ABSTRACT
Fruit of Allanblackia floribunda is an active ingredient in the pharmaceutical industry. Fruit production in the species is hindered by incidence of pathogenic fungi leading to economic loss. This study was conducted to investigate micro-fungi species associated with leaf spot of A. floribunda. Field surveys were carried out in natural stands containing matured A. floribunda trees located at Owu Ikija, Ogun State (6.80°N, 4.03°E) and Benin, Edo State (6.29°N and 5.58°E) in Southern Nigeria. Diseased leaf samples were collected during wet and dry seasons. Pure cultures of fungal isolates obtained from the leaf samples were examined to determine their cultural and morphological characteristics. Percentage incidence of micro-fungi in each location was estimated. Leaves of healthy seedlings were sprayed with 104 conidial/ml spore concentration of fungal isolates to determine their pathogenicity. Fourteen fungal species were isolated from leaves of A. floribunda across the two sites. Aspergillus spp., Macrophomina phaseolina, Penicillium species, Pestalotia palmarum, Rhizopus nigricans and Trichoderma pseudokoningii were isolated from both sites during both seasons. Fusarium oxysporum, Lasiodiplodia theobromae, Penicillium javanicum and Pythium aphanidermatum were present at Owu Ikija while Colletotrichum capsici, C. coccodes and Curvularia lunata were present at Benin with fungal incidence of 12.5, 12.5 and 4.17%, respectively. P. palmarum had modal fungal incidence (35.29%) at Benin followed by T. pseudokoningii at Owu-Ikija with frequency value of 18.75 and 17.54%, respectively. P. palmarum was the most prevalent out of all micro-fungi species associated with A. floribunda in all locations. Pathogenicity test was negative for all tested isolates, variety of micro-fungi are associated with A. floribunda.
Key words: Allanblackia floribunda, fruit, fungal isolates, leaf samples, pathogenicity.
Micro-organisms such as bacteria, fungi, viruses, and nematodes are integral parts of the forest ecosystems. They play important roles in every sphere of our human lives contributing to processes that involve food production, medicine, industrial development bioremediation and agriculture (Miles and Chang, 2004; Krzywinski et al., 2009). Plant development and health are affected by different pathogens at various stages in their life cycle and the combination of these agents make up the disease complex. Plant fungi are the predominant
pathogens responsible for tree diseases and thus have changed tree population diversity and ecosystem dynamics (Casadevall, 2007; Pujari et al., 2015). The devastating effect of these diseases on important forest trees has resulted in reduction in their quality, economic and aesthetic values as well as food production. Pseudocercospora ranjita was associated with leaf spot of Gmelina arborea (Wingfield and Robison, 2004); Fomes lignosus and Phellinus noxious (Corner) G. Cunn are the causative agents of root rot of Tectona grandis (Momoh, 1976; Moh’d Farid et al., 2009), while Cryphonectria parasitica (Murr) causing chestnut blight and Ceratocystic ulmi (Buism.) are the causal organism of Dutch elm diseases (USDA-APHIS, 2012). These fungi cause diseases in forest trees leaving discouraging results.
However, the seriousness of these diseases is often based on evaluation of the lethal effects of the diseases. For example, in the United States, the chestnut, which was once a major hardwood timber species is reportedly reduced to a less valuable bush species by chestnut blight (Manion, 1991) which is a result of the devastating fungus.
Allanblackia floribunda Oliv. is an evergreen, multi-purpose indigenous fruit tree with great potential as a source of alternate income to farmers and communities in tropical Africa (Munjuga et al., 2008). The fruits contain seeds that have large proportion of edible fat. The fat has a high melting point which solidifies at room temperature but thaws in the mouth which makes it a major raw material in food and pharmaceutical industries because it does not require further modification. The tree species is also used as a timber product and for medicinal purposes (Pye-Smith, 2009). However, fruit production in species such as A. floribunda is sometimes limited by the incidence of pathogens such as fungi leading to poor yield and economic loss. Also, there is dearth of information on micro-fungi associated with A. floribunda. Therefore, the study isolated micro-fungi species associated with A. floribunda leaves.
Study area
Field surveys were carried out at A. floribunda stands located at Owu Ikija, Ogun State (6.80°N, 4.03°E) and Benin, Edo State (6.29°N and 5.58°E) in Southern Nigeria. There are two seasons in the study area: rainy (March to November) and dry seasons (December to February). The average annual rainfall ranges from 1300 to 1600 mm while average annual temperature ranges from 26.5 to 28.9°C (FRIN, 2018).
Sample collection
Leaf samples with typical leaf spot symptoms were collected during the dry and rainy seasons. These samples were purposively selected. Prior to collection, each tree was examined thoroughly for signs and symptoms of diseases: necrotic symptoms on the leaves such as spot, blight, scorch and other symptoms associated with the leaves. At Owu-IKija, 25 disease trees were sampled while 35 were sampled at Benin. Samples were taken from diseased trees with at least two diseased trees in each sample plot. Collected leaf samples were kept in sterile sampling bags and taken for laboratory analysis at the Plant Pathology laboratory of Forestry Research Institute of Nigeria for isolation of associated organisms.
Isolation of associated fungi
Leaf samples were cut into 2 mm × 2 mm sizes, surface-sterilized in 1% sodium hypochlorite and rinsed in 5 changes of sterile distilled water. Cut sections were obtained from the boundary area between infected and healthy tissues. They were blot-dried and aseptically placed on PDA growth medium. The plates were replicated three times and incubated at 29±2°C. The plates were examined daily for fungal growth.
Identification of fungal isolates
The isolates were purified through sub-culture of fungal growth. The cultures were examined to determine their cultural and morphological characteristics. The isolates were identified as soon as sporulation was observed as their structures are best viewed at this period. Wet mounts of each isolate were prepared on slides and stained with lactophenol cotton blue. The mounts were then observed using the Olympus BX51M reflected light optical microscope. Identifications were carried out based on the cultural and morphological characteristics of the isolates using Standard Manual of Fungi as reference (Barnett and Hunter, 1998; CMI, 1972).
Determination of frequency of occurrence of isolates
The number of times each fungus was isolated from the diseased leaf samples was expressed as a percentage of all fungi isolated (Ilondu, 2011).
Pathogenicity test
Seven fungal pathogens namely Colletotrichum capsici, Colletotrichum coccodes, Curvularia lunata, Fusarium oxysporum, Macrophomina phaseolina, Lasiodiplodia theobromae and Pestalotia palmarum isolated from A. floribunda leaves were inoculated into healthy leaves in order to establish the actual causal organism of the disease and to satisfy Koch’s postulate. Two-year old A. floribunda seedlings were inoculated with inoculum suspension of pathogenic isolates in a screen house. Twelve seedlings were used for each isolate with three replicates. All leaves in each replicate were inoculated with spore solution of each fungal isolate using a high pressure hand sprayer till run-off Inoculum suspension was prepared by addition of distilled water to sporulating culture of the isolates. With the aid of a sterile inoculation loop, the culture was gently scraped into a beaker to dislodge spores from the aerial mycelium. This was repeatedly done to obtain enough quantity of inoculum suspension. The suspension was adjusted with sterile distilled water (1×104 spore/ml) after which two drops of tween 20 detergents (polyoxyethylene sorbitan mono-oleat) was added to reduce surface tension before the suspension was sprayed on leaves of the healthy seedlings.
Inoculated leaves were incubated for 48 h and then leaf spot disease symptom development was monitored. Control (that is, treatment without pathogen) was spray inoculated with sterile distilled water. Artificially inoculated leaves were taken back to the laboratory after 6 months for re-isolation of fungi.
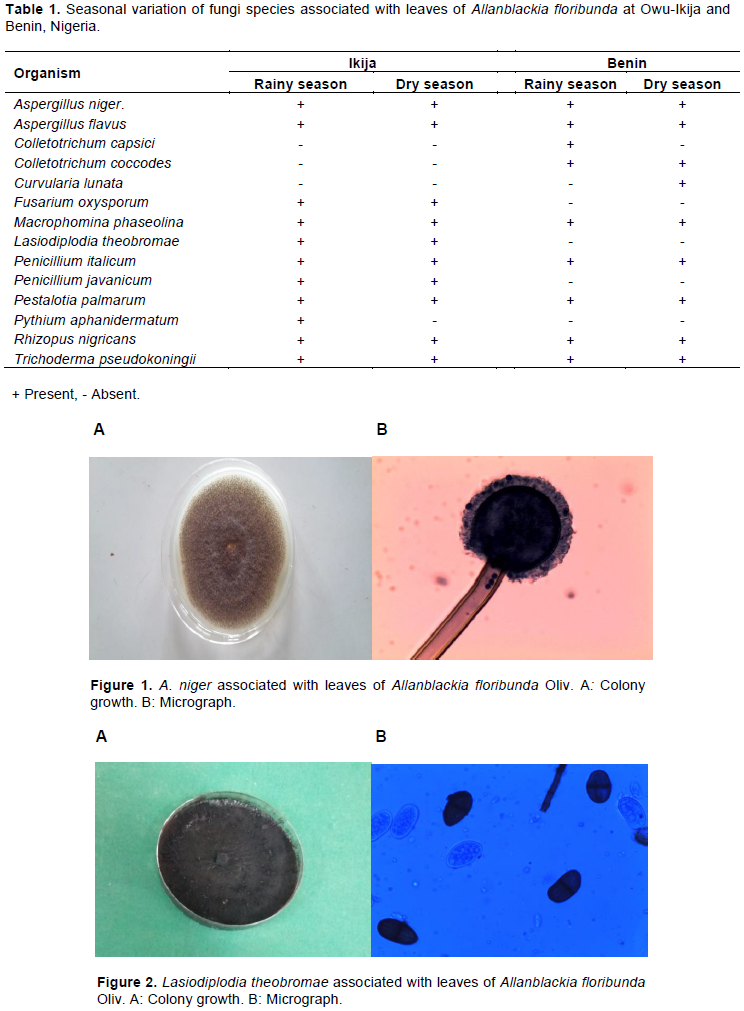
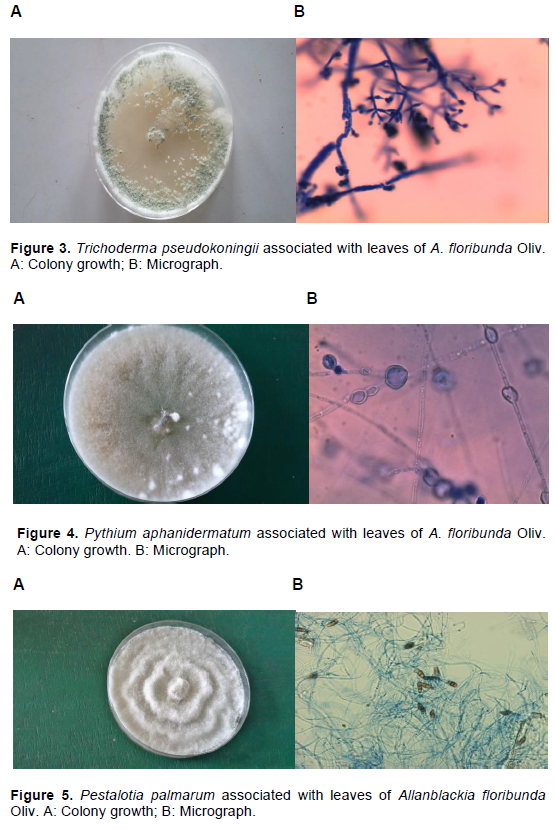
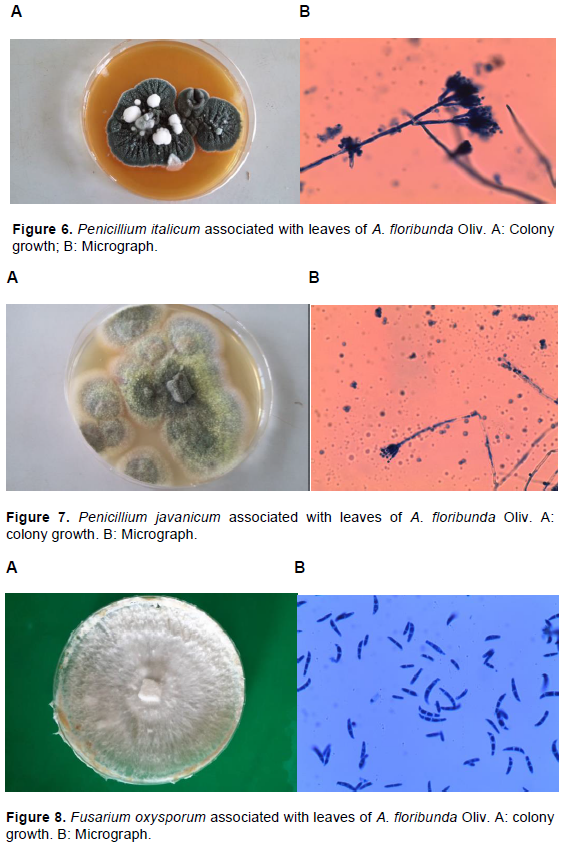
Fourteen fungi species were isolated from leaves of A. floribunda across the two sites (Table 1 and Figures 1 to 9). Micro-fungi species such as Aspergillus niger, Aspergillus flavus, Macrophomina phaseolina, Penicillium italicum, P. palmarum, Rhizopus nigricans and Trichoderma pseudokoningii were present at both study sites during the two seasons. Some of these organisms have been established to cause diseases in several forest species. For example, leaf blight of Terminalia catappa was caused by Fusarium solani (Rai and Mamatha, 2005); leaf spots of Aloe vera (Aloe barbadensis Miller) caused by Fusarium species (Avasthi et al., 2018) and leaf spot of Harungana madagascariensis was caused by Pestalotia harongae (Nsolomo and Venn, 1994). The occurrences of these pathogens always have adverse effect on the host tree species.
A similar finding was reported by Ukoima et al. (2013) while assessing the pathogens associated with seedlings of T. grandis. The study identified A. niger, Sclerotium rolfsii, A. flavus, Pythium debaryanum, Armillaria mallea, F. oxysporum, Rhizopus stolonifer, Penicillium species and Serratia species in all study sites evaluated in Akwa Ibom State.
P. palmarum was isolated in all study sites with high incidence of occurrence. These species are reported to be common in rainforest zone of Africa and are frequently associated with leaves of woody plants (Langenheim et
al., 1981; Fail and Langenheim, 1990). The highest percentage incidence was recorded during the dry season (35.29%) at Benin followed by T. pseudokoningii (18.75%) during the dry and raining season at Owu-Ikija (Table 2). A. niger also recorded relatively high incidence (17.64%) of occurrence at Benin during the dry season.
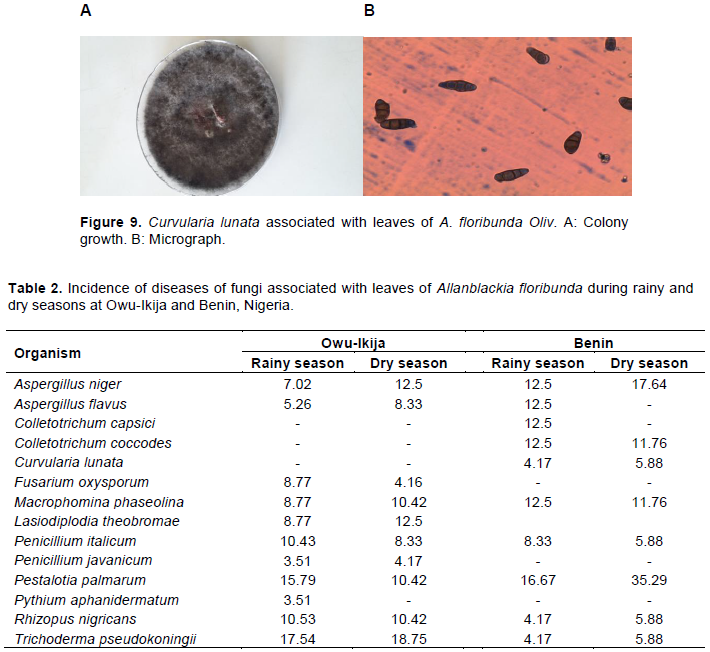
C. capsici, C. coccodes and C. lunata were also associated with A. floribunda though totally absent during both seasons at Owu-Ikija while F. oxysporum, L. theobromae and Pythium aphanidermatum were absolutely absent at Benin. The variation of these organisms could be attributed to biotic and abiotic components. The presence of two species of Colletotrichum in this study is in accordance with Freeman (2008) who reported that Colletotrichum species are broad range of pathogens, with many species affecting a single host and a single species infecting a diverse number of hosts. Bagwari et al. (2014) reported C. lunata associated with leaf spots of Populus deltoids. Other reports stated that the pathogen was associated with leaf blight of rice (Zhong et al., 2016).
A. niger, M. phaseolina, Penicillium spp., P. palmarum, R. nigricans and T. pseudokoningii were present at different percentage frequency at both Ikija and Benin. This result indicates that under favorable conditions several pathogens can co-exist to attack susceptible hosts like A. floribunda leaves.
Consequently, pathogenicity test result revealed that C. capsici, C. coccodes, C. lunata, F. oxysporum, M. phaseolina, L. theobromae and P. palmarum were not pathogenic on A. floribunda leaves. Many workers have also reported non pathogenicity of these organisms on various plant species (Arrhenius and Langenheim, 1986; Sanchez Hernadez et al., 1998; Slippers and Wingfield, 2007; Dania et al., 2010; Bagwari et al., 2014). These organisms may not be pathogenic under artificial conditions (seedling inoculation) because potted plants receive different levels of fertilization and moisture (Wingfield, 1996), this finding also corroborate previous results that, greenhouse trials have a moderate to weak correlation with those obtained from the field.
In addition, the biology of the specific pathogen may
influence the result. On the other hand, the tree species may be resistant as a result of production of antimicrobial molecules (phytoalexins) which is triggered immediately after attack by the pathogenic fungi (Bowyer et al., 1995), hence, prevent the disease spread. However, the disease-diversity hypothesis states that high species or high genetic diversity in a community confers disease resistance (Heybroek, 1982; Burdon, 2001). Positive or neutral ecosystem functioning effects on pathogen richness might also occur if additional plant species are important for completing the pathogens life cycles (Cheatham et al., 2009; Mundt et al., 2011). The incidence of microfungi such as Pestalotia, Macrophomina, Colletotrichum, Cercospora, Fusarium, Penicillium, and Aspergillus associated with leaves of A. floribunda may lead to reduction and loss of productivity. Although the associated organisms were not pathogenic on this important plant, sustainable management strategy should be adopted in order to forestall devastating effect of the associated organisms.
The authors have not declared any conflict of interests.
REFERENCES
|
Arrhenius SP, Langenheim JH (1986). The association of Pestalotia species with members of the leguminous tree genera Hymenaea and in the neotropics. Mycologia 78:673-676.
Crossref
|
|
|
|
Avasthi S, Guatham AK, Bhadauria R (2018). Isolation and characterization of Fusarium species causing leaf spot and root rot disease of Aloe vera. Journal on New Biological Reports 7(1):001-008.
|
|
|
|
|
Bagwari A, Singh YP, Kumar J, Dhiman RC (2014). First report of Curvularia eragrostidis leaf spot on Populus deltoildes. Forest Pathology 45(1):86-87.
Crossref
|
|
|
|
|
Barnett HL, Hunter BB (1998). Illustrated Genera of Imperfect Fungi.4th edition. APS Press, St Paul, Minnesota. ISBN-13: 978-0890541920. 218p.
|
|
|
|
|
Bowyer P, Clarke BR, Lunness P, Daniels MJ, Osbourn AE (1995). Host range of a plant pathogenic fungus determined by a saponin detoxifying enzyme. Science 276:371-373.
Crossref
|
|
|
|
|
Burdon RD (2001). Genetic diversity and disease resistance: Some considerations for research, breeding and deployment. Canadian Journal of Forest Research 31:596-606.
Crossref
|
|
|
|
|
Casadevall S (2007). Determinants of virulence in the pathogenic fungi. Fungi Biological Review 21(4):130-132.
Crossref
|
|
|
|
|
Cheatham MR, Rouse MN, Esker PD, Ignacio S, Pradel W, Raymundo R, Sparks AH, Forbes GA, Gordon TR, Garrett KA (2009). Beyond yield: Plant disease in the context of ecosystem services. Phytopathology 99:1228-1236.
Crossref
|
|
|
|
|
Commonwealth Mycological Institute (CMI) (1972). Descriptions of pathogenic fungi and bacteria. Eastern Press Ltd. London, UK. Set 35. 347p.
|
|
|
|
|
Dania VO, Ekpo EN, Nurudeen TA, Olasupo OO (2010). Preliminary investigations on the fungal diseases associated with Jatropha curcas leaves. African Journal of Agricultural Research and Development 3(1):41-44.
|
|
|
|
|
Fail GL, Langenheim JH (1990). Infection processes of Pestalotia subcuticularis on leaves of Hymeanaea courbaril. Phytopathology 80(11):1259-1265.
Crossref
|
|
|
|
|
Freeman S (2008). Management, survival strategies and host range of Colletotrichum acutatum on strawberry. Hortscience 43(1):66-68.
Crossref
|
|
|
|
|
Forestry Research Institute of Nigeria (FRIN) (2018). FRIN Meterology Research Station weather records. Available at:
View
|
|
|
|
|
Heybroek HM (1982). Monoculture versus mixture: Interactions between susceptible and resistant trees in a mixed stand. In: Heybroek HM, Stephan BR, von Weissenberg K (eds.), Resistance to disease and pests in forest trees. Pudoc, Wageningen, Netherlands, pp. 326-341.
|
|
|
|
|
Ilondu EM (2011). Evaluation of some aqueous plant extracts used in the control of pawpaw fruit (Carica papaya) rot fungi. Journal of Applied Biosciences 37:2419-2424.
|
|
|
|
|
Krzywinski M, Schein J, Birol I, Connors J, Gascoyne R, Horsman D, Jones SJ, Marra MA (2009). Circos: An information aesthetic for comparative genomics. Genome Research 19(9):1639-1645.
Crossref
|
|
|
|
|
Langenheim JS, Arrhenius SP, Nascimento JP (1981). The relationship of light intensity to leaf resin composition and yield in the tropical genera Hymenaea and Copaifera. Biological Systematics and Ecology 9(13):27-37.
Crossref
|
|
|
|
|
Manion PD (1991). Tree Disease Concepts. 2nd edition. Prentice-Hall. Englewood Cliffs, New Jersey. 402p.
|
|
|
|
|
Miles PG, Chang ST (2004). Mushrooms: cultivation, nutritional value, medicinal effect and environmental impact. Boca Raton, FL CRC Press. 436p.
|
|
|
|
|
Mundt CC, Sackett KE, Wallace LD (2011). Landscape heterogeneity and disease spread: Experimental approaches with a plant pathogen. Ecological Applications 21:321-328.
Crossref
|
|
|
|
|
Munjuga M, Ofori D, Sawe C, Asaah E, Anegbeh P, Peprah T, Mpand M, Mwaura L, Mtui E, Sirito C, Atangana, AH, Tchoundjeu Z, Jamnadass R, Simons AJ (2008). Allanblackia Propagation Protocol. Ian Dawson (ed). 39p.
|
|
|
|
|
Moh'd Farid A, Lee SS, Maziah Z, Patahayah M (2009). Pathogenicity of Rigidoporus microporus and Phellinus noxius against four major plantation tree species in Peninsular Malaysia. Journal of Tropical Crop Science 21(4):289-298.
|
|
|
|
|
Momoh ZO (1976). Status of root rot disease of Teak (Tectona grandis Linn. F.) in Nigeria. International Journal of Pest Management 22(1):43-48.
Crossref
|
|
|
|
|
Nsolomo VR, Venn K (1994). Forest fungal diseases of Tanzania: Background and current status. Norwegian Journal of Agricultural Sciences 8:189-201.
|
|
|
|
|
Pujari JD, Yakkundimath RI, Byadgi AS (2015). Image processing based detection of fungal diseases in plants. Procedia Computer Science 46:1802-1180.
Crossref
|
|
|
|
|
Pye-Smith C (2009). Seeds of Hope: A public-private partnership to domesticate a native tree, Allanblackia, is transforming lives in rural Africa. Nairobi: World Agroforestry Centre. 36pp. Available at:
View
|
|
|
|
|
Rai VR, Mamatha T (2005). Seedling diseases of some important forest tree species and their management. Working Pap. Finnish Forest Research Institute 11:51-64.
|
|
|
|
|
Sanchez-Hernandez MA, Ruiz-Davila A, Perez de Algaba, BlacoLopez MA, Trapero Casas A (1998). Occurrence and etiology of death of young Olive trees in Southern Spain. European Journal of Plant Pathology 104(4):347-357.
Crossref
|
|
|
|
|
Slippers B, Wingfield MJ (2007). Botryosphaeriaceae as endophytes and latent pathogens of woody plants: Diversity, ecology and their impact. Fungal Biology Review 21:90-106.
Crossref
|
|
|
|
|
Ukoima HN, Ikata M, Pepple GA (2013). Control of Lasiodiplodia theobromae on Rhizophora racemosa using plant extracts. American Journal of Biotechnology and Molecular Research 3(1):1-7.
Crossref
|
|
|
|
|
United States Department of Agriculture Animal and Plant Health Inspection Service (USDA-APHIS) (2012). List of hosts and plants associated with Phytophthora ramorum. Available at:
View
|
|
|
|
|
Wingfield MJ (1996). Pathogenicity of Leptographium procerum and L. terebrantis on Pinus strobus seedlings and established trees. European Journal of Forest Pathology Banner 6(5-6):299-308.
Crossref
|
|
|
|
|
Wingfield MJ, Robison DJ (2004). Diseases and insect pests of Gmelina arborea: Real insects and real opportunities. New Forest 28:227-243.
Crossref
|
|
|
|
|
Zhong LC, Ai YJ, Chun RH, Yi YD (2016). Identification of Curvularia lunata causing leaf spot of pineapple (Ananas comosus) in China. Canadian Journal of Plant Pathology 38(2):250-253.
Crossref
|
|