ABSTRACT
The flourishing to the war b/n pathogens and antibiotics is contingent on the diligent trial to find effective antibiotics; so that to lessen the fear faced by resistant microbes. The screening carried out manifested the presence of saponins, glycosides, tannins, reduced sugars, terpenoids, flavonoids and phenols. Alkaloids and steroids were not traced from any of the extracts. Aqueous and solvent extracts from leaves of Aloe elegans were inspected for antimicrobial activity against pathogens namely Klebsiella pneumonia, Salmonella thypherium, Bacillus subtilis, Escherichia coli, Staphylococcus aureus, Pseudomonas aeruginosa and Candida albicans by well and disc diffusion method. Disk diffusion method showed a better result than well diffusion method. The highest poisoning against all the microbes was seen by ethanol extract. The extract showed unsurpassed antifungal activity than antibacterial activity. Minimum inhibitory concentration (MIC) assay was ascertained for this extract against bacteria and fungi. Ethanol extract bespoke maximum toxicity against S. aureus at 0.78 mg/ml concentration succeeded by E. coli at 1.56 mg/ml concentration. The upshots provide justification for the possibility to utilize plant extracts to treat sundry infectious diseases.
Key words: Aloe elegans, minimum inhibitory concentration (MIC), antimicrobial, phytochemicals.
Plant materials remain a crucial source to fight stringent diseases in the world. The most important of these bioactive constituents of plants are alkaloids, tannins, flavonoids and phenolic compounds (Edeoga et al., 2005). The widespread use of a limited number of antimicrobial agents concomitantly with the reduced arsenal of drugs with antimicrobial function, has led to the development of resistance to drugs that oppose both fungal and bacterial infections, which has been an increasing problem (Zida et al., 2016). The medicinal plants are useful for healing as well as for curing of human diseases because of the presence of phytochemical constituents (Nostro et al., 2000). They are naturally occurring in the medicinal plants, leaves,and roots that have defense mechanisms and protect against various pathogens. Phytochemicals are primary and secondary compounds. Chlorophyll, proteins and common sugars are included in primary constituents and secondary compounds have terpenoids, alkaloids, and phenolic compounds (Krishnaiah et al., 2007). terpenoids exhibit various important pharmacological activities, that is, anti-inflammatory, anti-cancer, anti-malarial, inhibition of cholesterol synthesis, anti-viral and anti-bacterial activities (Mahato and Sen, 1997). Alkaloids are used as anesthetic agents and are found in medicinal plants (Herouart et al., 1988). Ergo, it is momentous to take action for the screening of different plants by considering their different floral parts in order to affirm their use as medicinal valued material and to bespeak the active principles by compartmentalization and characterisation of their constituents. In our investigation of Aloe elegans, an aqueous extract and three solvent extracts of leaves of A. elegans were screened for their phytochemical constituents and their possible antimicrobial activity.
Antimicrobial study of plants has gained prevalence and many researchers (Anzabi, 2014; Mariana et al., 2017) have shown that plants could be used as alternative medicines to combat the problem presented by resistant pathogens. Aloe vera, one of the species of Aloes, has been well studied in every aspect, it could be harnessed and has been used in the cosmetic industry. The successful and many uses of A. vera has propelled researchers to explore other plants with rigours. Aloe elegans is a regionally endemic species of the Aloeacea family which is only found in Eritrea and Ethiopia (Demissew and Nordal, 2010). To the best of our knowledge for this species, few or incomplete studies have been carried out in the struggle to find an alternative drug from plants. The present study investigated the potential use of A. elegans as a better source of antimicrobial agents.
Aloe species have been used for a long time as folk medicine for the treatment of constipation, burns and dermatitis. Recently, some species of Aloe, have been used in a wide range of skin and hair care products, and also form the basis of health drinks and tonics. The slimy gel inside the leaves consists of a complex mixture of polysaccharides, amino acids, minerals, trace element and other biologically active substances, such as enzymes. Aloe species have been the source of laxative drugs, the main purgative principle being an anthrone-C glycoside, aloin, which occurs at levels of between 18 and 30% of the dried product (British Pharmacopoeia, 1993, United States Pharmacopoeia, 1995). Recent research has indicated that aloe might kill the bacteria responsible for tuberculosis, Mycobacterium tuberculosis, and also the herpes virus causing herpes genitalis. Research has further shown that aloe inhibits growth of many common organisms such as yeasts, fungi, and bacteria infecting wounds (Demissew and Nordal, 2010).
Plant preparation
A. elegans leaves were gleaned from subzone of Mai-Nefhi, 20 km north east of Asmara. Aloe elegans was authenticated by a taxonomist in the Department of Botany, College of Mai-Nefhi, Prof. Gebrehiwet Medhanie. The plant material was washed to remove dirt and adhering materials and was sliced. The gel was obtained simply by rubbing using knife, and then dried in a shed. After being dried in a shade, the plant materials were ground in electric grinder. The powder was packed in plastic bag until further use.
Preparation of extracts
For water extraction, Parekh and Chanda (2006) method was followed with little modification. Air dried leaves of A. elegans, which was packed in plastic bag as a powder was summed to 500 ml of distilled water. This mixture was boiled with very low heat for about 120 min. After that it was cooled and filtered through 8 layers of muslin cloth for a sterility purpose, and finally centrifuged at 5000 g for 10 min and the supernatant was gleaned. This was done twice. Lastly, the supernatant was concentrated to make the final volume, one fourth of the original volume using a rotavapour and the crude extract was dissolved in distilled water and stored at 4°C for further usage (Parekh and Chanda, 2006).
For solvent extraction, Parekh and Chanda (2006) method was followed with some little modification. Air dried leaves of A. elegans (100 g), which was packed in plastic bag as a powder; was mixed with 500 ml of organic solvent (petroleum ether, ethyl acetate and ethanol) in 500 ml flask with cotton wool as a lid and then kept on rotary shaker at 220 rmp for 72 h. Succeeded by collecting the supernatant and filtered through 8 layers of muslin cloth for a sterility purpose and finally the solvent was evaporated to make the final volume one fourth of the original volume using a rotavapour and the crude extract was dissolved in dimithylsulfoxide (DMSO) and stored at 4°C (Parekh and Chanda, 2006).
Qualitative phytochemical screening
Phytochemical screening by standard methods were implemented to the newly groomed extracts to assure the absence or the presence of the proceeding phytochemicals such as tannins, steroids, alkaloids, glycosides, phenols, reduced sugars, alkaloids, saponins and flavonoids (Parekh and Chanda, 2006).
Test microorganisms
Microorganisms were given by National Health Laboratory of Eritrea. Two gram-positive bacteria: Bacillus subtilis and Staphylococcus aureus; three gram-negative bacteria: Klebsiella pneumonia, Pseudomonas aeruginosa, Escherichia coli and a fungi: Candida albicans were investigated.
Preparation of inoculum
Some colonies from nutrient agar of pure growth of each tested organism were transferred to 5 ml of Muller Hinton Broth for bacteria and Sabouraud dextrose broth for fungi. Both broths were incubated overnight at 37°C. The suspension was diluted with sterile distilled water to obtain approximately 106 to 108 CFU/ml (McFarland, 1907).
Preparation standard concentrations
One gram of each solvent and aqueous extract were dissolved into 5 ml of dimethylsulphooxide and 5 g of sterilized water, respectively. This harvested in 200 mg/ml of stock solution as a standard concentration of both types of extracts. Solvent extracts were pasteurized at 62°C while water extract was sterilized by using membrane filters.
Antimicrobial assay
The antimicrobial assay was implemented by dual procedures, namely, disk and well diffusion methods (Bauer et al., 1966; Perez et al., 1990). A 100 μl of inoculum (108 CFU) was inoculated with the Muller Hinton Agar and was made to be poured into sterile petri plates (HiMedia).
The well diffusion procedure was done by pipetting 100 μl of our test compound into the wells; which was built by sterilized borer of 0.6 cm. Similarly, the diffusion procedure was performed by pipetting our experiment compound (100 μl) onto the disc (0.6 cm) which was stuck in the agar.The plates were allowed to be incubated for 24 h at 37°C. Gentamycin (10 μg/disc) and nystatin discs (100 μg/disk) were used as positive controls for the bacteria and fungus, respectively. Negative control was prepared using respective solvent. Microbial growth was determined by measuring the diameter of zone of inhibition. The experiment was done in triplicate form and the mean values with their standard deviation are presented.
Minimum inhibitory concentration (MIC) assay
MIC procedure was exercised to an extract which was efficient in inhibiting the pathogens by both the well and disk diffusion method. MIC of ethanol extract was ascertained by INT calorimetric assay (a rapid p-Iodnitrotetrazolium chloride) (Eloff, 1998). Concisely, the test solvent sample was dissolved in DMSO which in turn was dissolved in MHB. The yielded solution was diluted serially by two fold in 96 well microtiter plate. An inoculum of 100 μl, which was calibrated to 1.5×106 CFU/ml groomed in MHB which was finally added. Plates with inoculums were sealed with a sterilized lid and mixed with a shaker to blend the composition, and was allowed to be incubated at 37°C for about 18 h. The amount of DMSO was made to be <2.5%, which means DMSO could not make an impact to the growth of microorganisms. A negative control was experimented parallel to the experiment, which contained a well with a 100 μl of inoculum+MHB+DMSO. Gentamycin was taken as a reference. After 18 h of incubation with 37°C, proceeded by addition of 40 μl INT (0.2 mg/ml), and incubated at 37°C for 30 min. The MIC of the ethanol extract was disclosed. Those microbials which were viable changed this yellow dye to pink. MIC is defined as it is the lowest concentration that precludes this mutability and results complete inhibition of the growth of microbes.
Phytochemical constituents such as phenols, flavonoids, tannins, alkaloids, saponins and many other aromatic compounds are secondary metabolites of plants that serve a defence mechanism against prediction by many microorganisms, insects and other herbivores (Bonjar et al., 2004). The phytochemical result is depicted in Table 1. The present study evinced the presence of medicinally active constituents of saponins, glycosides, tannins, terpenoids, flavonoids and phenols. Steroids and alkaloids were not present in the plant extracts studied. It is not surprising that ethanol extract of A. elegans showed significant inhibition of all tested bacterial and fungal organisms. Almost all the phytochemicals that fight against microbes have been contained in the ethanol extract. The bioactive compounds that are present in plants act by peculiar mechanism and exert antimicrobial activity. The antimicrobial activity of the extract may be due to the presence of phenols, tannins, terpenoids, and flavonoids. Phenols and tannins act their antimicrobial activity by binding to adhesins, enzyme inhibition, substrate deprivation, complex with cell wall, membrane disruption, and metal complexion. Terpenoids act their antimicrobial activity by membrane disruption. Flavonoids act in a way by binding to adhesins and complexing to cell wall of the microbes (Prashant et al., 2011). Next to ethanol extract, water extract showed an inhibition which cannot be underestimated; this might be due to the presence of flavonoids.
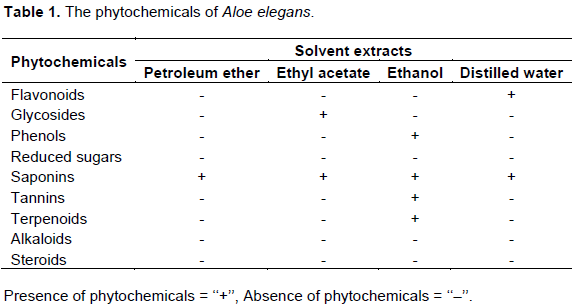
Out of the four extracts tested, ethanol extract was the best inhibitor of microbes. It showed inhibition to all organisms followed by an aqueous extract in both methods. The outcome for the antimicrobial results is depicted in Tables 2 and 3 as well as Figures 1 and 2, respectively. In this study, disk diffusion showed better inhibition than well diffusion method. The reason could be due to the plant extract added, which was able to diffuse in the bottom of the plate and thus be far from bacteria grown on the surface. Petroleum ether and ethyl acetate were the weakest extracts to show minimum inhibition in this study which might be caused by being weak solvents to dissolve the active components of the plant. Out of the five bacteria, S. aureus was the most susceptible organism which inhibited up to 22 mm, whilst Salmonella thypherium and E. coli proved to be the most resistant strain. Antifungal result was very promising; it showed up to 24 mm zone of inhibition in ethanol extract. The positive control for fungus showed its highest inhibition zone of up to 26 mm.Further study should be done to determine what would be their combined effect.In general, Gram negative bacteria were resistant strains compared to gram positive strains; the reason is simple; lipopolysaccharide (LPS) layer of gram-negative bacteria in outer membrane have a high hydrophobicity which acts as a strong permeability barrier against hydrophobic molecules. Hydrophobic molecules can pass through cell wall of gram positive bacteria easier than the gram-negative bacteria, because cell wall of the gram-positive bacteria contained only peptidoglycan (Ababutain, 2011).
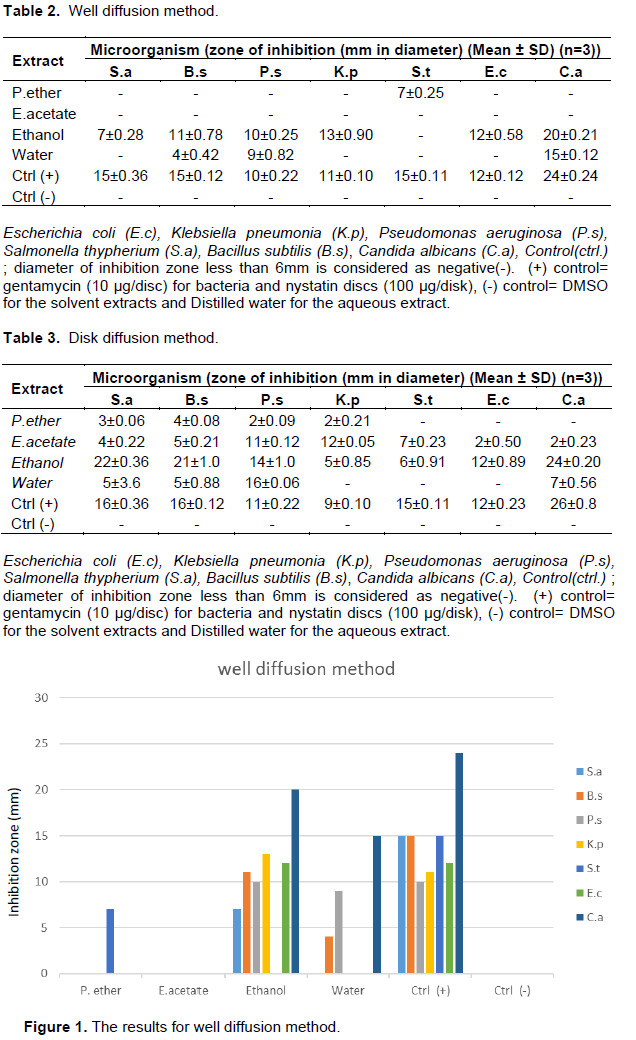
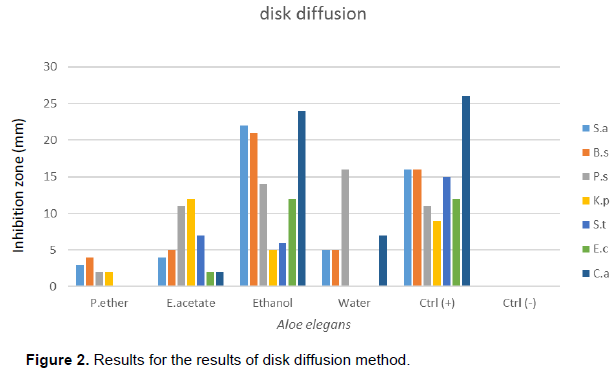
Ethanol extract showed the highest activity against pathogens which ultimately became the reason to be chosen for MIC test. Outcomes for MIC are shown in Table 4. Ethanol extract showed maximum toxicity against S. aureus at 0.78 mg/ml concentration which is succeeded by E. coli at 1.56 mg/ml. Poor toxicity was seen against B. subtilis at 25 mg/ml.
The upshots provides justification for the use of ethanol extract as a medicine to treat sundry infectious diseases and A. elegans might be a replacer plant for A. vera usage after more researches are done on this important plant.
The authors have not declared any conflict of interests.
REFERENCES
|
Ababutain IM (2011). Antimicrobial Activity of Ethanolic Extracts from Some Medicinal Plant. Aust. J. Basic Appl. Sci. 5:678-683.
|
|
|
|
Anzabi Y (2014). Evaluation of Antibacterial Activity of Aqueous Extracts of Onion and some Antibiotics on a Number of Important Bacteria in Terms of Food Hygiene. Crescent J. Med. Biol. Sci. 1(4):136-142.
|
|
|
|
Bauer AW, Kirby WMM, Sherris JC, Turck M (1966). Antibiotic susceptibility testing by a standardized single disk method. Am. J. Clin. Pathol. 45:493-496.
|
|
|
|
Bonjar GHS, Nik AK, Aghighi S (2004). Antibacterial and antifungal survey in plants used in indigenous herbal-medicine of south east regions of Iran. J. Biol. Sci. 4:405-412.
Crossref
|
|
|
|
Edeoga HO, Okwu DE, Mbaebie BO (2005). Phytochemical constituents of some Nigerian medicinal plants. Afr. J. Biotechnol. 4:685-688.
Crossref
|
|
|
|
Eloff JN (1998). A sensitive and quick microplate method to determine the minimal inhibitory concentration of plant extracts for bacteria. Planta Med. 64:711-713.
Crossref
|
|
|
|
Herouart D, Sangwan RS, Fliniaux MA, Sangwan- Norrrel BS (1988). Variations in the leaf alkaloid content of Androgenic Diploid Plants of Datura innoxia. Planta Med. 54:14-17.
Crossref
|
|
|
|
Krishnaiah D, Sarbatly R, Bono A (2007). Phytochemical antioxidants for health and medicine: A move towards nature. Biotechnol. Mol. Biol. Rev. 1:97-104.
|
|
|
|
Mahato SB, Sen S (1997). Advances in triterpenoid research, 1990-1994. Phytochemistry 44:1185-1236.
Crossref
|
|
|
|
Galeane MC, Martins CH, Massuco J, Bauab TI, Sacramento LI (2017). Phytochemical screening of Azadirachta indica A. Juss for antimicrobial activity. Afr. J. Microbiol. Res. 11(4):117-122.
Crossref
|
|
|
|
McFarland J (1907). The nephelometer: an instrument for estimating the number of bacteria in suspensions used for calculating the opsonic index and for vaccines. J. Am. Med. Assoc. 14:1176-1178.
Crossref
|
|
|
|
Nostro A, Germano MP, D'angelo V, Marino A, Cannatelli MA (2000). Extraction methods and bio autography for evaluation of medicinal plant antimicrobial activity. Lett. Appl. Microbiol. 30:379-384.
Crossref
|
|
|
|
Parekh J, Chanda S (2006). Antibacterial and phytochemical studies on twelve species of Indian medicinal plants: Afr. J. Biomed. Res. 10:175-181.
|
|
|
|
Perez C, Paul M, Bazerque P (1990). An Antibiotic assay by the agar well diffusion method. Acta. Biol. Med. Exp. 15:113-115.
|
|
|
|
Prashant T, Bimlesh K, Mandeep K, Gurpreet K, Harleen K (2011). A Review on phytochemical screening and extraction. Int. J. Pharm. Sci. 1:98-106.
|
|
|
|
Demissew S, Nordal I (2010). Aloes and Lilies of Ethiopia and Eritrea, 2nd ed., COLOPHON PAGE, University of Oslo.
|
|
|
|
Zida A, Bamba S, Yacouba A, Ouedraogo-Traore R, Guiguemdé RT (2016). Anti-Candida albicans natural products, sources of new antifungal drugs: A review. J. Mycol. Med. 650:19.
Crossref
|