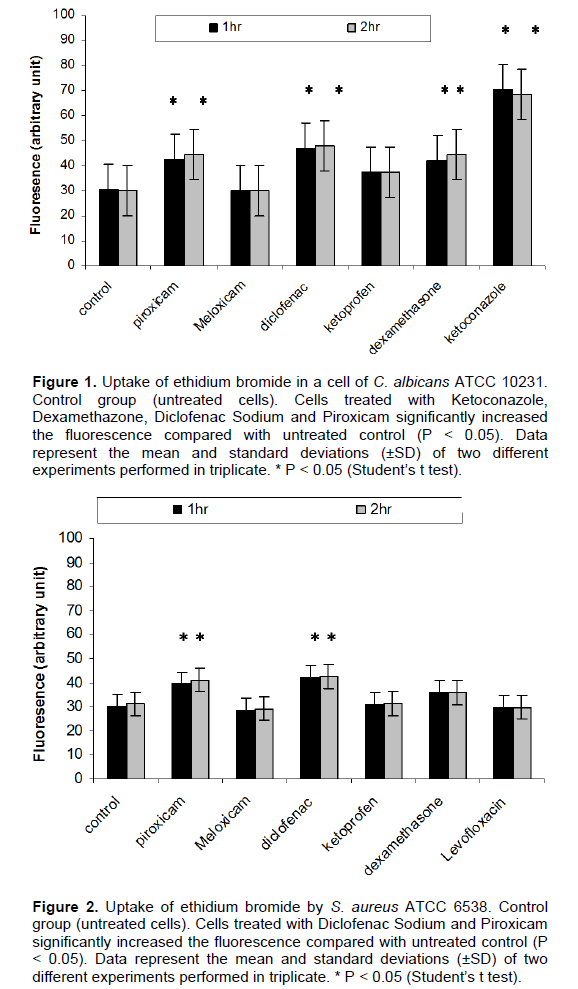
The increase in the uptake of ethidium bromide is an evidence to the effect of the tested agents on the membrane integrity in comparison to the unexposed cells
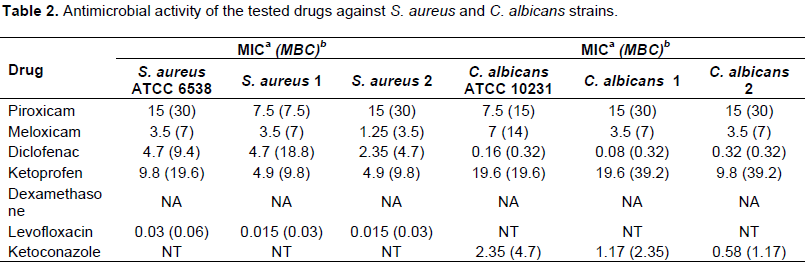
aMinimum inhibitory concentration in mM; bMinimum bactericidal concentration in mM; NA, no activity; NT, not tested.
(P<0.05). It was found that Meloxicam and Levofloxacin showed no increase in the uptake of ethidium bromide by S. aureus. For C. albicans, Meloxicam showed no effect while Ketoconazole showed the highest disruptive effect on the membrane integrity followed by Diclofenac sodium. As observed, Diclofenac sod had the highest effect on the membrane integrity of both S. aureus and C. albicans which may explain its antimicrobial effect on both organisms (Figures 1 and 2). Also, the antifungal activity of Diclofenac sodium against C. albicans may be due to that their log P (4.75) which are close to the log P of Ketoconazole (4.35). Niazi et al. (2010) explained that the antifungal activity of two Schiff bases may be due to their logP (3.77 and 3.74) which are close to Ketoconazole (4.35). Many studies agreed with our results, as they reported that the antimicrobial activity of NSAIDs may be due to their inhibition of bacterial DNA synthesis or impairment of membrane activity (Niazi et al., 2010; Dutta et al., 2004).
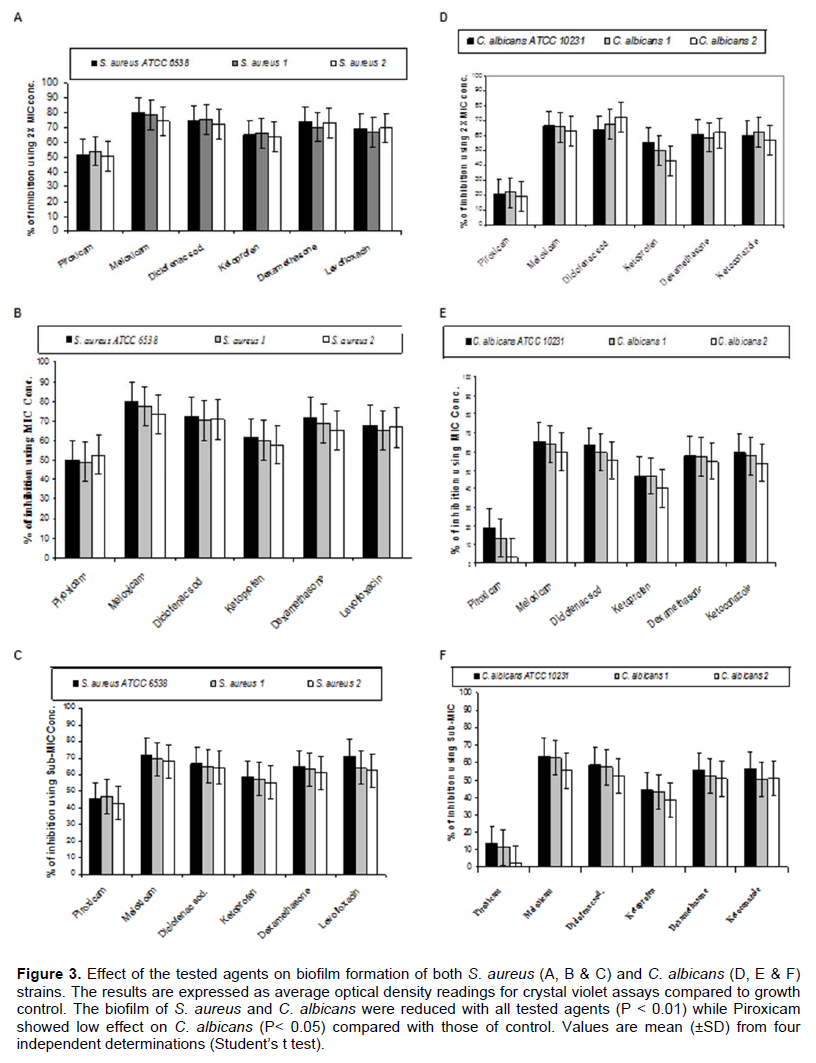
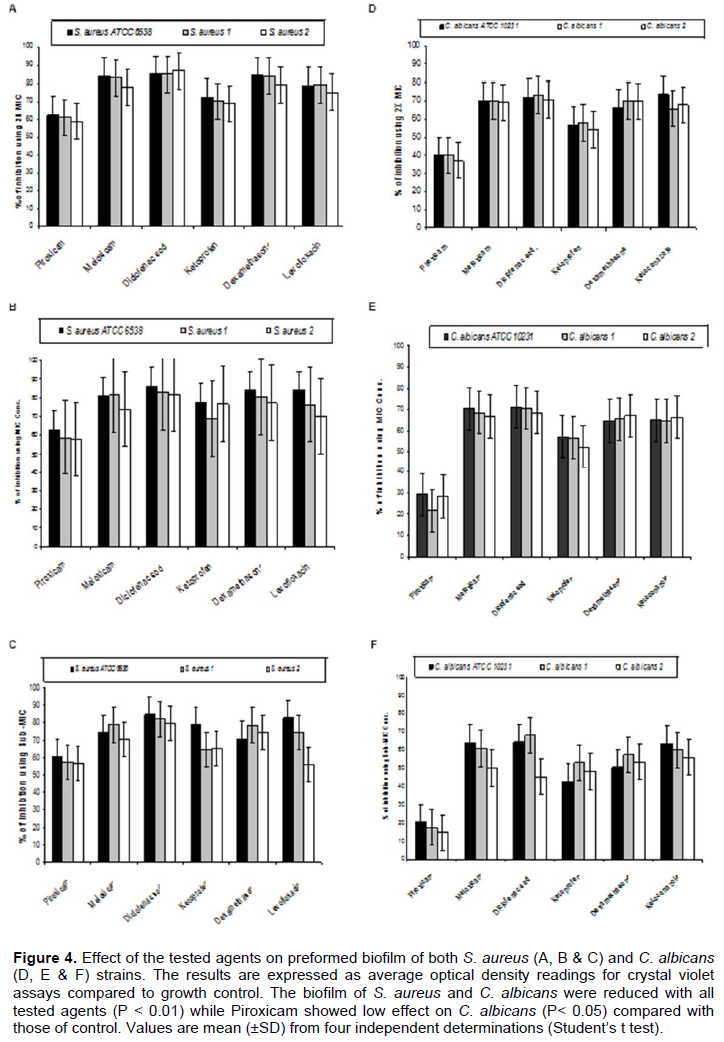
By testing the effect of NSAIDs, Dexamethazone, Levofloxacin and Ketoconazole on the ability of the tested strains to form biofilm, it was found that meloxicam showed the highest ability to inhibit biofilm formation by both S. aureus and C. albicans strains followed by Diclofenac sodium, Dexamethasone, Levofloxacin (in case of S. aureus) or Ketoconazole (in case of C. albicans), Ketoprofen and Piroxicam (Figure 3). On the other hand, Diclofenac sodium exhibited the highest disruptive effect on the preformed biofilm formed by the tested strains followed by Meloxicam, Dexamethasone, Levofloxacin (in case of S. aureus) or Ketoconazole (in case of C. albicans), Ketoprofen and Piroxicam (Figure 4).
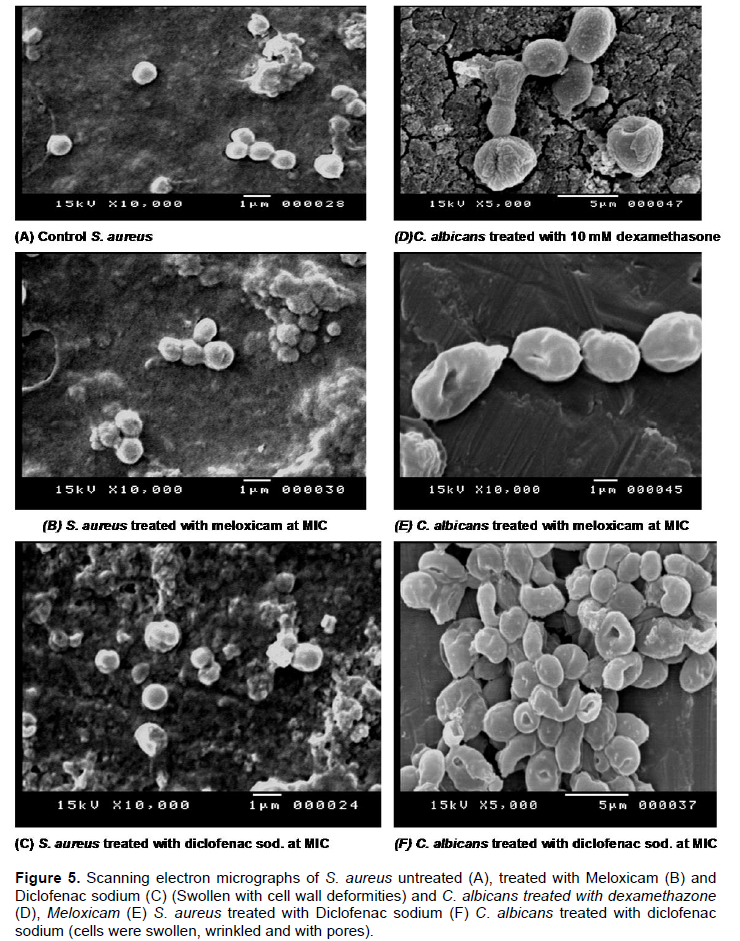
Our study showed that Dexamethasone affect the membrane permeability of the C. albicans while showed slight effect of S. aureus permeability in comparison to controls and its effect on biofilm formation and preformed biofilm are close to that shown by Ketoconazole and Levofloxacin. SEM was used to verify the effect of the tested drugs on the biofilm and the cell morphology of the tested strains. SEM graphs showed that the tested anti-inflammatory drugs caused morphological deformities (S. aureus and C. albicans) and pore formation on the surface of C. albicans. Also, the graphs showed that Dexamethazone caused swelling and pore formation to C. albicans cells (Figure 5). But Dexamethazone did not affect the cell morphology of S. aureus. The effect of NSAIDs on C. albicans and S. aureus cell morphology were also detected (Ashraf et al., 2015; Mohsen et al., 2015).
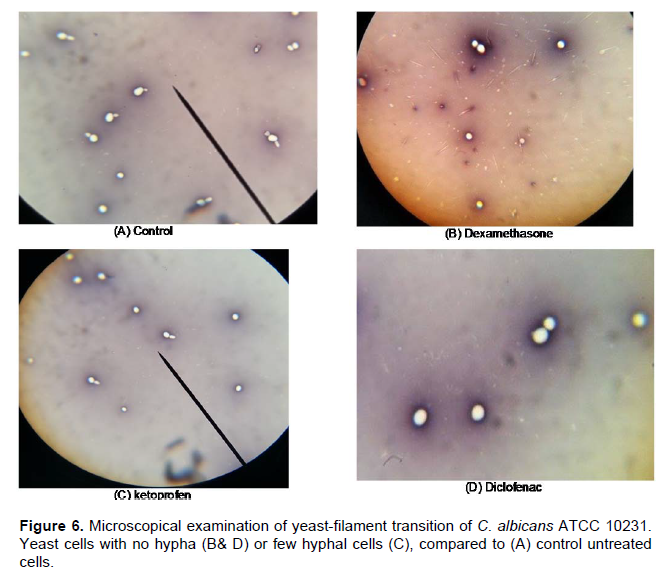
The ability of germ tube formation by C. albicans in the presence of tested drugs was determined. Diclofenac, Ketoconazole, Meloxicam (at MIC) and Dexamethazone (1 mg/ml) were found to inhibit germ tube formation but Ketoprofen and Piroxicam decreased the amount of germ tubes (Figure 6). Abdelmegeed and Shaaban (2013) reported that cultures treated with some NSAIDs exhibited a dose-dependent inhibition of germ tube formation following 3 h incubation at 37°C.
They reported significant reduction in germ tube formation under treatment with 1 and 10 mM anti-inflammatory drugs for 3 h especially with Ketorolac, Piroxicam and Diclofenac. Brooks and Day (1991) reported the antigerm tube effect of Diclofenac sodium suggesting that the antigerm tube effect observed in vitro might also be relevant in vivo (Brooks and Day, 1991).
Several studies demonstrated that local steroid treatment, even without antibiotics, can substantially reduce S. aureus colonization and the therapeutic effect of early combined therapy was superior to that in the steroid-only group; however, at the end of the treatment period, there was no significant difference between the 2 treatment groups in clinical scoring (Gong et al., 2006). According to our results, Dexamethazone had no antibacterial activity, slight effect on permeability of S. aureus cell membrane but had significant inhibitory effect on biofilm formation. So, we suggest that its effect may be due to low log P (partition coefficient) (1.83) which increase the wettability of tissue culture plate (TCP) surface and decrease the hydrophobic interaction between S. aureus surface and the plastic surface of TCP. Also, its low log P decreased its permeability through the hydrophobic membrane of S. aureus (Wang et al., 2012). Its effect on C. albicans may be due to its effect on prostaglandin production. The role of prostaglandin E2 (PGE2) and the fungal-produced prostaglandin EX (PGEx) in C. albicans in biofilm development and fungal pathogenesis has been demonstrated (Erb-Downward and Noverr, 2007). Moreover it has been investigated by Alem and Douglas (2005) that prostaglandin synthesis by both planktonic and biofilm cells was sensitive to the cyclooxygenase inhibitors aspirin, Diclofenac, and Etodolac. Also, they found that NSAIDs that showed more activity on COX2 (Meloxicam, Diclofenac sodium) had the highest ability to decrease germ tube and biofilm formation (Alem and Douglas, 2005). This explain the variation in activity between the tested drugs as meloxicam and Diclofenac sodium which showed higher effects on germ tube and biofilm formation while Piroxicam showed the lowest effect as it has a COX 1: COX 2 activity ratio of 250:1 (Frölich, 1997). Dexamethazone cause the repression of COX2 and prostaglandin E2 release in human pulmonary A549 cells by transcriptional and post transcriptional mechanisms. So, we suggested that the inhibitory effect of Dexamethazone may be due to its effect on COX2 and PGE2 that play an important role in hyphae formation and host cell damage (Mishra et al., 2014).
Due to the observed effect of the tested drugs on biofilm formation and the preformed biofilms, we tried to determine the potential molecular mechanism behind the ability of the tested agents to prevent biofilm formation by both C. albicans and S. aureus. We have provided evidence that there were variable effects of the tested drugs on the tested genes in comparison to control (C. albicans in the presence of serum). All tested drugs down-regulated the expression of the adhesion-related gene ALS1 (1.0643 to 2 fold). Dexamethasone was found to down-regulate the expression of ALS1 by 2 fold while Ketoconazole by 1.197 fold. On other hand, it was found
that Ketoprofen up-regulated the expression of HWP1 gene by 3.2 fold. Meloxican down-regulated HWP1 expression by 4.11 fold followed by Ketoconazole (3.89 fold) and Piroxicam (3.81) (Figure 7). The morphological switch from yeast-to-hypha is one of the most important biological features that enable C. albicans to colonize, invade, and survive in the host tissues during infection. C. albicans, the yeast-to-hypha transition is triggered by various environmental causes, such as serum, N-acetylglucosamine, neutral pH, high temperature, starvation, CO2, and adherence (Biswas et al., 2007). In responses to a wide variety of stresses, including nutritional and environmental stresses, C. albicans initiate morphological changes which are controlled by a complex network of parallel pathways (e.g. MAPK and cAMP-PKA pathways) (Garcia-Rodriguez et al., 2005). Thus, any interference with the expression of genes involved in the MAPK cascades and cAMP-PKA pathway can block filamentation in C. albicans. The ALS family gene is related to the growth and morphological change of C. albicans. ALS family especially, ALS1 and ALS3 are important in the yeast-to-hypha transformation of C. albicans (Hoyer, 2001). Another well-characterized C. albicans hypha specific gene is the glycosylphosphatidylinositol (GPI)-linked cell surface protein HWP1. Functional analyses showed that this protein is required for the adherence of fungal cells to epithelial cells as well as for normal biofilm and hypha formation repression effect of Diclofenac sodium on the regulation of genes controlling C. albicans morphogenesis was also reported (Loza et al., 2004; Zhou et al., 2012).
S. aureus biofilm formation requires the production of polymeric N-acetylglucosamine that is controlled by icaABCD operon. Meloxicam was found to down-regulate the expression of icaA gene by 17.46 fold followed by levofloxacin (15.62 fold) and diclofenac sod. (7.042 fold) but dexamethasone showed no significant effect on its expression (Table 3).

NSAIDs have antibacterial and antifungal activity, can affect membrane permeability, germ tube formation and the expression of some genes controlling adhesion. Dexamethazone showed significant effect on biofilm formation of both S. aureus and C. albicans which may be a result of its Log P and the inhibition of PGEs release and affected gene expression of ALS1 and HWP1 of C. albicans. These effects of the tested drugs in addition to its anti-inflammatory properties may result in good response to the antimicrobial therapy in-vivo.
The authors have not declared any conflict of interests.
REFERENCES
|
Abdelmegeed E, Shaaban MI (2013). Cyclooxygenase inhibitors reduce biofilm formation and yeast-hypha conversion of fluconazole resistant Candida albicans. J. Microbiol. 51(5):598-604.
Crossref
|
|
|
|
Alem M A, Douglas L J (2005). Prostaglandin production during growth of Candida albicans biofilms. J. Med. Microbiol. 54:1001-1005.
Crossref
|
|
|
|
|
Ashraf A, Yousri F, Taha N, Abd El-Waly O, Ramadan A, Ismail E (2015). Effect of Some Non-steroidal Anti-Inflammatory Drugs on Growth, Adherence and Mature Biofilms of Candida spp. Am. J. Microbiol. Res. 3(1):1-7.
Crossref
|
|
|
|
|
Auphan N, Di Donato J A, Rosette C, Helmerg A, Karin M (1995). Immunosuppression by glucocorticoids: inhibition of NF-kappa B activity through induction of I kappa B synthesis. Science 270:286-290.
Crossref
|
|
|
|
|
Benyahya M, Senaud J, Bohatier J (1992). Etude en microscopie électronique. Ann. Sci. Nat. 13:103-119.
|
|
|
|
|
Biswas S, Van Dijck P, Datta A (2007). Environmental sensing and signal transduction pathways regulating morphopathogenic determinants of Candida albicans. Microbiol. Mol. Biol. Rev. 71:348-376.
Crossref
|
|
|
|
|
Brooks PM, Day RO (1991). Non-steroidal anti-inflammatory drugs–differences and similarities. New Engl. J. Med. 324:1716–125.
Crossref
|
|
|
|
|
CLSI-Clinical and Laboratory Standards Institute (2008). Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. Approved standard. M7-A7. 7 edition. Wayne, PA: CLSI.
|
|
|
|
|
Cox SD, Mann CM, Markham JL, Bell HC, Gustafson JE, Warmington JR, Wyllie SG (2000). The mode of antimicrobial action of the essential oil of Melaleuca alternifolia (tree oil). J. Appl. Microbiol. 88:170-175.
Crossref
|
|
|
|
|
Crump JA, Collignon PJ (2000). Intravascular catheter-associated infections. Eur. J. Clin. Microbiol. Infect. Dis. 19:1-8.
Crossref
|
|
|
|
|
Ding X, Liu Z, Su J, Yan D (2014). Human serum inhibits adhesion and biofilm formation in Candida albicans. BMC Microbiol. 14:80.
Crossref
|
|
|
|
|
Dominic RM, Shenoy S, Baliga S (2007). Candida biofilms in medical devices: Evolving trends. Kathmandu Univ. Med. J. 5:431-436.
|
|
|
|
|
Douglas LJ (2003). Candida biofilms and their role in infection. Trends Microbiol. 11:30-36.
Crossref
|
|
|
|
|
Dutta NK, Kumar KA, Mazumdar K, Das-tidar SG (2004). In vitro and in vivo anti mycobacterial activity of anti-inflammatory drug, Diclofenac sodium. Indian J. Exp. Biol. 42(9):922-927.
|
|
|
|
|
Erb-Downward JR, Noverr MC (2007). Characterization of prostaglandin E2 production by Candida albicans. Infect. Immun. 75:3498-505.
Crossref
|
|
|
|
|
Frölich JC (1997). A classification of NSAIDs according to the relative inhibition of cyclooxygenase isoenzymes. Trends Pharmacol Sci. 18(1):30-34.
Crossref
|
|
|
|
|
Garcia-Rodriguez LJ, Valle R, Duran A, Roncero C (2005). Cell integrity signaling activation in response to hyperosmotic shock in yeast. FEBS Lett. 579:6186-6190.
Crossref
|
|
|
|
|
Golia S, Hittinahalli V, Sangeetha KT, Vasudha CL (2011). Study of biofilm formation as a virulence marker in Candida species isolated from various clinical specimens. JEMDS 1:1238-1246.
Crossref
|
|
|
|
|
Gong JQ, Lin L, Lin T, Hao F, Zeng FQ, Bi ZG (2006). Skin colonization by Staphylococcus aureus in patients with eczema and atopic dermatitis and relevant combined topical therapy: A double-blind multicentre randomized controlled trial. Br. J. Dermatol. 155(4):680-687.
Crossref
|
|
|
|
|
Gotz F (2002). Staphylococcus and Biofilms. Mol. Microbiol. 43(6):1367-1378.
Crossref
|
|
|
|
|
Hoyer LL (2001). The ALS gene family of Candida albicans. Trends Microbiol. 9:176-180.
Crossref
|
|
|
|
|
Ji G, Beavis RC, Novick RP (1995). Cell Density Control of Staphylococcal Virulence Mediated by an Octa- peptide Pheromone. Proc. Natl. Acad. Sci. USA 92(26):12055-12059.
Crossref
|
|
|
|
|
Klotz SA, Chasin B (2007). Powell B, Gaur NK, Lipke PN. Polymicrobial bloodstream infections involving Candida species: Analysis of patients and review of the literature. Diagn. Microbiol. Infect. Dis. 59(4):401-406.
Crossref
|
|
|
|
|
Kojic EM, Darouiche RO (2004). Candida infections of medical devices. Clin. Microbiol. Rev. 17:255-267.
Crossref
|
|
|
|
|
Liu H, Kohler J, Fink GR (1994). Suppression of hyphal formation in Candida albicans by mutation of a STE12 homolog. Science 266: 1723-1726.
Crossref
|
|
|
|
|
Loza L, Fu Y, Ibrahim AS, Sheppard DC, Filler SG, Edwards JE (2004). Functional analysis of the Candida albicans ALS1 gene product. Yeast 21(6):473-482.
Crossref
|
|
|
|
|
Mishra NN, Ali S, Shukla PK (2014). Arachidonic acid affects biofilm formation and PGE2 level in Candida albicans and non-albicans species in presence of sub-inhibitory concentration of fluconazole and terbinafine. Braz. J. Infect. Dis. 18(3):287-293.
Crossref
|
|
|
|
|
Mohsen A, Gomaa A, Mohamed F, Ragab R, Eid M, Ahmed A, Khalaf A. (2015). Antibacterial, Anti-biofilm Activity of Some Non-steroidal Anti-Inflammatory Drugs and N-acetyl Cysteine against Some Biofilm Producing Uropathogens. Am. J. Epidemiol. Infect. Dis. 1:1-9.
Crossref
|
|
|
|
|
Niazi S, Javali C, Paramesh M, Shivaraja S (2010). Study of influence of linkers and substitutions on antimicrobial activity of some Schiff bases. Int. J. Pharm. Pharm. Sci. 2:108-112
|
|
|
|
|
O'Gara JP (2007). ICA and beyond: biofilm mechanisms and regulation in Staphylococcus epidermidis and Staphylococcus aureus. FEMS Microbiol. Lett. 270(2):179-188.
Crossref
|
|
|
|
|
Paniagua-Contreras G, Sáinz-Espu-es T, Monroy-Pérez E, Raymundo J R, Arenas-Aranda D, Negrete-Abascal E, Vaca S (2012). Virulence Markers in Staphylococcus aureus strains isolated from hemodialysis catheters of Mexican patients. Adv. Microbiol. 2(4):476-487.
Crossref
|
|
|
|
|
Parker MG, Doebbeling BN (2012). The challenge of Methicillin-resistant Staphylococcus aureus prevention in hemodialysis therapy. Semin. Dial. 25(1):42-49.
Crossref
|
|
|
|
|
Pfaller MA, Diekma DJ (2007). Epidemiology of invasive candidiasis: A persistent public health problem. Clin. Microbiol. Rev. 20:133-163.
Crossref
|
|
|
|
|
Pina-Vaz C, Sansonetty F, Rodrigues AG, Martinez-De-Oliveira J, Fonseca AF, Mardh PA (2000). Antifungal activity of ibuprofen alone and in combination with fluconazole against Candida species. J. Med. Microbiol. 49:831-840.
Crossref
|
|
|
|
|
Scheinman RI, Cogswell PC, Lofquist AK, Baldwin ASJ (1995). Role of transcriptional activation of I kappa B alpha in mediation of immunosuppression by glucocorticoids. Science 270:283-286.
Crossref
|
|
|
|
|
Seo YS, Lee DY, Rayamahji N, Kang ML, Yoo HS (2008). Biofilm-forming associated genotypic and phenotypic characteristics of Staphylococcus spp. Isolated from animals and air. Res. Vet. Sci. 85(3):433-438.
Crossref
|
|
|
|
|
Viale P, Stefani S (2006). Vascular Catheter-Associated Infections: A Microbiological and Therapeutic Update. J. Chemother. 18(3):235- 249.
Crossref
|
|
|
|
|
Wang JD, Douville NJ, Takayama S, Elsayed M (2012). Quantitative Analysis of Molecular Absorption into PDMS Microfluidic Channels. Ann. Biomed. Eng 40(9):1862-1873
Crossref
|
|
|
|
|
Wei GX, Campagna AN, Bokek LA (2006). Effect of MUC7 Peptides on the growth of bacteria and on Streptococcus mutans biofilm. J. Antimicrob. Agents 57:1100-1109.
Crossref
|
|
|
|
|
Zhou Y, Wang G, Li Y, Liu Y, Song Y, Zheng W, Zhang N, Hu X, Yan S, Jia J (2012). In vitro interactions between aspirin and amphotericin B against planktonic cells and biofilm cells of C. albicans and C. parapsilosis. Antimicrob. Agents Chemother. 56:2350-2360.
Crossref
|
|