Full Length Research Paper
ABSTRACT
In this experiment, the action mechanism of the gene Ssc1 was studied in the process of interaction with plants through heterologous expression, subcellular localization and fluorescent PCR technology. It was found that the gene Ssc1 could enhance the pathogenicity of Botrytis cinerea through heterologous expression. Fusing the promoter, SP and CTP of Ssc1 with GFP and expressing in tobacco, it was found that the fusion protein could be secreted into plant cells and located in chloroplasts. Trypan blue staining and fluorescence detection found that in the early stage after inoculation and in the areas outside of the scab, GFP fluorescence could be detected in the tobacco leaves despite the trypan blue staining being negative. Additionally, it was proved by quantitative PCR that the gene Ssc1 was highly expressed in the early infection stages. Taken together, these results indicated that the effector Ssc1 was an important pathogenic factor, which could locate in chloroplasts and mainly play the role in the early stage during the interaction between S. sclerotiorum and plants.
Key words: Sclerotinia sclerotiorum; effector; chorismate mutase; heterologous expression; subcellular localization.
INTRODUCTION
Sclerotinia sclerotiorum (Lib. de Bary) is an important necrotrophic phytopathogenic fungus with a wide host range of more than 400 plants, including rape, sunflower, legumes, Cucurbitaceae and Solanaceae (Boland and Hall, 1994; Derbyshire, 2022), with infection mechanism similar to that of Botrytis cinerea (Pers.) (Amselem et al., 2011). S. sclerotiorum is intensely destructive to its host and oxalic acid and cell wall-degrading enzymes play important roles in its infection process (Bateman and Beer, 1965). In addition to cell wall-degrading enzymes, oxalic acid and other traditional pathogenic factors, researchers have found that secretory effector play an important role in the interaction of S. sclerotiorum with plants (Hancock, 1966; Riou et al., 1991; Kim et al., 2011). For example, Guyon et al. (2014) identified 78 potential effector candidates by secretome analysis and analyzed the expression patterns of 16 secretory proteins in different plants, Lyu et al. (2016) demonstrated that a small molecular effector protein, SsSSVP1, could be beneficial to self-infection by affecting plant energy metabolism. Wang Xin-yu et al. (2009) found that the effector protein Sspg1 could interact with IPG-1, a small protein in plants, playing an important role in early pathogenic stage. Zhu et al. (2013) found that SSITL, a secretory effector protein, could inhibit the disease resistance mediated by jasmonic acid in the early stages of infection. Fan et al. (2021) Found that a novel effector protein SsERP1 inhibits plant ethylene signaling to promote Sclerotinia sclerotiorum infection.
Chorismate mutase catalyzes the conversion of chorismate to prephenic acid, providing precursors for the synthesis of phenylalanine and tyrosine (Andrew, 2003). Although chorismate mutase is ubiquitous in microorganisms and plants and is required for the synthesis of some essential amino acids, not all plant pathogenic microorganisms have this enzyme (Romero et al., 1995). Moreover, the Cmu1 gene, which is homologous to chorismate mutase, has been shown to be closely related to pathogenicity in the biotrophic pathogenic fungi Ustilago maydis. Cmu1 was also found to influence the salicylic acid (SA) level and then weaken the disease resistance signal (Armin et al., 2011). A novel chorismate mutase from Erysiphe quercicola performs dual functions of synthesizing amino acids and inhibiting plant salicylic acid synthesis (He et al., 2021). For nematodes, in addition to providing nutritional needs, CM also provides some help for its parasitic life (Lander et al., 2020).
Previous studies (Dickman Marty Lab.) discovered that the effector Ssc1, which is homologous to chorismate mutase, is related to the pathogenicity of S. sclerotiorum through deletion mutation (unpublished), but the mechanism is not clear.
Thus, in order to further explore the action mechanism of the gene Ssc1, the secretory, subcellular localization, and specific expression at different infection stages were analyzed in this experiments. The results showed that the gene Ssc1 could enhance the pathogenicity of B. cinerea through heterologous expression. Also it was found that Ssc1 proteins could be secreted into the host cells and colocalized with the chloroplast. Finally, through GFP fluorescence detection, trypan blue staining and quantitative PCR, it was proved that the gene Ssc1 were highly expressed in the early infection stages. All these indicated that the gene Ssc1 was an important pathogenicity factor and mainly play the role in the early stage during the interaction between S. sclerotiorum and plants.
MATERIALS AND METHODS
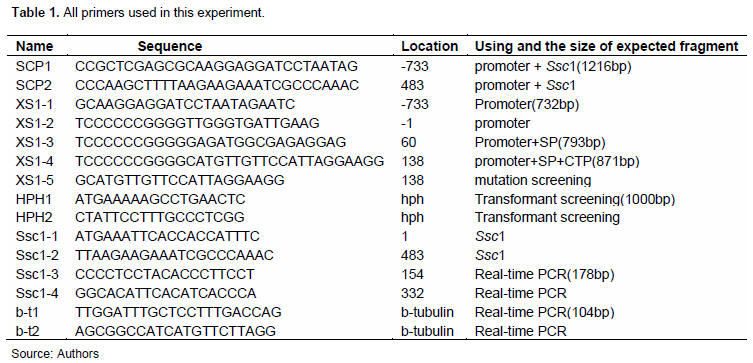
Materials and primers
S. sclerotiorum 1980, B. cinerea T4 and the vector pCX62, pBluntNAT-GFP1-1 were obtained from Prof. Dickman Marty of Texas A&M University. The Escherichia coli derivative DH5α was used for cloning purposes. S. sclerotiorum 1980 and B. cinerea T4 were routinely cultured on potato dextrose agar (PDA) at 25°C. All chemicals used were of analytical grade. All the primers used in this experiment are listed in Table 1.
Molecular techniques and sequence analysis of the Ssc1 gene
Fungal RNA and DNA were extracted using the TRIzol or cetyl trimethylammonium bromide (CTAB) protocol. The plasmid DNA was isolated using the plasmid kit (OMIGA) according to the protocol. Phylogenetic tree generation, and DNA and protein sequence alignment and analysis were conducted using DNAman7.0 software (Lynnon Biosoft). Primers were designed using Primer Premier 5.0 (Premier). Fungi were transformed using the method of restriction enzyme-mediated integration (REMI) based on the same method described previously (Zhao et al., 2010; 2011).
Cloning of the Ssc1 gene and heterologous expression in B. cinerea
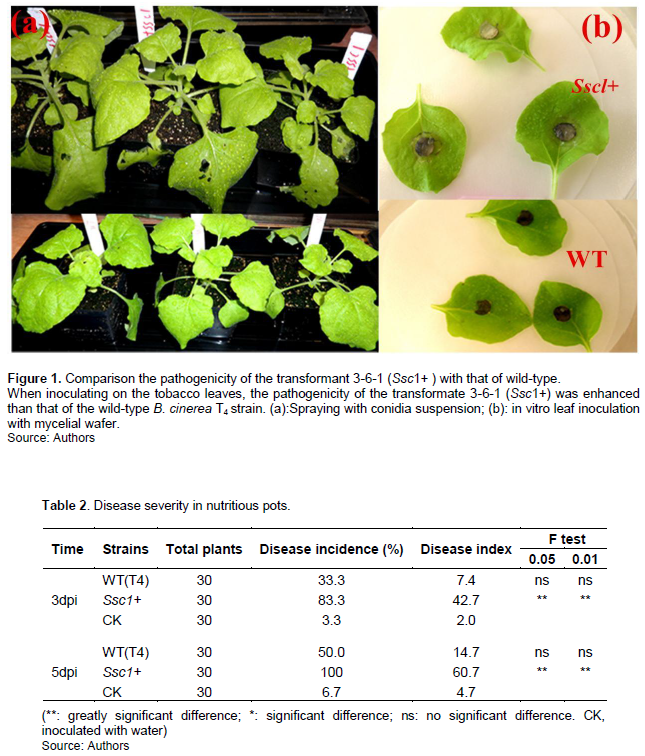
The DNA fragment of the Ssc1 gene and its promoter was cloned using the primers SCP1 and SCP2, after which the expression vector pCX62-Ssc1 was constructed by inserting the fragment into pCX62. They were then digested by Xho I and Hind III, respectively, and then transformed into the protoplast of B. cinerea T4 strains as mentioned above (Zhao et al., 2010; 2011). Transformants were selected on PDA plates using hygromycin B at 250 ug/mL, and then the integration of gene Ssc1 into the genome of B. cinerea and its normal expression were confirmed by PCR and RT-qPCR, respectively. Finally, the transformants were inoculated onto tobacco using the methods of in vitro leaf inoculation with mycelial wafer and spraying with conidial suspension as described previously (Zhao et al., 2018; Xu et al., 2011). The culture dishes and nutrient plates were placed in a 25°C, 85% relative humidity, 14 h light /10 h dark cycle phytotron. The lesions were studied from 12 h, and the disease severity was determined by calculating the disease index. The disease grades were as follows: grade 0: no symptoms; grade 1: small infection spots identified on 1–2 leaves; grade 2: small infection spots identified on 3–5 leaves; grade 3: 1–2 leaves began rotting; grade 4: 3–4 leaves began to rot; grade 5: the whole plant has begun to rot.
Cloning and confirming the promoter of Ssc1
The promoter element and the cis element were analyzed using the online software Promoter 5.0. The DNA sequence of the promoter was cloned by PCR using the XS1-1 and XS1-2 primers, after which the GFP fusion vector was constructed and transformed in the protoplast of S. sclerotiorum. The GFP fluorescence in the hyphae of the transformant was detected by confocal fluorescence microscope.
Analysis of the secretory and subcellular localization of the effector
The signal peptide and localization peptide were predicted using online bioinformatics software, including the SignalP 4.1 Server, PredictNLS and ChloroP 1.1 Server. The fragments containing the promoter+signal peptide (SP) and the promoter+signal peptide (SP) +localization peptide (CTP) were then cloned by PCR using the primer pairs XS1-1/XS1-3 and XS1-1/XS1-4, respectively. Nextly, the GFP fusion vector was constructed and transformed into the protoplast of S. sclerotiorum as mentioned above. When inoculating tobacco with the transformant, the fusion protein localization was detected through confocal fluorescence microscope.
Detecting the secretory time and tissue specificity of the Ssc1 effector through trypan blue staining
At different times after inoculation with the transformant, in which the GFP fusion protein could be expressed, the leaves of tobacco were dyed using trypan blue and then analyzed by confocal fluorescence microscope.
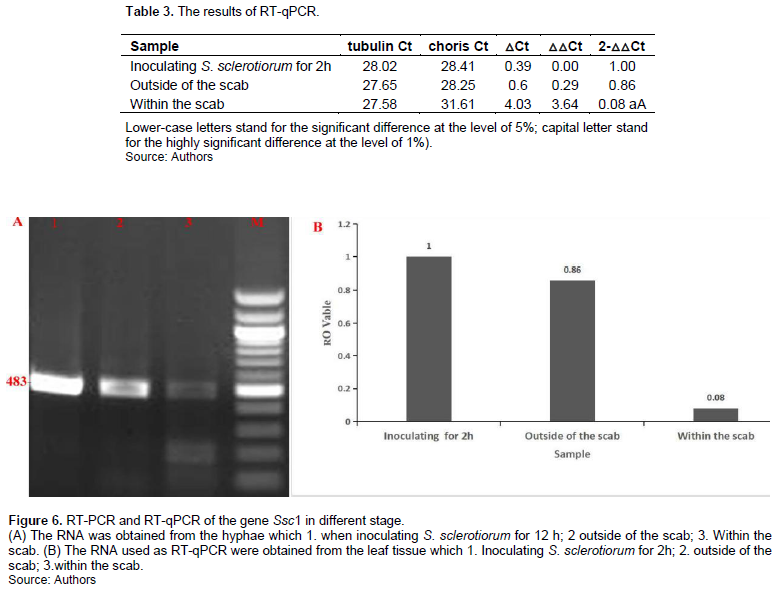
Detection of transcriptional differences of Ssc1 gene at different stages and locations
Following inoculation on tobacco with the wild type of S. sclerotiorum, the mycelia were collected from the areas of the early stage of inoculation site, within the scab and outside the scab, after which RT-PCR of the Ssc1 gene was conducted using the primer set Ssc1-1/Ssc1-2. Additionally, RT-qPCR of the gene Ssc1 was performed according to the manufacturer’s suggestions, primers were designed according to the sequence of the gene (Table1), and tubulin was used as reference gene for fluorescent qPCR.
RESULTS
The gene Ssc1 could heterologously enhance the pathogenicity of B. cinerea
A 1216 bp DNA fragment containing the Ssc1 gene and its promoter was obtained (Supplementary data 1) by PCR using the primers SCP1 and SCP2 (Table1). The fragment was inserted into the vector pCX62 and transformed into the protoplast of the pathogenic fungus B. cinerea T4 strains. The gene was confirmed to have been integrated into the genome of B. cinerea and could be expressed normally by PCR and RT-PCR (Supplementary 2). The pathogenicity of the transformant 3-6-1 was enhanced comparing with that of the wild type following inoculation of tobacco leaves (Figure 1 and Table 2).
The promoter of Ssc1 was obtained
The promoter element was analyzed using bioinformatics software, which revealed the presence of a TATA box and a CAAT box in the upstream portion of the gene. The promoter was predicted as shown below.
Promoter predictions for seq 0–2000 bp:
Start End Score
1888 1938 0.98
Promoter Sequence (transcription start shown in bold):
CTCCTTTCTGGTGGCTGTCATATAAGTACGCTCCCAACCTCAATGTTCAA
A 733-bp DNA fragment upstream of ATG was cloned by PCR using the XS1-1 and XS1-2 primers (the sequence is shown in Supplementary 2), after which the GFP fusion vector was constructed based on the pBluntNAT-GFP plasmid according to the roadmap (Supplementary 3) and transformed into the protoplast of S. sclerotiorum. Some transformants in which the GFP fluorescence was detected through confocal fluorescence microscopy were then obtained (Supplementary 4). Taken together, these findings indicated that the 733 bp DNA fragment upstream of the ATG codon could act as the promoter.
The effector could be secreted and colocalized with the chloroplasts of tobacco
Online bioinformatics software revealed a predicted signal peptide (SP) with 20 amino acids and a chloroplast target peptide (CTP) with 26 amino acids in the gene sequence of Ssc1 (Supplementary 5).
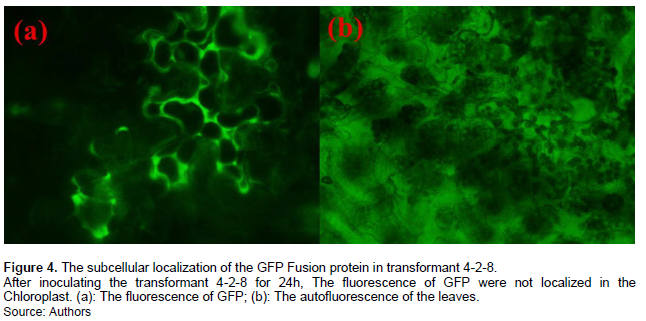
Two PCR products that contained promoter+SP and promoter+SP+CTP were cloned by PCR using the primer pairs XS1-1/XS1-3 and XS1-1/XS1-4, respectively. The GFP fusion vector was then constructed according to the roadmap (Supplementary 3), after which it was transformed into the protoplast of S. sclerotiorum as mentioned above. A few transformants were obtained, and the recombinant vector was confirmed to have been integrated into the genome by PCR using the primers of HPH1 and HPH2 by PCR in the transformant 3-7-5 and transformant 4-2-8 (Supplementary 6).
Finally, inoculation on the tobacco leaves with transformant 3-7-5, in which GFP was fused with promoter+SP+CTP, revealed that the GFP fusion protein could be expressed, secreted into the host cell, and localized in the chloroplast (Figures 2 to 3). However, the GFP could not be co-localized with the chloroplast when the transformant 4-2-8 was inoculated on tobacco, as the GFP fusion protein had no chloroplast target peptide (Figure 4).

The Ssc1 protein were secreted in plant cells in the early infection stage
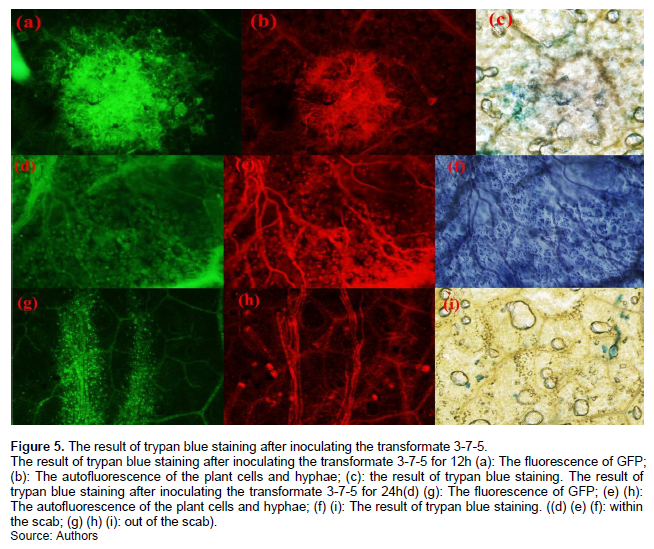
Following inoculation on tobacco with transformant 3-7-5 for 12 h, GFP fluorescence was detected in the leaves by confocal fluorescence microscopy, despite no scab emerging on the leaf and negative trypan blue staining results (Figure 5). After inoculation for 24 h, a scab began to form, and the leaf tissues of the tobacco and pathogenic hyphae within the scab could be stained by trypan blue, which indicated that the plant cells had died. On the contrary, in the areas outside the scab, although the trypan blue staining of tobacco tissue and the fungal hyphae was negative, GFP fluorescence could be detected (Figure 5). In combination, these findings indicate that although the plant cells were alive in the early infection stage, the fungi had begun to expand; also the effector had begun to be secreted into the plant cells.
The gene Ssc1 was highly expressed in the early infection stage
Analysis by RT-PCR and RT-qPCR revealed that the gene Ssc1 was highly expressed in the early infection stage and in the areas outside the scab, but its expression was relatively low within the scab, in which plant cells have died (Table 3 and Figure 6).
DISCUSSION
In this study, the gene Ssc1 homologous to chorismate mutase in S. sclerotiorum was proved to be closely associated with pathogenicity through heterologous expression in B. cinerea. The gene encodes a small protein, which could be secreted into plant cells and localized in the chloroplast, playing a role in the early stages of infection.
The shikimate pathway is a fundamental metabolic pathway in plants and microorganisms. The final product of this pathway is chorismate, which is the precursor of many important compounds, including aromatic amino acids (phenylalanine, tryptophan, and tyrosine), salicylic acid (SA), indole-3-acetic acid (IAA) and other secondary metabolites (Strack, 1997). These chorismate-derived compounds (CDCs) play important roles in plant growth, development, defense, and interaction with other organisms (Romero et al., 1995).
Chorismate mutase catalyzes the conversion of chorismate to pre-phenylic acid, providing precursors for the synthesis of phenylalanine and tyrosine (Andrew, 2003). For parasitic pathogenic microorganisms, phenylalanine and tyrosine can be obtained from the host, and thus chorismate mutases may not be necessary for their amino acid metabolism.
However, BLAST searches of the NCBI database revealed that only some pathogenic microorganisms have genes homologous to chorismate mutases, such as nematodes, U. maydis, Erysiphe quercicola, etc., which have been proved to play a role in the interaction of plant pathogens and plants (Armin et al., 2001; He et al., 2021; Lander et al., 2020).
Armin et al. (2011) found that the Cmu1 gene homologous to chorismate mutase was closely related to pathogenicity through affecting the SA level and the resistance signal. It was usually considered that the SA pathway typically plays an important role in the defense against biotrophic pathogens but does not play a major role in resistance to necrotrophic pathogens in plants (Strack, 1997; Govrin and Levine, 2000; Yang et al., 2015). Unlike U. maydis, S. sclerotiorum is a necrotrophic fungus, as for how this effector play a role in necrotrophic fungus in the process of infection the plant is an interesting question.
Previous studies by the Dickman Lab (unpublished) on the deletion mutation of Ssc1 indicated that the Ssc1 effector was related to pathogenicity. In this study, this gene was found to enhance the pathogenicity of the pathogen B. cinerea through heterologous expression. These findings suggested that the gene is a pathogenic factor rather than a gene necessary for amino acid metabolism. Both B. cinerea and S. sclerotiorum are necrotrophic pathogens with similar pathogenic mechanisms. Thus, further investigation is needed to determine why only S. sclerotiorum retains this important factor related to the interaction between fungi and plants.
In this experiment, within 12 h after inoculation of the transformants 3-7-5 onto tobacco, the mycelia and plant cells could not be stained by trypan blue. Negative trypan blue staining indicated that the plant cells were alive during the early stages of infection. However, GFP fluorescence could be detected in the tobacco leaves despite the trypan blue staining being negative. Through fluorescence detecting, it could be found that the pathogenic hypha had begun to expand near the inoculation point before trypan blue staining positive. It was reported that the necrotrophic pathogens may have a semi-living biotrophic stage, in which stage S. sclerotiorum expanded into the plant tissue through its adaptation to the plant environment and inhibition of host defense (Mehdi et al., 2015). Effectors produced by the fungus may help its own growth and inhibit host resistance during this semi-living biotrophic stage. It was speculated that the Ssc1 effector may influence the SA level and resistance signal by affecting the metabolic pathway of chorismate during this stage, similar to that of the Cmu1 gene, and then the mycelium grew and expanded as proposed in Figure 7.
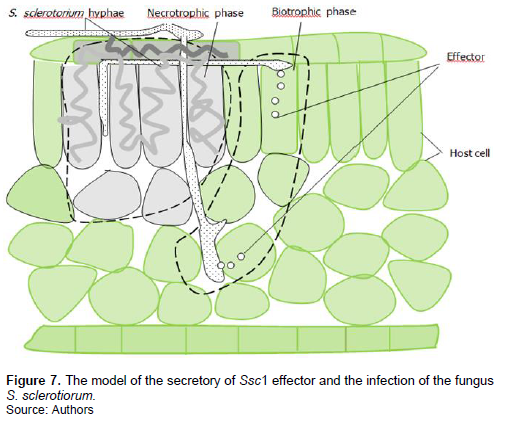
At 24 h after inoculation with the transformant 3-7-5, scabs began to form, and the trypan blue staining of plant cells and pathogenic hypha within the lesion range was positive, indicating that the cells had died. During this stage, GFP fluorescence was obviously co localized with the chloroplasts of the tobacco, indicating that effector proteins accumulated in chloroplasts (Figure 3). Previous research showed that the response of plants to various stress factors first occurs in the membrane system (Renu et al., 2014). Stress factors, including pathogenic organisms, cause metabolic disorders and accelerate the variation in the biochemical and biophysical structures of membranes, as well as initiate some secondary metabolism related to the stress response (Renu et al., 2014). The chloroplast is an organelle specialized for carrying out photosynthesis in plants, and the Calvin-Benson cycle occurring in the chloroplasts can provide chorismate precursors, such as D-erythritose-4 phosphoric acid and sedum phosphate heptaose (Wang et al., 2006). Perhaps under the pressure of pathogen infection, the chloroplast membrane structure was first disordered, and then the accumulation of some enzymes, including exogenous chorismate mutase, on the imperfect chloroplast affects the secondary metabolism related to plant defense, such as the metabolism related to salicylic acid (SA), IAA and aromatic amino acids (Baier and Dietz, 2005). However, further studies are necessary to determine why the effector protein was located in the chloroplast as well as its role in this organelle.
CONFLICT OF INTERESTS
The authors have not declared any conflict of interests.
ACKNOWLEDGEMENT
The authors would like to thank Prof. Dickman M. B. for offering the strains of S. sclerotiorum 1980 and Botrytis cinerea T4. This work was supported by Nature Science Fund of Shandong Province (ZR2020MC125?ZR2013CM006); Key R and D plan of Shandong Province (2019GNC106018); and Shandong Provincial Key Laboratory of Agricultural Microbiology Open Fund (SDKL2017015).
REFERENCES
|
Amselem J, Cuomo CA, van Kan JA, Viaud M, Benito EP, Couloux A, Coutinho PM, de Vries RP, Dyer PS, Fillinger S, Fournier E, Gout L, Hahn M, Kohn L, Lapalu N, Plummer KM, Pradier J-M, Quévillon E, Sharon A, Simon A, Have A, Tudzynski B, Tudzynski P, Wincker P, Andrew M, Anthouard V, Beever RE, Beffa R, Benoit I, Bouzid O, Brault B, Chen Z, Choquer M, Collémare J, Cotton P, Danchin EG, Silva DC, Gautier A, Giraud C, Giraud T, Gonzalez C, Grossetete S, Güldener U, Henrissat B, Howlett BJ, Kodira C, Kretschmer M, Lappartient A, Leroch M, Levis C, Mauceli E, Neuvéglise C, Oeser B, Pearson M, Poulain J, Poussereau N, Quesneville H, Rascle C, Schumacher J, Ségurens B, Sexton A, Silva E, Sirven C, Soanes DM, Talbot NJ, Templeton M, Yandava C, Yarden O, Zeng Q, Rollins JA, Lebrun M-H, Dickman M (2011). Genomic analysis of the necrotrophic fungal pathogens S. sclerotiorum and Botrytis cinerea. PLOS Genetics 7(8):1-27. |
|
|
Andrew RK (2003). The biosynthesis of shikimate metabolites. Natural Product Reports 20(1):119-36. |
|
|
Armin D, Kerstin S, Matthias M, Regine K (2011). Metabolic priming by a secreted fungal effector. Nature 478:394-398. |
|
|
Baier M, Dietz KJ (2005). Chloroplasts as source and target of cellular redox regulation: A discussion on chloroplast redox signals in the context of plant physiology. Journal of Experimental Botany 56(416):1449-1462. |
|
|
Bateman DF, Beer SV (1965). Simultaneous production and synergistic action of oxalic acid and polygalacturonase during pathogenesis by Sclerotinia rolfsii. Phytopathology 55:204-211. |
|
|
Boland GJ, Hall R (1994). Index of plant hosts of S. sclerotiorum. Canadian Journal of Plant Pathology 696(16): 93-108. |
|
|
Derbyshire MC, Newman TE, Khentr YY, Taiwo AO (2022). The evolutionary and molecular features of the broad?host?range plant pathogen Sclerotinia sclerotiorum. Molecular Plant Pathology 23(8):1075-1090. |
|
|
Fan HX, Yang WW, Nie JY, Zhang W, Wu J, Wu D, Wang Y (2021). A novel effector protein SsERP1 inhibits plant ethylene signaling to promote Sclerotinia sclerotiorum infection. Journal of Fungi 7(10):825-832. |
|
|
Govrin EM, Levine A (2000). The hypersensitive response facilitates plant infection by the necrotrophic pathogen Botrytis cinerea. Current Biology 10(13):751-757. |
|
|
Guyon K, Balagué C, Roby D, Raffaele S (2014). Secretome analysis reveals effector candidates associated with broad host range necrotrophy in the fungal plant pathogen S. sclerotiorum. BMC Genomics 15:336-354. |
|
|
Hancock JC (1966). Degradation of pectic substances associated with pathogenesis by S. sclerotiorum in sunflower and tomato stems. Phytopathology 56: 975-979. |
|
|
He QG, Liu Y, Liang P, Liao XM, Li X, Li X, Shi D, Liu W, Lin C, Zheng F, Miao (W 2021). A novel chorismate mutase from Erysiphe quercicola performs dual functions of synthesizing amino acids and inhibiting plant salicylic acid synthesis. Microbiological Research 242(9):1-12. |
|
|
Kim HJ, Chen C, Kabbage M (2011). Identification and characterization of S. sclerotiorum NADPH oxidases. Applied and Environmental Microbiology 77(21):7721-7729. |
|
|
Lander B, Tina K, Tim DM, Kris M, Boerjan W, Lefevere H, Gheysen G (2020). Chorismate mutase and isochorismatase, two potential effectors of the migratory nematode Hirschmanniella oryzae, increase host susceptibility by manipulating secondary metabolite content of rice. Molecular Plant Pathology 21(12):1634-1646. |
|
|
Lyu X, Shen C, Fu Y, Xie J, Jiang D, Li G, Cheng J (2016). A Small Secreted Virulence-Related Protein Is Essential for the Necrotrophic Interactions of S. sclerotiorum with Its Host Plants.PLoS Pathogy 12(2):1-28. |
|
|
Mehdi K, Oded Y, Dickman MB (2015). Pathogenic attributes of S. sclerotiorum Switching from a biotrophic to necrotrophic Lifestyle. Plant Science 233:53-60. |
|
|
Renu S, Yan D, Stephen HH (2014). Stress sensing in plants by an ER stress sensor/transducer, bZIP28. Plant Science 5:59-67. |
|
|
Riou C, Freyssinet G, Fevre M (1991). Production of cell wall-degrading enzymes by the phytopathogenic fungus S. sclerotiorum. Applied and Environmental Microbiology 57(5):1478-1484. |
|
|
Romero RM, Roberts MF, Phillipson JD (1995). Chorismate mutase in microorganisms and plants. Phytochemistry 40(4):1015-1025. |
|
|
Strack D (1997). Phenolic metabolism. Plant Biochemistry[M]. New York: Academic Press 387-416. |
|
|
Wang XY, Li Q, Niu XW, Chen HY, Xu LL, Qi CK (2009). Characterization of a canola C2 domain gene that interacts with PG, an effector of the necrotrophic fungus S. sclerotiorum. Journal of Experimental Botany 60(9):2613-2620. |
|
|
Wang Z, Zhu XG, Chen Y, Li Y, Hou J, Li Y, Liu L (2006). Exploring photosynthesis evolution by comparative analysis of metabolic networks between chloroplasts and photosynthetic bacteria. BMC Genomics 30(7):100-106. |
|
|
Xu HJ, Wang YZ, Zhao PB, Zhang YB, Xu RY, Li DC (2011). A cAMP-Dependent Protein Kinase Gene, aapk1, Is Required for Mycelia Growth, Toxicity and Pathogenicity of Alternaria alternata on Tobacco. Journal of Phytopathology 159(4):208-216. |
|
|
Yang YX, Ahammed GJ, Wu C (2015). Crosstalk among jasmonate, salicylate and ethylene signaling pathwaysin plant disease and immune responses. Current Protein and Peptide Science 16(5):450-461. |
|
|
Zhao PB, Ren AZ, Xu HJ, Li DC (2010). A cAMP-dependent serine-threonine Protein Kinase Gene (fpk1) in Fusarium verticillieides is required for hyphal development, cell wall con struction and Plant Infection. Journal of Microbiology and Biotechnology 20(1): 208-216. |
|
|
Zhao PB, Ren AZ, Li DC (2011). The FUS3/KSS1 -Type MAP Kinase Gene FPK1 Is Involved in Hyphal Growth, Conidiation and Plant Infection of Fusarium proliferatum. Journal of Molecular Microbiology and Biotechnology 4(8):1-10. |
|
|
Zhao PB, Ren AZ, Dong P, Sheng YS, Chang, X (2018). The antimicrobial peptaibol trichokonin IV promotes plant growth and induces systemic resistance against Botrytis cinerea infection in moth orchid. Journal of Phytopathology 166(5):346-354. |
|
|
Zhu WJ, Wei W, Fu YP, Cheng JS, Xie JT, Li GQ, Yi X, Kang Z, Dickman MB, Jiang D (2013). A Secretory Protein of Necrotrophic Fungus S. sclerotiorum that Suppresses Host Resistance. PLoS One 8(1):1-19. |
|
Copyright © 2024 Author(s) retain the copyright of this article.
This article is published under the terms of the Creative Commons Attribution License 4.0