ABSTRACT
Thermophiles are excellent sources of enzymes that can withstand and carry out reactions efficiently under high temperatures. This study isolated and characterised thermotolerant bacteria that produce enzymes of potential industrial value from five hot springs in Eritrea. A total of 65 bacterial isolates were obtained from the five hot springs. Out of the 65 isolates; 19 isolates produced a positive reaction for amylases, 36 for carboxymethyl cellulases, eight for proteases, 10 for xylanases and 11 for pectinases. More than half (36 out of 65) were able to produce carboxymethyl cellulases. Six isolates which showed carboxymethyl cellulase activity were from the genus Bacillus, while those belonging to Brevibacillus were seven. BLAST analysis of the partial sequences showed that 19 out of the 24 isolates sequenced showed high similarity (> 99%) to those of reference strains of the genera Bacillus and Brevibacillus available in the Genebank and EZ-taxon databases. The five isolates (E5, G2, G8, M1 and M13) that showed moderate similarities (97.2-99%) to strains from the Genebank and EZ-taxon databases were further characterized. Physiological characterization of the five selected isolates based on tolerance to NaCl, temperature and production of hydrolytic enzymes indicated that these isolates are potentially novel. Isolates G8 and M13 showed significantly higher amylase activity (p < 0.05) than the other three isolates. Caseinase activity recorded by the five isolates was the highest (p < 0.05) compared to other enzyme activities. The enzymes produced by thermotolerant bacteria from the five hot springs may be potentially useful for catalysis under harsh operational conditions encountered in industrial processes.
Key words: Thermphilic, bacteria, thermozymes, hot springs, Eritrea.
The discovery of extremophiles has been a remarkable impetus for biotechnology industries. The products secured from extremophiles such as proteins, enzymes (extremozymes) and compatible solutes have great biotechnological potential. The inherent stability of thermostable enzymes, which have been isolated mainly from thermophilic or thermotolerant organisms, has made them suitable candidates for a number of commercial applications (Singh et al., 2011). The chemical production of polymer intermediates, pharmaceuticals, speciality chemicals and agrochemicals is hindered by expensive processes due to low selectivity and undesired byproducts (Angelaccio, 2013). The lack of enzyme stability of mesophilic enzymes renders them inefficient for the harsh reaction conditions required in industrial processes. For this reason, the use of biocatalysts in organic reactions represents only a small fraction of the potential industrial market (Meyer et al., 2012). Thermotolerant bacteria have attracted industrial and biotechnological attention as their enzymes are well suited for harsh industrial processes (Abdel-Rahman et al., 2016; Archna et al., 2015; Verma et al., 2015). Proteases obtained from thermophilic or thermotolerant (Adhikari et al., 2015; Bozoglu et al., 2015; Lele and Deshmukh, 2016; Panda et al., 2013; Remigio et al., 2012; Verma et al., 2014)bacteria, for example, have found applications such as hide dehairing in the leather industry and stain removal in laundry detergents (Chandrashekhar and Narayan, 2015; Dudhgara et al., 2015; Jaouadi et al., 2013). Cellulases have showed great potential in the production of bioethanol and other speciality chemicals from renewable agricultural residues (Hardiman et al., 2010). Brewing and sugar production require α-amylases that are stable at high temperatures for gelatinization and liquefaction of starch to run processes at a relatively low cost (Rasooli et al., 2008). Xylanases active at high temperature and pH have attracted special interest in the pulp and paper industry as they reduce the need for toxic chlorinated compounds (Srinivasan and Rele, 1999). Pectinases are of importance in cotton scouring in textile industries (Dhiman et al., 2008). However, only a few of actual applications of these biocatalysts have reached the market (Coker, 2016).
Hot springs are potential habitats for thermophilic microorganisms. During the last few decades, hot spring environments from around the world have been targeted for the isolation of novel thermotolerant or thermophilic microorganisms that produce stable thermozymes (Lele and Deshmukh, 2016; Shahinyan et al., 2015; Verma et al., 2014). Even though Eritrea is endowed with plenty of thermal springs, aside from their prospects in geothermal energy, the thermal hot springs in Eritrea have not yet been studied with respect to biotechnological prospects. In Eritrea, thermal springs are located scattered in the eastern low lands. This study aimed to isolate and characterize thermotolerant bacteria from hot springs in Eritrea that produce enzymes such as amylases, cellulases, pectinases, xylanases and proteases.
Sampling
Five hot springs; Maiwooi, Akwar, Garbanabra, Gelti and Elegedi were selected for sampling from three different locations, Gahtelai area, Irafayle and Alid area (Figure 1). Maiwooi (15° 32′ 53″N 39° 06′ 38″ E) and Akwar (15° 33′ 34″N 39° 05′ 37″ E) are located near Gahtelai at elevations of 330.1 and 344.5 m, with temperatures of 51.9 and 49.0°C and pH range of 7.54 and 6.97 respectively (Figure 1). These are low energy hot springs which discharge near-neutral bicarbonate waters (Yohannes, 2010). Garbanabra (15° 03′ 38″N 39° 46′ 27″ E) and Gelti (15° 03′ 39″N 39° 46′ 46″ E) are located near Irafayle on the shore of Gulf of Zula at elevations of 0.0 and 0.0 m, temperatures of 51.0 and 52.7°C and pH range of 7.05 and 7.01 respectively. Elegedi (14° 52′ 55″N 39° 55′ 37″ E) is located in Alid volcanic center at elevation of 512.7 m, with a temperature of 100°C and pH range of 7.19. Elegedi which is located about 30 km south of the Gulf of Zula and is associated with a high temperature geothermal system underlying the Alid volcanic centre in the northern Danakil depression of Eritrea (Yohannes, 2010). The bubbling water discharged from this hot spring is typical of the fumarolic steam condensate with high temperatures. Triplicate samples of water, wet sediment and microbial mat were collected from each hot spring.
Samples were collected in 20-ml autoclaved test tube containers and immediately placed in a thermoflask to keep the temperature of the water samples constant (Khalil, 2011). The samples were transported to the College of Health Science Laboratory in Asmara, Eritrea for further processing.
Isolation and enumeration of thermotolerant bacteria
Five millilitre from each water sample was used to inoculate 100 ml of culture media in 250-ml Erlenmeyer flask. The enrichment culture media contained; 5 g of NaCl (BLULUX), 9 g of peptone (BLULUX) and 2 g of yeast (BLULUX) per litre. The inoculated flasks were incubated at 55ºC with shaking at 240 rpm for 94 h.
One hundred microlitre of culture from each flask was spread on agar media containing; 5 g of NaCl (BLULUX), 9 g of peptone (BLULUX), 2 g of yeast (BLULUX) and 20 g of bacteriological agar (BLULUX) per litre and was incubated at 55°C in a bench top incubator for 24 to 48 h (Khalil, 2011). To obtain pure cultures, distinctive colonies were picked, transferred to fresh agar medium and incubated at 55°C for 24 and 48 h. Purified colonies were grown on tryptic soy broth (Difco) and stored in 20% glycerol at -80°C.
Morphological characterization
Colony and cell morphology were performed according to the standard protocols. Isolates were grown for 24-72 h at 50°C on the agar media described above. All the 65 isolates were examined using binocular microscope (BX100 Olympus) and characterised by Gram and spore staining (Moses et al., 2009).
Detection of enzymes
Hydrolase production by thermotolerant bacterial isolates was screened by plating on starch (Khalil, 2011), carboxymethyl cellulose (Teather and Wood, 1982), skimmed milk (Zilda et al., 2012), pectin (Huang et al., 2012)and xylan (Gessesse and Gashe, 1997)agar plates for amylase, cellulose, protease, pectinase and xylanase activity respectively. All the assays were conducted at 55°C for 48 h.
Molecular characterization using partial 16S rRNA gene
Bacterial isolates were grown in Luria Broth medium (Tryptone, 10 g/L; yeast extract, 5 g/L; NaCl, 10 g/L; pH 7.0) at 55°C for 48 h. The cultures were centrifuged at 10,000 × g for 1 min, and the supernatant was removed and the pellet was retained. DNA was extracted as previously described (Sambrok and Russell, 2001)and was stored at -80°C until further analysis. Bacterial universal primers 8F (5'-AGRCTTTGATCCTGGCTCAG-3') and 1492R (5'-CGGCTACCTTGTTACGACTT-3') were used to amplify the 16S rDNA from genomic DNA (Heuer et al., 1997). Polymerase chain reaction (PCR) was performed in a thermocycler (PeQLab, VWR, Germany). Each reaction mixture (50 µl) contained; 25 μl of 10× PCR master mix (BIOLINE), 2.5 U of Taq DNA polymerase (BIOLINE), 0.2 μM of each primer and 25 ng of template DNA. The amplification was performed as follows. Initial denaturation for 5 min at 94°C, 30 cycles each of denaturation for 30 s at 94°C, annealing for 30 s at 55°C, primer extension for 1.5 min at 72°C and final extension for 10 min at 72°C. The PCR products were checked by gel electrophoresis using 1.2% (w/v) agarose gels stained with ethidium bromide (10 mg/l) and stored at -20°C.
Sequencing of PCR products of the 65 bacterial isolates was carried out by Macrogen, South Korea. Sequencing was conducted in one direction using the forward primer (27 F) as previously described (Sanger et al., 1977). The BioEdit program was used to remove ambiguity and comparisons were done with the NCBI GenBank databases using Basic Local Alignment Tool (BLAST) algorism (Altschul et al., 1990). Sequences were submitted to the GenBank database and were assigned the accession numbers KX549080- KX549103. The differences in the nucleotides were converted into distance matrices using Maximum Likelihood method (Saitou and Nei, 1987). A phylogenetic tree was constructed using MEGA 7 (Kumar et al., 2016).
Biochemical and physiological characterization of the selected isolates
Biochemical tests including indole production, motility test, catalase, oxidase, sugar fermentation test, casein hydrolysis test, Tween 20 hydrolysis test and gelatin liquefaction test were performed on the five isolates designated E5, M1, M13, G2 and G8 (Sneath et al., 1986). The ability to grow at different temperatures was evaluated by inoculating the isolates into LB agar medium (Difco) at 15, 20, 30, 40, 50, 60 and 70°C (Aanniz et al., 2015). The pH range for growth was determined by growing the isolates at 55°C in 10 ml LB broth (Difco) adjusted to pH 4, 5, 6, 7, 8, 9, 10, 11, 12 and 13 using HCl or NaOH (Allan et al., 2005). Salt tolerance was determined by growing the cultures in 10 ml LB broth supplemented with NaCl to total concentrations of 1 to 13% (w/v) at 55°C (Nakamura and Swezey, 2017). Turbidities of both pH and salt tolerance series were determined using a dual-beam spectrophotometer (Versamax, Germany) set at 680 nm, at 24 h intervals.
Enzyme activities of the selected isolates
Direct quantification of enzyme activities by measuring the diameter of clearing zones on petri dishes was used to compare the five selected isolates. The enzyme activity assay included α-amylase (Khalil, 2011), cellulase using cellulose powder and carboxymethyl cellulose (CMC) as substrates (Teather and Wood, 1982), caseinase (Adhikari et al., 2015), pectinases (Huang et al., 2012)and xylanases (Gessesse and Gashe, 1997). Incubation was done at 55°C for 48 h. The diameter of the clear zones was measured using a ruler. Multivariate analysis (MANOVA) was used to compare enzyme activity between the five selected isolates. Significance was tested at p = 0.05.
Morphological and cellular characterization of isolates
Based on colony morphology, 65 thermotolerant bacterial isolates were obtained from the five hot springs in Eritrea. The highest number (21) of the isolates was obtained from Garbanabra while the lowest (5) was from Gelti. The colony forming units of thermotolerant bacteria that grew at 55ºC varied from 5.4 × 103 in Akwar to 1.4 × 104 cfu ml-1 in Elegedi (Table 1).
The number of isolates obtained from Akwar and Maiwooi were almost twice those from Gelti and Elegedi. The 65 isolates were Gram positive, rod-shaped, and endospore forming (Table 2). The spores observed were terminal as shown in Figure 2.
Hydrolase activity
A positive result for hydrolase activity was indicated by the clear zone formed around the colony (Figure 2). Out of the 65 isolates; 19 isolates produced a positive reaction for amylases, 36 for carboxymethyl cellulases, eight for proteases, 10 for xylanases and eleven for pectinases (Table 2). More than half (36 out of 65) were able to produce carboxymethyl cellulases. Six isolates which showed CMCase activity were from the genus Bacillus, while seven belonged to Brevibacillus. The five selected isolates; E5, G2, G8, M1 and M13 showed CMCase activity. However, only E5, G2 and M1 showed positive activity when cellulose powder was used as a substrate.
16S rRNA analysis
Genomic DNA was extracted from all the 65 thermotolerant isolates. 16S rRNA gene amplification with bacterial specific primers yielded an amplification product of approximately 1500 bp. From the 65 amplified PCR products sent for sequencing, only 24 appeared to be unambiguous and were considered for phylogenetic analysis. The 24 isolates were submitted to the NCBI database and were assigned accession numbers (KX549080-KX549102).
Sequence comparison with the Genebank and EZ-taxon databases using BLAST pairwise alignment was done and the affiliations of the 24 isolates to the closest reference strain were determined. The identification of the 24 isolates based on the sequence comparison with the Genebank, NCBI and reference strains belonging to genera Bacillus and Brevibacillus is shown in Table 3. BLAST analysis of the partial sequences showed that 19 out of the 24 isolates showed high similarity (> 99%) to reference strains of the genera Bacillus and Brevibacillus available in the Genebank and EZ-taxon databases. Five isolates (E5, G2, G8, M1 and M13) showed moderate sequence similarity (97.2 - 99%) to reference strains. Isolates G8, G9, M9 and M12 showed similarity (99.0 - 99.6%) to Bacillus aerius strain 24K (AJ831843) described by Shivaji et al. (2006). Four isolates (E5, G4, G5 and M5) were shown to affiliate (98.0 - 99.7%) with Bacillus sonorensis strain NRBC AYTN01000016 (Palmisano et al., 2001). M13 was the only isolate which affiliated with Bacillus licheniformis strain 9945A (Gwinn and Thorne, 1964). The other 15 isolates (G1, G2, G3, G6, G11, G12, G14, G18, G19, G20, M1, M3, M10, M11 and M14) showed 98.1 to 100% similarity to Brevibacillus borstelensis strain NRRL (Shida et al., 1996).
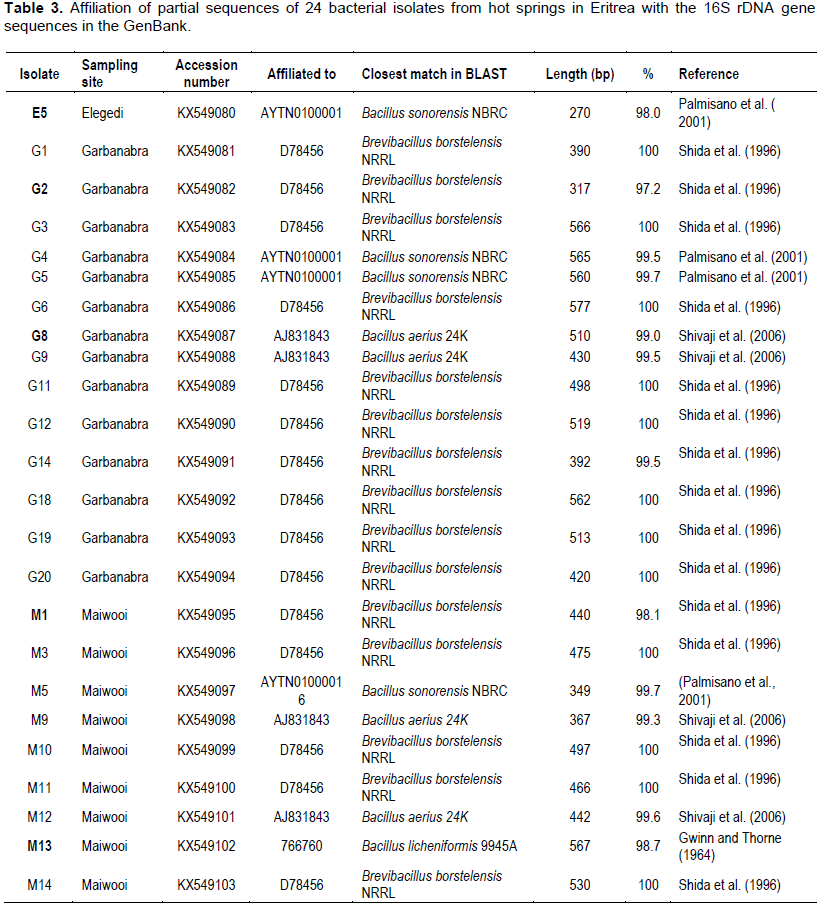
The five isolates that showed moderate similarity are indicated in bold letters. Out of 13 isolates sequenced from Garbanabra, nine affiliated with the genus Brevibacillus while the other five belonged to Bacillus. In Maiwooi, five out of nine isolates had shown similarity (98.1 to 100%) to Brevibacillus.
The phylogenetic tree of the 16S rRNA partial sequences of the 24 isolates revealed two major clusters (Figure 3). One cluster containing strains belonging to the genus Bacillus and the other containing strains affiliated with Brevibacillus with bootstrap values of 92 and 94 respectively.
Characterization of five bacterial isolates with moderate similarity
The five isolates (E5, G2, G8, M1 and M13) that showed moderate similarities (97.2 - 99.0%) with strains in Genebank and EZ-taxon databases were further characterized. The partial sequences of E5, G8 and M13 revealed similarity with Bacillus (98.0 - 99%) while G2 and M1 affiliated with the genus Brevibacillus (97.2 - 98.1%). The five isolates were Gram positive endospore forming rods. The growth of E5 was observed to occur between 20 - 60°C (Table 4).
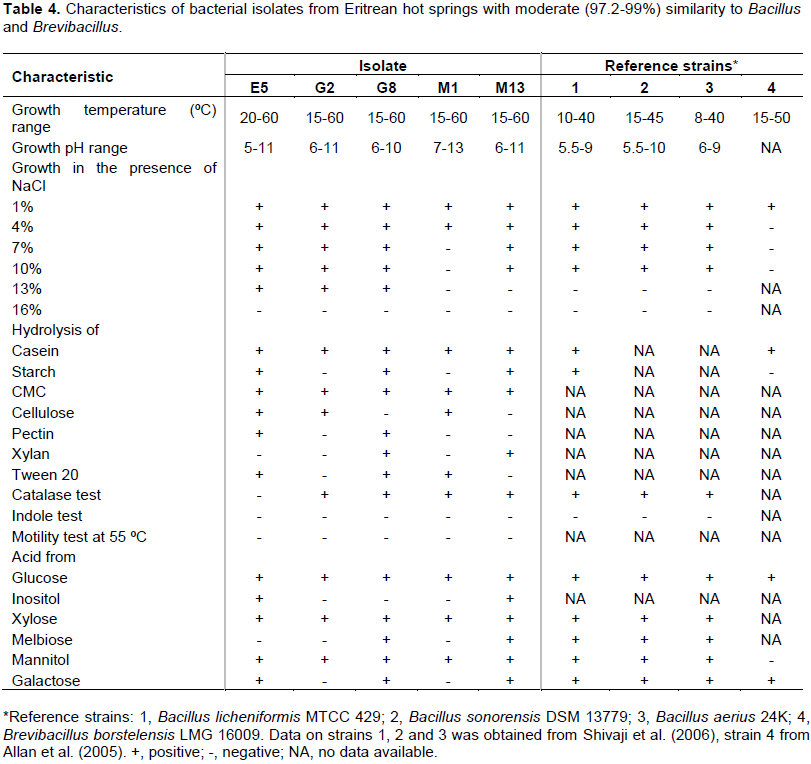
E5 grew at a temperature range of 20 - 60°C. The others; G2, G8, M1 and M13 grew at a temperature range between 15 and 60°C. The isolates E5, G8 and M13 formed brown colonies on casein agar plates, whereas G2 and M1 formed white colonies. Isolate E5 grew between pH 5 and pH 11, G2 and M13 between pH 6 and 11, G8 between pH 6 - 10 and M13 between pH 7 and 13. Isolates E5, G2 and G8 tolerate up to 11.6% NaCl while M17 and M13 tolerated up to 10% NaCl. The five selected isolates were indole negative and showed no motility at 55ºC. All except E5 were catalase positive. All the five isolates produced acid from glucose, xylose and mannitol. E5, G8 and M13, whose sequence affiliated with the genus Bacillus, also produced acid from galactose, inositol as well as melibiose and were shown to hydrolyze Tween 20.
Enzyme activity assay
Halo size, a semi-quantitative method, indicates the efficiency of a colony in producing particular enzyme (Scorsetti et al., 2012). The agar diffusion method was employed to quantify enzyme activities by measuring the diameter of the zone of clearance (Figure 4). E5, G8 and M13, affiliated with the genus Bacillus, showed amylase activity while G2 and M1, belonging to the genus Brevibacillus, exhibited no amylase activity. Isolates G8 and M13 showed significantly higher amylase activity (p < 0.05) than the other three isolates.
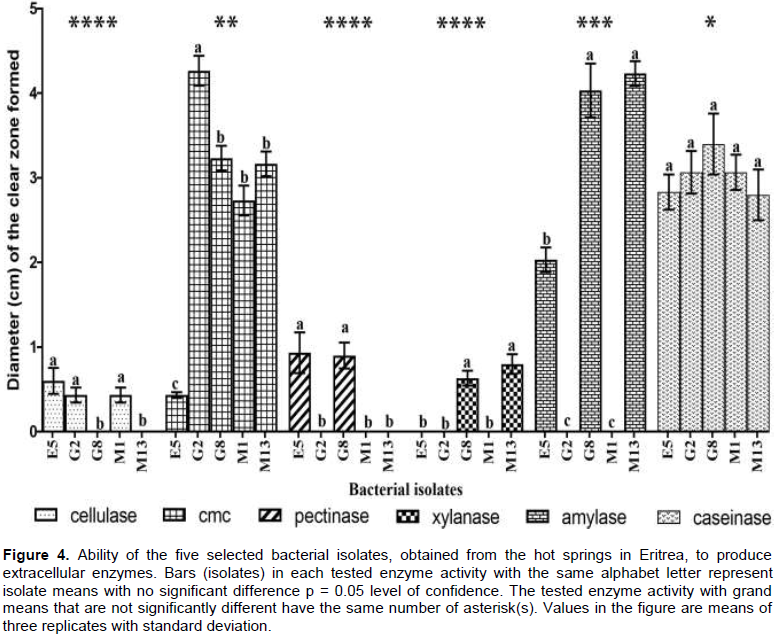
All the five isolates, E5, G2, G8, M1 and M13, were able to produce extracellular CMCases. Isolate G2 recorded significantly higher activity (p < 0.05) while isolate E5 recorded the least activity. There was no significant difference in caseinase activity between five isolates (p < 0.05). All the five isolates showed positive caseinase activity. Cellulase activity was recorded for isolates E5, G2 and M1, while G8 and M13 showed no activity. G8 and M13 were the only isolates that showed xylanase activity. Pectinase activity was only registered by isolates E5 and G8. Caseinase activity was significantly the highest compared to other substrates (p < 0.05). Amylase and CMCase activities were also
significantly higher (p < 0.05) than cellulase, pectinase and xylase activities.
The hydrolase activities, tolerance to high temperature, pH range and high NaCl concentrations were used to rank the effectiveness of the five isolates (Table 5). From the five selected isolates E5 had the highest score (7) followed by G8 (6). M13 had the lowest score of 3.
A score of one (1) was assigned to the positive reactions of the six hydrolase activities. Temperature tolerance of the five isolates were similar and hence was not considered in the ranking. A score of one (1) was assigned for those isolates that grew at pH 5 or 13. An isolate that grew above 10% NaCl was also given a score of one (1).
A total of 65 thermotolerant bacterial isolates were isolated from five hot springs in Eritrea. Thermotolerant bacteria were present in all samples analyzed. The cfu counts per ml ranged from 5.4 × 103 in Akwar to 1.4 × 105 cfu/ml in Elegedi. Relatively higher cfu counts were retrieved from Elegedi, a boiling hot spring than the other hot springs. The total counts in this study were higher than 50-5000 cfu/ml recorded in Morrocan hot springs (Aanniz et al., 2015)and 170-1330 cfu/ml recorded in the geothermal springs in Saudi Arabia (Khiyami et al.,
2012).
The sequences for 24 isolates were without ambiguities. All the 24 isolated microbes belonged to the domain bacteria, phylum Firmicutes, class Bacilli, order Bacillales, within two different families: Bacillaceae and Paenibacillaceae. Among these were; B. sonorensis, B. licheniformis, B. aerius and B. borstelensis. Bacilli were previously isolated from hot springs in Saudi Arabia using the same culture medium (Khalil, 2011). This indicates that the enrichment culture medium used in this study is suitable for cultivation of the bacilli group. The other reason, which cannot be ruled out, is that Bacillus species are spore-forming ubiquitous bacteria in thermal hot springs. They were observed to be the predominant groups isolated from hot springs in Indonesia using other enrichment medium such as spring water enriched with nutrient broth (Yohandini, 2015). Bacillus have also been isolated using nutrient broth supplemented with 1% Tween or Olive oil from geothermal springs in Armenia (Shahinyan et al., 2015), Castenholz TYE medium from hot springs in India (Verma et al., 2014), Tryptone Soy Agar (TSY) from hot springs in Morocco (Aanniz et al., 2015)and nutrient agar from hot springs in Fiji (Narayan et al., 2008). The genus Bacillus and related genera are reported to be widely distributed in nature. It includes thermophilic, psychrophilic, acidophilic, alkaliphilic and halophilic bacteria that utilize a wide range of carbon sources for heterotrophic growth or are the autotrophs (Panda et al., 2014). In the present study, the isolates were recovered from five hot springs in Eritrea with different temperature regimes (49 - 100°C), as well as different sodium concentration levels ranging from 0.06 to 3640 mg/lt at near neutral pH.
The sequences of the 24 isolates were shown to form two clusters on the phylogenetic tree. One cluster included the genus Bacillus and nine other isolates. Included in the other cluster are isolates represented in the genus Brevibacillus and the 14 other isolates. All the 14 isolates showed similarity to B. borstelensis species. Brevibacillus and Bacillus are known to co-inhabit in diverse environments including rocks, dust, aquatic environments, guts of various insects and animals (Nicholson, 2002).
The thermotolerant bacteria isolated from the Eritrean hot springs, in the present study, were shown to produce hydrolytic enzymes such as amylases, cellulases, proteases, pectinases and xylanases at 55°C. Plate assays revealed that 19 of the isolates were amylase producers and 45 were protease producers. The increased stability of thermophilic enzymes at high temperature, chemical denaturants and pH changes makes them suitable for harsh industrial processes. Higher reaction rates and process yields of enzymatic reactions are achieved at high temperatures because of the decrease in viscosity, the increase in the diffusion coefficient of substrates as well as an increase in the solubility of substrates and products (Haki and Rakshit, 2003). Screening of microorganisms for amylase production allows for the discovery of novel amylases required for specific industrial applications. Thermostability is the most important property of an effective amylase, because liquefaction and saccharification of starch are performed at high temperature. In the present study, out of 65 isolates from the hot springs in Eritrea, 19 were positive for amylase production. Among the amylase positive isolates, seven were affiliated to Bacillus based on their partial 16S rRNA gene sequence similarity and three to Brevibacillus. Isolates E5, G8 and M13 which tested positive for amylase activity possessed thermostability and halotolerability. E5 was also able to grow at slightly acidic levels (pH 5). The three of them were also observed to thrive at pH 11. This suggests that the enzymes obtained from these isolates are promising in their application in starch, detergent and textile industries.
Proteases have a long history of application in the food and detergent industries. They are also used in the leather industry for dehairing and bating of hides as a substitute for toxic chemicals. The use of the Bacillus species for protease production offers several advantageous like significant activity, stability, substrate specificity, short period of fermentation, mere downstream purification and low cost production (Aqel et al., 2012). In the present study, skimmed milk as well as casein were used as substrates to assess protease activity of the isolates. Eight of the isolates in the present study showed positive protease activity in media supplemented with skimmed milk as a substrate while 45 isolates showed positive activity using casein. The five possible novel isolates exhibited protease activity in medium supplemented with casein as a substrate.
Xylanase and pectinase screening of the isolates was also observed using xylan and pectin as a carbon source. Ten isolates were xylanase positive while those that showed positive pectinase activity were eleven. Isolates E5, G8, M9 and M13 belonging to the genus Bacillus were among those that showed positive xylanase and pectinase activities. E5 was shown to grow at a temperature of 60°C and slightly acidic pH of 5. Therefore, its xylanase and pectinase may have potential uses in industries such as detergent, food, pharmaceutical, leather, agriculture, kraft pulp prebleaching process and molecular biology reagents.
Physiological and biochemical characterization of the five isolates revealed some differences from strains in the Genebank and EZ-taxon databases. Isolate E5 was shown to grow at a maximum temperature of 60°C and 5-11 pH range, while B. sonorensis DSM 13779 which had shown 98% BLAST similarity with E5 did not show growth above 45°C (Shivaji et al., 2006). Notably, E5 isolated from the boiling hot spring with a temperature of 100°C did not grow in cultures above 60°C. Bacillus spp. are known to form spores and become dormant during extreme environmental conditions. This could explain why E5 did not grow above 60°C while it was isolated from the boiling hot spring. The other four isolates (G2, G8, M1 and M13), like E5, were shown to grow at 60°C. Isolate E5 was able to grow in the presence of 13% NaCl while B. sonorensis DSM 13779 was not able to grow above 10% NaCl (Shivaji et al., 2006). Isolates G2 and M1 showed moderate similarities (97.2 and 98.1, respectively) with strain B. borstelensis LMG. The maximum growth temperature (60°C) of the isolates G2 and M1 was 10°C higher than the B. borstelensis LMG 16009 (Allan et al., 2005). The isolates G2 and M1, unlike B. borstelensis LMG 16009, were shown to produce acid from mannitol. These observations could indicate the potential that these isolates could be novel.
To the best of our knowledge, the present study investigated for the first time thermotolerant bacteria that produce enzymes from five hot springs in Eritrea using culture methods. Most of the isolates were thermotolerant and showed positive hydrolase activities. The sequences of the 24 isolates showed similarity with Bacillus and Brevibacillus from the phylum Firmicutes. Five isolates (E5, G2, G8, M1 and M13) showed moderate similarities with strains in Genebank and EZ-taxon databases. Moreover, the physiological and biochemical behavior of these isolates was not similar to the strains of the same species. When the five isolates were ranked based on hydrolase activities, tolerance to high temperature, acidic or alkaline pH levels and high NaCl concentrations, E5 was observed to be the most effective followed by G8, G2, M1 and M13. Taken together, the 16S rDNA data, physiological and biochemical characteristics provide possible evidence for the novel nature of the five isolates.
The authors have not declared any conflict of interests.
REFERENCES
|
Aanniz T, Ouadghiri M, Melloul M, Swings J, Elfahime E, Ibijbijen J, Ismaili M, Amar M (2015). Thermophilic bacteria in Moroccan hot springs, salt marshes and desert soils. Brazilian Journal of Microbiology 46(2):443-53.
Crossref
|
|
|
|
Abdel-rahman MA, El-din MN, Refaat BM, Abdel-shakour EH, Ewais EE, Alrefaey HMA (2016). Biotechnological application of thermotolerant cellulose-decomposing bacteria in composting of rice straw. Annals of Agricultural Sciences 61(1):135-143.
Crossref
|
|
|
|
|
Adhikari H, Ghimire S, Khatri B, Yuvraj KC (2015). Enzymatic screening and molecular characterization of thermophilic bacterial strains isolated from hotspring of Tatopani, Bhurung, Nepal. International Journal of Applied Sciences and Biotechnology 3(3):392-397.
Crossref
|
|
|
|
|
Allan RN, Lebbe L, Heyrman J, De Vos P, Buchanan CJ, Logan NA (2005). Brevibacillus levickii sp. nov. and Aneurinibacillus terranovensis sp. nov., two novel thermoacidophiles isolated from geothermal soils of northern Victoria Land, Antarctica. International Journal of Systematic and Evolutionary Microbiology 55:1039-1050.
Crossref
|
|
|
|
|
Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ (1990). Basic local alignment search tool. Journal of Molecular Biology 215:403-410.
Crossref
|
|
|
|
|
Angelaccio S (2013). Extremophilic SHMTs: From structure to biotechnology. BioMed Research International 2013:851428.
Crossref
|
|
|
|
|
Aqel H, Al-quadan F, Yousef TK (2012). A novel neutral protease from thermophilic Bacillus strain HUTBS62. Journal of BioScience and Biotechnology 1(2):117-123.
|
|
|
|
|
Archna S, Priyank V, Nath YA, Kumar SA (2015). Bioprospecting for extracellular hydrolytic enzymes from culturable thermotolerant bacteria isolated from Manikaran thermal springs. Research Journal of Biotechnology 10(4):33-42.
|
|
|
|
|
Bozoglu C, Hundur S, Alaylar B, Karadayi M, Gulluce M (2015). Isolation and molecular characterization of thermophilic bacteria with xylanase activity from thermal springs in Erzurum. Journal of Life Sciences and Technologies 3(1):32-35.
Crossref
|
|
|
|
|
Chandrashekhar SH, Narayan G (2015). Thermostable alkaline protease from Bacillus sp. and its potential applications. IOSR International Journal of Pharmacy and Biological Sciences 10(5):58-56.
|
|
|
|
|
Coker JA (2016). Extremophiles and biotechnology: current uses and prospects. F1000Research 5:396.
Crossref
|
|
|
|
|
Dhiman SS, Sharma J, Batan B (2008). Pretreatment processing of fabric by alkalothermophilic xylanase from Bacillus stearothermophilus SDX. Enzyme and Microbial Technology 43:262-269.
Crossref
|
|
|
|
|
Dudhgara PR, Sunil B, Anjana G (2015). Hide dehairing and laundry detergent compatibility testing of thermostable and solvents tolerant alkaline protease from hot spring isolate Bacillus cohnii U3. Online Journal of Biological Sciences 15(3):152-161.
Crossref
|
|
|
|
|
Gessesse A, Gashe BA (1997). Production of alkaline xylanase by an alkaliphilic Bacillus sp. isolated from an alkalinesoda lake. Journal of Applied Microbiology 83:402-406.
Crossref
|
|
|
|
|
Gwinn DD, Thorne CB (1964). Transformation of Bacillus licheniformis. Journal of Bacteriology 87(3):519-526.
|
|
|
|
|
Haki GD, Rakshit SK (2003). Developments in industrially important thermostable enzymes: a review. Bioresource Technology 89:17-34.
Crossref
|
|
|
|
|
Hardiman E, Gibbs M, Reeves R, Bergquist P (2010). Directed evolution of a thermophilic beta-glucosidase for cellulosic bioethanol production. Applied Biochemistry and Biotechnology 161:302-312.
Crossref
|
|
|
|
|
Heuer H, Krsek M, Baker P, Smalla K, Wellington EMH (1997). Analysis of actinomycete communities by specific amplification of genes encoding 16S rRNA and gel-electrophoretic separation in denaturing gradients. Applied and Environmental Microbiology 63(8):3233-3241.
|
|
|
|
|
Huang S, Sheng P and Zhang H (2012). Isolation and identification of cellulolytic bacteria from the gut of Holotrichia parallela larvae (Coleoptera: Scarabaeidae). International Journal of Molecular Sciences 13:2563-2577.
Crossref
|
|
|
|
|
Jaouadi NZ, Rekik H, Badis A, Trabelsi S, Belhoul M, Yahiaoui AB, Aicha H Ben, Toumi A, Bejar S, Jaouadi B (2013). Biochemical and molecular characterization of a serine keratinase from Brevibacillus brevis US575 with promising keratin-biodegradation and hide-dehairing activities. PLoS One 8(10):e76722.
Crossref
|
|
|
|
|
Khalil A (2011). Isolation and characterization of three thermophilic bacterial strains (lipase, cellulose and amylase producers) from hot springs in Saudi Arabia. African Journal of Biotechnology 10(44):8834-8839.
Crossref
|
|
|
|
|
Khiyami MA, Serour EA, Shehata MM, Bahklia AH (2012). Thermo-aerobic bacteria from geothermal springs in Saudi Arabia. African Journal of Biotechnology 11(16):4053-4062.
|
|
|
|
|
Kumar S, Stecher G, Tamura K (2016). MEGA7: Molecular Evolutionary Genetics Analysis version 7.0 for bigger datasets. Molecular Biology and Evolution 33(7):1870-1874.
Crossref
|
|
|
|
|
Lele OH, Deshmukh P V (2016). Isolation and characterization of thermophilic Bacillus sp. with extracellular enzymatic activities from hot springs of Ganeshpuri, Maharashtra, India. International Journal of Applied Research and Technology 2(5):427-430.
|
|
|
|
|
Meyer H-P, Eichhorn E, Hanlon S, Lütz S, Schürmann M, Wohlgemuth R, Coppolecchia R (2012). The use of enzymes in organic synthesis and the life sciences: perspectives from the Swiss Industrial Biocatalysis Consortium (SIBC). Catalysis Science and Technology 3(1):29-40.
Crossref
|
|
|
|
|
Moyes RB, Reynolds J, Breakwell DP (2009). Differential staining of bacteria. Current Protocols in Microbiology 15(1): A.3C.1-A.3C.8.
|
|
|
|
|
Nakamura LK, Swezey J (2017). Taxonomy of Bacillus circulans Jordan 1890: base composition and reassociation of deoxyribonucleic acid. International Journal of Systematic Bacteriology 33(1):46-52.
Crossref
|
|
|
|
|
Narayan V, Hatha MM, Morgan HW, Rao D (2008). Isolation and characterization of aerobic thermophilic bacteria from the Savusavu hot springs in Fiji. Microbes and Environments 23(4):350-352.
Crossref
|
|
|
|
|
Nicholson WL (2002). Roles of Bacillus endospores in the environment. Cellular and Molecular Life Sciences 59:410-416.
Crossref
|
|
|
|
|
Palmisano MM, Nakamura LK, Duncan KE, Istock CA, Cohan FM (2001). Bacillus sonorensis sp. nov., a close relative of Bacillus licheniformis, isolated from soil in the Sonoran Desert, Arizona. International Journal of Systematic and Evolutionary Microbiology 51:1671-1679.
Crossref
|
|
|
|
|
Panda AK, Bisht SS, DeMondal S, Senthil Kumar N, Gurusubramanian G, Panigrahi AK (2014). Brevibacillus as a biological tool: a short review. Antonie Van Leeuwenhoek 105:623-639.
Crossref
|
|
|
|
|
Panda MK, Sahu MK, Tayung K (2013). Isolation and characterization of a thermophilic Bacillus sp. with protease activity isolated from hot spring of Tarabalo, Odisha, India. Iranian Journal of Microbiology 5(2):159-165.
|
|
|
|
|
Rasooli I, Astaneh SDA, Borna H, Barchini KA (2008). A thermostable α-Amylase producing natural variant of Bacillus spp. isolated from soil in Iran. American Journal of Agricultural and Biological Sciences 3(3):591-596.
Crossref
|
|
|
|
|
Remigio Z, William M, Olle H, Wilson P (2012). Isolation and characterization of a protease-producing thermophilic bacterium from an African hot spring. African Journal of Biotechnology 11(62):12571-12578.
|
|
|
|
|
Saitou N, Nei M (1987). The neighbor-joining method: a new method for reconstructing Phylogenetic trees. Molecular Biology and Evolution 4(4):406-425.
|
|
|
|
|
Sambrok J, Russell D (2001). Molecular cloning: A laboratory manual. Third. (J Argentine, N Irwin, KA Janssen, S Curtis, M Zierler, N Mclnerny, D Brown and S Schaefer, Editors). New York: Cold Spring Harbor Laboratory Press. 2100 p.
|
|
|
|
|
Sanger F, Nicklen S, Coulson AR (1977). DNA sequencing with chain-terminating inhibitors. Proceedings of the National Academy of Sciences of the United States of America 74(12):5463-5467.
Crossref
|
|
|
|
|
Shahinyan GS, Margaryan AA, Panosyan HH, Trchounian AH (2015). Isolation and characterization of lipase-producing thermophilic bacilli from geothermal springs in Armenia and Nagorno-Karabakh. Biological Journal of Armenia 2(67):6-15.
|
|
|
|
|
Shida O, Takagi H, Kadowaki K and Komagata K (1996). Proposal for two new genera, Brevibacillus gen. nov. and Aneurinibacillus gen. nov. International Journal of Systematic Bacteriology 46(4):939-946.
Crossref
|
|
|
|
|
Shivaji S, Chaturvedi P, Suresh K, Reddy GSN, Dutt CBS, Wainwright M, Narlikar JV, Bhargava PM (2006). Bacillus aerius sp. nov., Bacillus aerophilus sp. nov., Bacillus stratosphericus sp. nov. and Bacillus altitudinis sp. nov., isolated from cryogenic tubes used for collecting air samples from high altitudes. International Journal of Systematic and Evolutionary Microbiology 56(2006):1465-1473.
Crossref
|
|
|
|
|
Singh G, Bhalla A, Ralhan PK (2011). Extremophiles and extremozymes: importance in current biotechnology. International Journal of the Bioflux Society 3(1):46-54.
|
|
|
|
|
Sneath PHA, Mair NS, Sharpe ME, Holt JG (1986). Bergey's Manual of Systematic Bacteriology. Baltimore, USA: Williams and Wilkins. 2:965-1599.
|
|
|
|
|
Srinivasan MC, Rele M (1999). Microbial xylanases for paper industry. Current Science 77(1):137-142.
|
|
|
|
|
Teather RM, Wood PJ (1982). Use of Congo red-polysaccharide interactions in enumeration and characterization of cellulolytic bacteria from the bovine rumen. Applied and Environmental Microbiology 43(4):777-780.
|
|
|
|
|
Verma A, Gupta M, Shirkot P (2014). Isolation and characterization of thermophilic bacteria in natural hot water springs of Himachal Pradesh (India). The Bioscan 9(3):947-952.
|
|
|
|
|
Verma P, Yadav AN, Shukla L, Saxena AK, Suman A (2015). Hydrolytic enzymes production by thermotolerant Bacillus altitudinis IARI-MB-9 and Gulbenkiania mobilis IARI-MB-18 isolated from Manikaran hot springs. International Journal of Advanced Research 3(9):1241-1250.
|
|
|
|
|
Yohandini H (2015). Isolation and phylogenetic analysis of thermophile community within Tanjung Sakti hot spring, South Sumatera, Indonesia. HAYATI Journal of Biosciences 22:143-148.
Crossref
|
|
|
|
|
Yohannes E (2010). Geothermal exploration in Eritrea. Explor Geotherm Resouces, Oct 29-November 19, 2010, Kenya. 16 pp.
|
|
|
|
|
Zilda DS, Harmayani E, Widada J, Asmara W, Irianto HE, Patantis G, Fawya YN (2012). Screening of thermostable protease producning microrganisms isolated from Indonesian hotspring. Squalen 7(3):105-114.
|
|