Full Length Research Paper
ABSTRACT
Cryptosporidiosis is a diarrhoeal disease caused by Cryptosporidium species. It has become a more recognised pathogen especially in immunocompromised patients. It is an important opportunistic infection responsible for significant morbidity and mortality in Human Immunodeficiency Virus (HIV) and AIDS patients. The main aim of this study is to compare microscopy using modified Ziehl-Neelsen (MZN) with ELISA in the detection of Cryptosporidium in stool. The study was conducted at the HIV treatment clinic in Ahmadu Bello University Teaching hospital. It was a prospective and cross-sectional type. A total of 183 stool samples were collected and processed for the detection of the Cryptosporidium oocyst using modified Zeihl Neelsen stain and the antigen using ELISA. Kappa test was used which showed strong agreement between the two methods in the detection of Cryptosporidium in stool. Out of the 183 stool samples analysed, MZN stain identified 7 (3.8%) while ELISA identified 9 (4.9%) of the Cryptosporidia. The sensitivity, specificity, negative predictive value and positive predictive value of ELISA as compared to MZN for the detection of Cryptosporidium were 66.9, 99.4, 98.3 and 85.7%, respectively. This study showed that both MZN and ELISA can be used in the detection of Cryptosporidium in stool samples, even though ELISA had a higher specificity than MZN.
Key words: Cryptosporidium, ELIZA, modified Ziehl Neelsen, HIV/AIDS.
INTRODUCTION
Cryptosporidiosis is an infection caused by Cryptosporidium species which are intestinal pathogens (Saha et al., 2019a). It serves as a common cause of diarrhoea in humans and animals (Elsawey et al., 2020). Cryptosporodium associated diarrhoea has become well known as a result of its severe manifestation in acquired immunodeficiency disease syndrome (AIDS) patients (Agholi et al., 2013). It is an important opportunistic infection and is responsible for significant morbidity and mortality in Human Immunodeficiency Virus (HIV) and AIDS patients (Sarkar et al., 2014).
The clinical spectrum seen in HIV infected individuals ranges from asymptomatic infections to severe diseases that are characterized by profuse watery diarrhoea, which may be accompanied by vomiting and abdominal pain.(Nkenfou et., 2013; Petrova et al., 2017).
Effective methods to diagnose cryptosporidiosis especially among immunocompromised have been of great challenge in most laboratories in developing countries like Nigeria. Without a proper diagnostic tool, the burden of the infection and its effect on growth, malnutrition and mortality remains underappreciated. Over the years microscopy using modified Ziehl Neelsen (MZN), has been the main diagnostic method in detecting the oocyst in diarrhoeal stool (Ojuromi et al., 2012). Because of their tiny size (about 4-6 µm), detecting the parasite in diarrhoeal stool using MZN has been a problem (Ojuromi et al., 2012; Kumarya and Gwarzo, 2013). This is because the oocysts are usually confused with other faecal materials, pollen and yeast cells. Another disadvantage of this technique is that large number of oocysts (50,000-500,000) per gram of stool need to be present for detection (Ahmed and Karanis, 2018). Where low oocyst numbers are expected in samples, concentration methods are used to increase the sensitivity of detection (Radfar et al., 2013). Due to its limitations, other more effective methods such as antigen detection using enzyme linked immunosorbent assay (ELISA) which can detects low level of oocysts in a gram of stool, have been developed to give more reliable results (Ghoshal et al., 2018). This method of diagnosing cryptosporidiosis is becoming increasingly used because of their increase in sensitivity and specificity (Gawad et al., 2018).
Studies on cryptosporidiosis remain scanty in Northern Nigeria, especially amongst immunocompromised patients. The greatest burden of cryptosporidiosis is in children and HIV patients (Costa et al., 2018; Karshima and Karshima, 2021). It has been estimated in India among children <2 years, that cryptosporidiosis leads to 3.9-7.1 million diarrheal episodes, 66.4-249.0 thousand hospitalizations, and 5.8-14.6 thousand deaths each year (Sarkar et al., 2014). In HIV seropositive patients, it is a major cause of chronic diarrhoea which subsequently leads to malnutrition and death in severe cases (Iqbal et al., 2012). Therefore, prompt and reliable methods of diagnosis are essential to help curb the severity in such groups of patients.
The main aim of this study is to compare microscopy using Modified Ziehl-Neelsen (MZN) with ELISA in the detection of Cryptosporidium in stool.
MATERIALS AND METHODS
The study was a cross sectional and prospective type. A total of 183 HIV seropositive patients attending the HIV treatment clinic were recruited to participate in the study.
Collection and processing of samples
Stool samples were collected in a clean wide mouthed screw capped plastic containers, which were transported to the Medical Microbiology laboratory of ABUTH, Zaria for processing. The samples were preserved and concentrated using the formalin-ether concentration method (Allam et al., 2021). In this method, 2 ml of watery stool was collected in a tube and then 10 ml of 10% formalin added. This was mixed well then filtered using a coffee strainer into another tube and faecal particles collected on the sieve were discarded. Three millilitres of ether were added to the filtrate and solution was vortexed for 15 s. This was to allow adequate mixing of ether to the filtrate and centrifuged immediately at low speed (500 g) for 1 min. After centrifuging, four layers were formed; topmost layer represented the ether, then faecal debris layer followed by formol water layer and lastly the sediment layer. The layer of the formol water was carefully transferred to another clean tube using a Pasteur pipette. One millilitre of this was transferred into a clean plain bottle for ELISA analysis, and the remaining was then centrifuged at 1000 g for 10 min. The supernatant was discarded while the sediment was used for making the smear (Tahvildar-biderouni and Salehi, 2014).
Sample analysis using Modified Zeihl Neelsen staining technique
The smears were allowed to air dry and then fixed using 95% ethanol. After fixing, the slides were stained with concentrated carbol fuchsin, after which it was heated from below until vapour was seen. This stain was left for 5 min before it was rinsed with water. This was followed by decolourization using 1% sulfuric acid for 1 min and then washed. Lastly 1% methylene blue was used to counterstain for 1 min, and finally washed. The smeared slide was then air dried and viewed under X 100 objectives. Under oil immersion, Cryptosporidium oocyst appeared as oval pinkish red on a blue background.
Sample analysis using ELISA
The supernatant from the previously preserved stool sample was used for this procedure using ProspecTTM (Oxoid, Basingstoke, UK). This is a rapid ELISA kit for detection of Cryptosporidium antigen in the stool. Test was carried out according to the manufacturer’s instructions.18
First, required number of the micro wells was identified, including two additional wells for positive and negative controls. Four drops of the negative and four drops of the positive control were added in wells A1 and B1, also 200 µl of the supernatant from stool sample was added to subsequent wells. The plate was covered using a microplate cover and incubated for 60 min at room temperature. The contents of the wells were then discarded into a waste container, washed 3 times using a diluted wash buffer (1 part of wash buffer concentrate to 9 parts distilled water). After the last wash, the plate was tapped on a paper towel to remove excess wash buffer. Four drops (200 µl) of enzyme conjugate were added to each well and incubated for 30 min at room temperature. Contents of the wells were discarded into a waste container, washed using diluted wash buffer for 5 times and still excess wash buffer removed as earlier done. Four drops of colour substrate were added into each well and incubated for 10 min at room temperature. Finally, a drop (50 µl) of stop solution was added to each well and results read within 10 min at room temperature (Thermofisher, 2017).
Washing of wells consisted of filling each well to overflowing and decanting contents. After the last fill, contents were discarded into a container, and the plate turned upside down on a paper towel. Results were read both visually and using an ELISA reader. Visually, positive result was seen as pale to deep yellow while negative wells will remain colourless. Wells were read at 450 nm. Absorbance of 0.15 OD and above was read as positive while absorbance reading at less than 0.15 OD was recorded as negative.
RESULTS
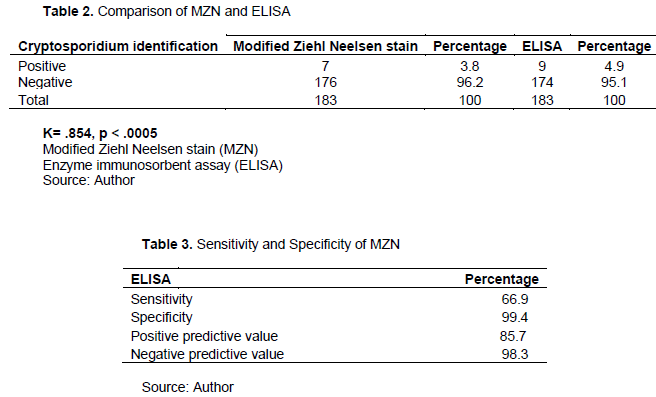
A total of 183 diarrheal stool samples were collected from March to December 2017 from HIV seropositive patients attending the HIV treatment clinic. Ninety-two (50.3%) participants were in the age group 26-35 years while the 36-45 years group had 46 (25.2%). Only 7 (3.8%) of the participants were from the age group 56-65 years. Out of the 183 participants, 132 (72.1%) were females and 51 (27.9%) were males (Table 1). +Modified Ziehl-Neelsen stain identified 7 (3.8%) Cryptosporidia in stool of the HIV seropositive while ELISA identified 9 (4.9%) of these cases (Figure 1). However, using a Kappa test there is strong agreement between the two methods in the detection of Cryptosporidium in the stool.
The sensitivity, specificity, negative predictive value and positive predictive value of ELISA as compared to MZN for the detection of Cryptosporidium were 66.9, 99.4, 98.3 and 85.7%, respectively.

DISCUSSION
In this study, all samples were analysed using MZN and ELISA. Out of the 183 stool samples obtained, MZN and ELISA detected 7 (3.8%) and 9 (4.9%) cases, respectively of Cryptosporidium in HIV seropositive cases in this study (Table 2). There was no statistically significant difference between MZN and ELISA (p<0.05) in the detection of Cryptosporidium in stool. However, test of agreement between MZN and ELISA in the detection of Cryptosporidium showed a significant degree of agreement between the two tests (k= 0.854, p< 0.0005). These results indicate that both tests can be equally used in the detection of Cryptosporidium in stool, although this study suggests that ELISA may have a slightly higher rate of detection.
The sensitivity and specificity results showed that ELISA has a significantly higher sensitivity than MZN in the detection of Cryptosporidium in stool; although the specificity of MZN is, still high (Table 3). These findings are buttressed by several studies in various parts of the world that revealed the higher sensitivity and specificity of ELISA as compared to MZN (Cengiz et al., 2016; Aly Shalash et al., 2016; Omoruyi et al., 2014). However, one study by Mittal et al. (2014) revealed that MZN staining is a more sensitive method than ELISA for detection of Cryptosporidium in stool samples but that the specificity of ELISA was more than MZN (Mittal et al., 2014; Saha et al., 2019b). Another study in India showed that MZN and ELISA have comparative sensitivity and specificity. They also concluded that ELISA may be used as a reliable substitute for microscopy in setups where the case load is higher or expertise in special staining techniques is unavailable and that the cost of the ELISA kits can only be justified if the sample load is sufficiently high or if immunocompromised patients form a significant patient population (Ghoshal et al., 2018; O’Leary et al., 2021).
Furthermore, MZN may detect some false positives as indicated by the positive predictive value of 85.7% and above. One of the major advantages of ELISA is in its detection of Cryptosporidium antigens during asexual multiplication which cannot be picked on MZN (Ghoshal et al., 2018). In this study, 2 cases of Cryptosporidium were detected in stools which were reported as negative by MZN, further buttressing the relative superiority of ELISA over MZN. Despite being a bit costly, ELISA should be considered for diagnosis in situation where there is a low concentration of parasite in the faecal samples and to overcome the lack of experienced technicians who can carry out MZN (Hassan et al., 2021).
CONCLUSION
This study showed that both MZN and ELISA can be used in the detection of Cryptosporidium in stool samples as both tests showed strong agreement with each other. However, ELISA can be used as a confirmatory test for the diagnosis of cryptosporidiosis.
CONFLICT OF INTERESTS
The authors have not declared any conflict of interests.
REFERENCES
|
Agholi M, Hatam GR, Motazedian MH (2013). HIV/AIDS-Associated Opportunistic Protozoal Diarrhea. AIDS Research and Human Retroviruses 29(1):35-41. |
|
|
Ahmed SA, Karanis P (2018). Comparison of current methods used to detect Cryptosporidium oocysts in stools. International Journal of Hygiene and Environmental Health 221(5):743-763. |
|
|
Allam AF, El-Taweel HA, Farag HF, Mohamed AFI, Khalil SS, Shehab AY (2021). Performance of formalin ethyl acetate, percoll sedimentation and ELISA for the detection of Cryptosporidium infection in asymptomatic children. Journal of Parasitic Diseases 45(2):319-323. |
|
|
Aly Shalash IR, Zalat R, El-Enain G, EL-Mohandes M, EL-Faramawy M, Aly E. Comparison between Modified Acid Fast Staining and Antigen Detection Assay as Diagnostic Techniques for Cryptosporidium parvum. World Journal of Medical Sciences 13(1):72-8. |
|
|
Cengiz ZT, Yilmaz H, Sahin IH, Kapmaz M, Ekici P (2016). The Frequency of Cryptosporidium spp. in Immunocompromised Patients by Modified Acid-Fast Staining, Cassette Kit and ELISA Methods: Comparison of the Diagnostic Techniques. Jundishapur Journal of Microbiology 10(2). |
|
|
Costa D, Razakandrainibe R, Sautour M, Valot S, Basmaciyan L, Gargala G (2018). Human cryptosporidiosis in immunodeficient patients in France (2015-2017). Experimental parasitology 192:108-112. |
|
|
Elsawey A, Suzan E, Magied S Abdel, Mosaad Y, Nabih N (2020). Prevalence of Cryptosporidium species among immunocompetent and immunocompromised Egyptian children: comparative study. Parasitologists United Journal 13(2):114-20. |
|
|
Gawad SSA, Ismail MAM, Imam NFA, Eassa AHA, Abu-Sarea EY (2018). Detection of Cryptosporidium spp. in Diarrheic Immunocompetent Patients in Beni-Suef, Egypt: Insight into Epidemiology and Diagnosis. The Korean Journal of Parasitology 56(2):113. |
|
|
Ghoshal U, Jain V, Dey A, Ranjan P (2018). Evaluation of enzyme linked immunosorbent assay for stool antigen detection for the diagnosis of cryptosporidiosis among HIV negative immunocompromised patients in a tertiary care hospital of northern India. Journal of Infection and Public Health 11(1):115-119. |
|
|
Hassan EM, Örmeci B, DeRosa MC, Dixon BR, Sattar SA, Iqbal A (2021). A review of Cryptosporidium spp. and their detection in water. Water Science and Technology 83(1):1-25. |
|
|
Iqbal A, Lim YAL, Mahdy MAK, Dixon BR, Surin J (2012). Epidemiology of Cryptosporidiosis in HIV-Infected Individuals: A Global Perspective. Open Access Scientific Reports 1(9):1-15. |
|
|
Karshima SN, Karshima MN (2021). Epidemiology of Cryptosporidium Infections among People Living with HIV/AIDS in Nigeria: Results of Systematic Review and Meta-analysis. Acta Parasitology 66(1):60-74. |
|
|
Kumarya AS, Gwarzo MY (2013). Cryptosporidiosis in HIV infected patients with diarrhoea in Kano state, North-western Nigeria 5(8):301-305. |
|
|
Thermosfisher (2017). Cryptosporidium Antigen Detection Microwell ELISA Directions for Use for In Vitro Diagnostic Use 310:1-6. |
|
|
Mittal S, Sharma M, Chaudhary U, Yadav A (2014). Comparison of ELISA and Microscopy for detection of Cryptosporidium in stool. Journal of Clinical and Diagnostic Research 8(11):DC07. |
|
|
Nkenfou CN, Nana CT, Payne VK (2013). Intestinal parasitic infections in HIV infected and non-infected patients in a low HIV prevalence region, West-Cameroon. PLoS One 8(2):e57914. |
|
|
O'Leary JK, Sleator RD, Lucey B (2021). Cryptosporidium spp. Diagnosis and Research in the 21st Century. Food and Waterborne Parasitology 24:e00131. |
|
|
Ojuromi OT, Izquierdo F, Fenoy S, Fagbenro-Beyioku A, Oyibo W, Akanmu A (2012). Identification and Characterization of Microsporidia from Fecal Samples of HIV-Positive Patients from Lagos, Nigeria. PLoS ONE 7(4):e35239. |
|
|
Omoruyi B, Nwodo U, Udem C, Okonkwo F (2014). Comparative Diagnostic Techniques for Cryptosporidium Infection. Molecules 19(2):2674-83. |
|
|
Petrova S, Koemdzhiev D, Iliev P, Nikolov D, Stoyanova K (2017). Comparison Between Three Laboratory Methods for the Diagnosis of Cryptosporidium. Scripta Scientifica Vox Studentium 1(0). |
|
|
Radfar MH, Gowhari MA, Khalili M (2013). Comparison of capture ELISA and modified Ziehl-Neelsen for detection of Cryptosporidium parvum in feces of camel (Camelus dromedarius ) in Iran. Scientia Parasitologica 14(3):147-152. |
|
|
Saha R, Saxena B, Jamir ST, Shekhar S (2019). Prevalence of cryptosporidiosis in symptomatic immunocompetent children and comparative evaluation of its diagnosis by Ziehl-Neelsen staining and antigen detection techniques. Tropical Parasitology 9(1):18. |
|
|
Sarkar R, Tate JE, Ajjampur SSR, Kattula D, John J, Ward HD (2014). Burden of diarrhea, hospitalization and mortality due to cryptosporidial infections in Indian children. PLoS Neglected Tropical Diseases 8(7):e3042. |
|
|
Tahvildar-biderouni F, Salehi N (2014). Detection of Cryptosporidium infection by modified ziehl-neelsen and PCR methods in children with diarrheal samples in pediatric hospitals in Tehran 7(4):125-30. |
|
Copyright © 2024 Author(s) retain the copyright of this article.
This article is published under the terms of the Creative Commons Attribution License 4.0