ABSTRACT
Tuberculosis (TB) has become a global health challenge; it is a serious problem in sub-Saharan Africa, where rates of Human Immunodeficiency Virus (HIV) co-infection and drug resistance are high. The prevalence and rifampicin (RIF) resistance of Mycobacterium tuberculosis (MTB) among patients attending Federal Medical Centre Makurdi, Benue State was investigated. Sputum samples collected from 200 patients were tested for acid-fast Bacilli (AFB) using Ziehl-Neelsen stain. GeneXpert machine was used to test the resistance of the AFB to rifampicin. The prevalence of TB was 25.5% and the infection rate was higher in males (28.3%) than in females (23.1%), even though the difference was not statistically significant (ï£2=0.684; p>0.05). The prevalence of TB among HIV patients was also investigated. Prevalence of TB in HIV sero-positive patients was 22.2 and 28.9% in HIV sero-negative patients. This difference, however, was not statistically significant (ï£2=4.453; p>0.05). Most (70.6%) of the AFB positive specimens were susceptible to rifampicin; only a few (29.4%) were resistant to it (ï£2=43.377; p<0.05). With the high prevalence of TB and the high rifampicin resistance (MDR-TB) in Benue State, policy makers and government should increase budgetary allocations for TB control and prevention through sourcing or appealing to international organizations for finance. In addition, the government should encourage research for the development of new and better TB vaccines by financing the project.
Key words: Prevalence, resistance, Mycobacterium tuberculosis, Makurdi.
Tuberculosis (TB) is a disease caused by the bacterium Mycobacterium tuberculosis. It generally affects the lungs, but can also affect other parts of the body (WHO, 2005). TB which occurs in the lungs is known as pulmonary TB while extrapulmonary TB occurs outside of the lungs, although extrapulmonary TB may coexist with pulmonary TB (Mandell et al., 2010). TB is transmitted through the air when people with active TB cough, spit, speak, or sneeze (WHO, 2005; CDC, 2011). Symptoms of active TB are chronic cough with blood-stained sputum, fever, night sweats, weight loss, chills, loss of appetite and fatigue (Mandell et al., 2010).
One-third of the world's population is infected with TB (WHO, 2005). New infections occur in about 1% of the population each year (WHO, 2009). There were more than 10 million cases of active TB which resulted in 1.3 million deaths in 2016, making it the number one cause of death from an infectious disease (WHO, 2017). TB is one of the leading causes of death with people living with HIV/AIDS, HIV patients may be more susceptible to TB and HIV promotes latent TB to active TB disease (WHO, 2010; Iliyasu and Babashana, 2009). In 2014, 480,000 new cases of Multi Drug Resistant Tuberculosis (MDR-TB) were estimated, while 190,000 people died of MDR-TB (WHO, 2015).
TB is a serious public health problem in Nigeria. Nigeria has the second highest TB disease burden in Africa, ranking fifth among the high TB burden countries in the world (WHO, 2008). As high as 210,000 new cases of TB occurred in the country in 2010, with Lagos, Kano, Oyo and Benue State having the highest prevalence rates (WHO, 2012). Benue State is one of the states with a high level of TB infections. People with HIV/AIDS are mostly vulnerable to the disease with some of them infected with the MDR-TB (NMA, 2013).
M. tuberculosis does not retain any common bacteriological stain due to high lipid content in the cell wall, thus it is neither Gram-positive nor Gram-negative. Ziehl-Neelsen staining or acid fast staining is used (Nester et al., 2007). GeneXpert MTB/RIF is also used (WHO, 2008, 2011). The GeneXpert MTB/RIF test is a new molecular test for TB which diagnoses TB by detecting the presence of TB bacteria, as well as testing for resistance to the drug rifampicin (WHO, 2010). Standard anti-TB drugs have been in use for years now and resistance to the drugs had been discovered. Disease strains that are resistant to a single anti-TB drug have been discovered in every country surveyed. Primary resistance occurs when a person becomes infected with a resistant strain of TB. A person with susceptible TB may also develop secondary or acquired resistance during therapy because of inadequate treatment, not taking the prescribed treatment appropriately or lack of compliance on the part of the patient, or using low-quality medication (O’Brien, 1994). MDR-TB is caused by bacteria that do not respond to, at least, isoniazid and rifampicin, the two most powerful first-line anti-TB drugs (Peter et al., 2009). Extensively drug-resistant TB (XDR-TB) is also resistant to three or more of the six classes of second-line drugs (CDC, 2009). XDR-TB is a term sometimes used to define extensively resistant TB and constitutes one in ten cases of MDR-TB. Cases of XDR TB have been identified in more than 90% of countries (Kielstra, 2014). Another term is totally drug-resistant TB which is resistant to all currently used drugs (Maryn, 2012). It was first observed in 2003 in Italy (Milgliri et al., 2007). It was not widely reported until 2012 (Maryn, 2012; WHO, 2016).
Justification of the study
A number of factors make people more susceptible to TB infections. The most important risk factor globally is HIV; 13% of all people with TB are infected by the virus (WHO, 2011). This in particular is a serious problem in sub-Saharan Africa, where rates of HIV are high (Chaisson and Martinson, 2008). 30% of those co-infected with HIV develop the active disease (Gibson et al., 2005). Drug resistance has made treatment of TB much more difficult. Therapy requires the use of second-line drugs which have a greater risk of adverse effects and lower potency than first-line drugs (Gler et al., 2012; WHO, 2011). Drug resistant TB is a significant and growing public health threat. The additional costs of drug-sensitivity testing, second-line drugs and the medical care associated with them are expensive; it was discovered that the cost of treating a patient carrying MDR strains is hundreds of times greater than that for patients carrying drug sensitive strains. Since most cases of TB infection result in latent infection, it was estimated that there may be as many as 50 million people who are now infected with MDR-TB (Zumla et al., 2013; WHO, 2005, 2010).
TB was declared a “global health emergency” in 1993 by the WHO (2017). In 2006, the Stop TB Partnership developed a global plan to stop TB that aimed to save 14 million lives between its launch and 2015 (WHO, 2011). It was discovered that targeted objectives were not achieved by 2015, due to the increase in HIV co-infection and the emergence of MDR-TB (WHO, 2017).
The purpose of this study was to determine the prevalence of TB and rifampicin resistance among the M. tuberculosis clinical isolates in Makurdi metropolis.
Study area
Makurdi, Benue State, Nigeria, was the study area. Makurdi metropolis is located in North Central Nigeria along the River Benue. It lies at Latitude: 7° 43' 32" N and Longitude: 8° 33' 51" E. Makurdi is the capital of Benue State and covers an area of 34,059 km2 and has an estimated population of 500,797.
Research design
This study was a three month hospital based prospective longitudinal and cross-sectional survey which was carried out in the laboratory of Federal Medical Centre (FMC) Makurdi, Benue State, Nigeria.
Sample size
Sample size was determined using this formula (Araoye, 2004):
n=Z2 P q/d2
Where, n=desired sample size, Z=standard and normal deviation usually set at 1.96 or approximately 2.0 which correspond to 95% (0.05) confidence level, P=proportion in the target population estimated to have a particular characteristics, q=1.0-p, d=degree of accuracy usually set at 0.05, p=144/10000 people are infected with TB in Benue State (NMA, 2013).
Study population
The study population consists of 200 out patients who attended Federal Medical Centre, Makurdi for diagnosis and treatment between February and May, 2015. Ethical clearance was sought and obtained at FMC, Makurdi.
Sample collection
Three sputum specimens were collected from each patient and were examined in the laboratory for tubercle bacillus. Each patient produced 10 ml of sputum for each sputum container (20 ml). Sputum specimens were collected in an air-tight sputum container. Patients were asked to inhale deeply and cough from within and before carefully spitting into the container to avoid contamination from the outside part. After sputum collection, the containers were firmly closed and the patient’s hands washed with soap and water. Sputum specimens were stored in the refrigerator until test was to be carried out. Protective precautionary laboratory procedures like using of laboratory coats, hand gloves, face mask as well as frequent hand washing with the use of disinfectants were ensured.
Laboratory test
The diagnosis was carried out using Ziehl-Neelsen staining technique on triplicate early morning sputum samples collected under standard bio-safety procedures (Morello et al., 2006; Nester et al., 2007).
M. tuberculosis GeneXpert test was used to test for rifampicin resistance among the M. tuberculosis clinical isolates.
The Xpert MTB/RIF has been developed by the foundation for innovative new diagnostics (FIND), who have partnered with the Cepheid Corporation and the University of Medicine and Dentistry of New Jersey (WHO, 2011).
The sample to be put in the GeneXpert machine was prepared by pouring the sputum into the already prepared MTB/RIF cartridge and mixing vigorously. After clicking “create test” in the GeneXpert DS system window, the scan barcode dialog box appeared and the barcode on the xpert MTB/RIF cartridge was scanned by the scanning machine.
The sample ID was typed in the sample ID box. This sample ID was associated with the test results and was shown in the “view results” window of all the reports. The password was typed in when the dialog box appears, and this happened after clicking “start test”. The instrument module door with the blinking green light was opened and the cartridge loaded. The door was closed and the test starts when the green light stopped blinking. When the test is finished, the lights went off on their own and the system released the door lock at the end of the run. The module door was then opened to remove the cartridge.
To view results on the GeneXpert software, “view results” was clicked on the menu bar in the GeneXpert DS system window and the results appears with the sample ID and could read MTB detected low, MTB detected medium or high and RIF resistance detected or not detected. It was then instructed to print out the result (WHO, 2011).
Statistical analysis
Data collected were analyzed using descriptive and inferential statistics. Chi square test was used to determine associations between variables; correlation coefficients were used to determine relationships between variables. Significance was held at 0.05 level.
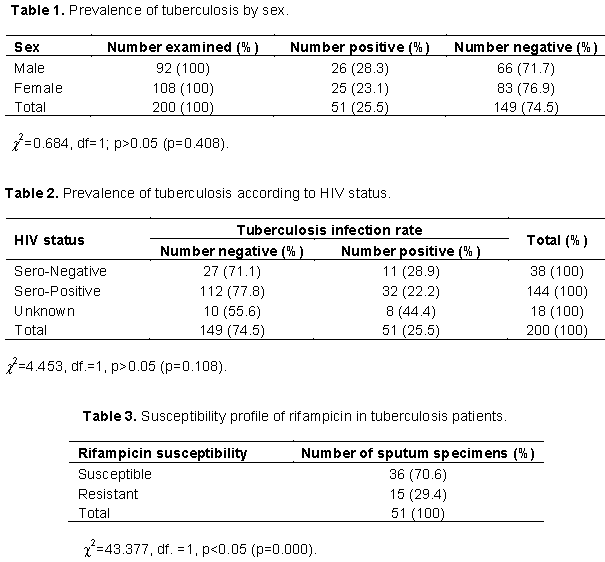
Two hundred patients were screened for M. tuberculosis (MTB). The prevalence of TB among them was 25.5% (n= 51). As shown in Table 1, the prevalence of TB was 28.3% in the male and 23.1% in the female. The difference in the prevalence of M. tuberculosis was however not statistically significant.
The prevalence of TB among HIV patients is shown in Table 2. Prevalence of TB in HIV sero-positive patients was 22.2%, while it was 28.9% in HIV sero-negative patients.
The susceptibility profile of the acid fast bacilli (AFB) to rifampicin is shown in Table 3. Most of the AFB positive specimens were susceptible to rifampicin (70.6%), while 29.4% were resistant to rifampicin.
This study was designed with the aim to determine prevalence of TB and rifampicin resistance among the M. tuberculosis clinical isolates. Findings of this study confirms the high prevalence of TB among the patients in the state, although WHO (2009) and NMA (2013), had earlier reported that Benue State is one of the states with a high prevalence of the disease. Prevalence rate of TB was observed to be 25.5% in this study. This high prevalence in the state could be attributed to the high HIV prevalence. This study observed as high as 22.2% co-infection with HIV sero-positive patients. Previous studies had also reported high prevalence among the HIV sero-positive patients (Kumar et al., 2007; WHO, 2009, 2010; NMA, 2013). This has made screening of TB patients for HIV mandatory. Ojiezeh et al. (2015) recorded 14.0% co-infection with HIV sero-positive patients in Ondo State. Studies from other parts of Nigeria showed lower prevalence, (Nwobu et al., 2004; Onubogu et al., 2014) from Edo and Lagos states, respectively. Tsaku et al. (2011) recorded a high rate of 24.2% of patients with HIV/TB co-infection in Nasarawa State. Aweke et al. (2016) also recorded as high as 27.7% of HIV/TB co-infection in Ethiopia. Lowering of immunity due to HIV/AIDS could enhance TB infection (Iliyasu and Babashani, 2009; Aweke et al., 2016). Other studies also recorded high prevalence of TB/HIV co-infection (Ifebunandu et al., 2012; Rajasekaran et al., 2007). Awoyemi et al. (2002) recorded as high as 32.8% TB/HIV co-infection. TB is among the living causes of death for people living with HIV (WHO, 2009).
This study also recorded a higher prevalence of TB among males than females. This agrees with the study of Ojiezeh et al. (2015). They stressed that males are highly exposed to the bacilli than females.
Rifampicin resistance appeared to be higher than that reported by Hatfull and Jacobs (2000) and WHO (2013). Drug resistant TB is a significant and growing public health threat. According to Gursimrat (2011), MDR-TB and HIV are among the major challenges facing the control of TB. Crofton (1959) observed that MDR-TB and XDR-TB could threaten the success of TB control. In 2007, the United States recorded 36% of MDR-TB and 56% of XDR-TB. MDR-TB and XDR-TB have very complex pathway of diagnosis and treatment. They are resistant to a large number of medications, care was also complicated and patients were highly infectious (CDC, 2014). Treatment also takes a longer time (not less than 2 years), and also requires hospitalization. Drug resistance has made treatment of TB more difficult and expensive (CDC, 2014). Treatment uses the second-line drugs which have a greater risk of adverse effects and lower potency than first-line drugs (Gler et al., 2012; WHO, 2011).
Prevalence of TB was high in Makurdi. It was also observed that there was a high rate of MDR-TB and TB/HIV co-infection in the state. These factors could generate new challenges, complicate treatment greatly, and make it difficult to eliminate TB. More attention should be given to financing the development of new TB drugs by governments and other institutions.
The authors have not declared any conflict of interests.
The authors acknowledgement goes to the staff of Federal Medical Centre Makurdi where the research was conducted and the patients who completed the surveys. In particular, they would like to thank the ethical committee, laboratory technologists and scientists of F.M.C, Makurdi.
REFERENCES
|
Araoye MO (2004). Research Methodology with Statistics for Health and Social Sciences, 1st edition, Ilorin, Nathadex publishers; 115-125.
|
|
|
|
Aweke M, Zelalem D, Essey M, and Demeke W (2016). Prevalence and associated factors of TB/HIV co-infection among HIV infected patients in Amhara region, Ethiopia. African Health Sciences 16(2):588-595.
Crossref
|
|
|
|
Awoyemi OB, Ige M, Onadeko BO (2002). Prevalence of active pulmonary tuberculosis in human immunodeficiency virus seropositive adult patients in University College Hospital, Ibadan, Nigeria. African Journal of Medicine and Medical Sciences 31:329-332.
|
|
|
|
Centers for Disease Control and Prevention (CDC) (2011). Vaccine and Immunizations: TB Vaccine (BCG)"
|
|
|
|
Centers for Disease Control and Prevention (CDC) (2009). Division of Tuberculosis Elimination (DTBE).
|
|
|
|
Chaisson RE, Martinson NA (2008). Tuberculosis in Africa-combating an HIV-driven crisis. The New England Journal of Medicine 358(11):1089-1092.
Crossref
|
|
|
|
Crofton J (1959). Chemotherapy of pulmonary tuberculosis. British Medical Journal 1:1610-1614
Crossref
|
|
|
|
Gibson PG, Abramson M, Wood-Baker R, Volmink J, Hensley M, Ulrich Costabel (2005). Evidence-Based Respiratory Medicine (1st ed.). BMJ Books. 321.
|
|
|
|
Gler MT, Podewils LJ, Munez N, Galipot M, Quelapio MI, and Tupasi DT (2012). Impact of patient and program factors on default during treatment of multidrug resistant tuberculosis. The International Journal of Tuberculosis and Lung Disease 16(7):955-960.
Crossref
|
|
|
|
Gursimrat KS (2011). Tuberculosis: Current Situation, challenges and overview of its control programs in India. Journal of Global Infectious Diseases 3(2):143-150.
Crossref
|
|
|
|
Hatfull GF, Jacobs WR (2000). Molecular Genetics of Mycobacteria. Washington, D.C. ASM Press. 7-18.
|
|
|
|
Ifebunandu NA, Ukwaja KN, Obi SN (2012). Treatment outcome of HIV-associated tuberculosis in a resource poor setting. Tropical Doctor 42:74–76.
Crossref
|
|
|
|
Iliyasu Z, Babashani M (2009). Prevalence and Predictors of TB co-infection Among HIV Seropositive Patients attending Aminu Kano Teaching Hospital, Northern Nigeria. International Journal of Epidemiology 19(2):81-87.
|
|
|
|
Kielstra P (2014). Zoe Tabary, ed. "Ancient enemy, modern imperative – a time for greater action against tuberculosis". Economist Insights. The Economist Group.
|
|
|
|
Kumar V, Abbas AK, Fausto N, Mitchell RN (2007). Robbins Basic Pathology. Saunders Elsevier. 8th ed. P 935.
|
|
|
|
Mandell GL, Bennett JE, Dolin R (2010). Mandell, Douglas, and Bennett's principles and practice of infectious diseases (7th ed.). Philadelphia, PA: Churchill Livingstone/Elsevier. 250:11
|
|
|
|
Maryn M (2012). Totally Resistant TB: Earliest Cases in Italy". Wired. Archived from the original on 14 January 2012.
View
|
|
|
|
Migliori GB, De Iaco G, Besozzi G, Centis R, Cirillo DM (2007). First tuberculosis cases in italy resistant to all tested drugs. Eurosurveillance. 12(5):E070517.1.
|
|
|
|
Morello, Josphine A, Paul A, Granato, Mario E, Wilson, Verna Morton (2006). Laboratory Manual and Workbook in Microbiology: Applications to patients care. 10th ed. Boston. McGrawHill Higher Education New York
|
|
|
|
Nester EW, Anderson DN, Roberts EC, and Nester MT (2007). Microbiology. A Human Perspective. 5th edition, Boston MA, McGraw Hill. pp. 455-457.
|
|
|
|
Nigerian Medical Association (NMA) (2013). 27,000 Nigerians die of tuberculosis yearly.
View
|
|
|
|
Nwobu GO, Okodua MA, Tatfeng Y, Mirabeau (2004). Comparative study of HIV associated pulmonary tuberculosis in Chest Clinics from two regions of Edo State, Nigeria. Journal of Health and Allied Sciences 3:4.
|
|
|
|
O'Brien R (1994). Drug-resistant tuberculosis: Etiology, management and prevention. Seminars in Respiratory Infections 9(2):104-112.
|
|
|
|
Ojiezeh TI, Ogundipe OO, Adefosoye VA (2015). A retrospective study of the incidence of pulmonary tuberculosis and human immunodeficiency virus co- infection among patients attending national tuberculosis and leprosy control programme, Owo Centre. Pan African Medical Journal 20:1.
Crossref
|
|
|
|
Onubogu CC, Kunle-Ope CN, Onyejepu N, Nwokoye NN, Raheem TY, Igbasi UT, Tochukwu NE, Omoleye RM, Ejezie CO, Musa AZ, Odunukwe NN, Onwujekwe DI, Idigbe EO (2010). Prevalence of tuberculosis and human immune deficiency Virus (TB/HIV) co-infection amongst patients with broncho pulmonary disorders in Lagos. African Journal of Microbiology Research 4(18):1904-1908.
|
|
|
|
Peter R, Donald B, Chan M, and Paul D (2009). The global burden of tuberculosis-combating drug resistance in difficult times. The New England Journal of Medicine 360:2393-2395.
Crossref
|
|
|
|
Rajasekaran S, Mahilmaran A, Annadurai S, Kumar S, Raja K (2007). Manifestation of tuberculosis in patients with human immunodeficiency virus: A large Indian study. Annals of Thoracic Medicine 2:58-60.
Crossref
|
|
|
|
Tsaku IM, Akyala I, Amuta EU (2011). A retrospective study on the mortality rate of human immunodeficiency virus (HIV) and pulmonary tuberculosis (PTB) co-infected individuals in Nasarawa State, Nigeria. Journal of Biology, Agriculture and Healthcare 3(7):218-224.
|
|
|
|
World Health Organization (WHO) (2005). Anti-Tuberculosis Drug Resistance in the World: The WHO/IUATLD Global Project on Anti-Tuberculosis Drug Resistance Surveillance. Report No. 3. Prevalence and Trends. WHO/HTM/TB/2004.343. Geneva, Switzer-land.
|
|
|
|
World Health Organization (WHO) (2008). Implementing the WHO Stop TB Strategy: a Handbook for National TB Control Programmes. Geneva: 179.
|
|
|
|
World Health Organization (WHO) (2009). Epidemiology. Global tuberculosis control: epidemiology, strategy, financing mates of TB burden. 6-33.
|
|
|
|
World Health Organization (WHO) (2010). Tuberculosis. In: Raviglione Mario C., editor. The Essentials. Fourth Edition. Geneva Switzerland: World Health Organization, 2010.
|
|
|
|
World Health Organization (WHO) (2011). WHO report 2011: Global tuberculosis control. Geneva, Switzerland: WHO; 2011. WHO/HTM/TB/2011.16.
Crossref
|
|
|
|
World Health Organization (WHO) (2012). Global Tuberculosis Report.
View
|
|
|
|
World Health Organization (WHO) (2015). Global Strategy and Targets for Tuberculosis Prevention, Care and Control after 2015 (Geneva, Switzerland).
|
|
|
|
World Health Organization (WHO) (2016). Totally Drug-Resistant TB: a WHO consultation on the diagnostic definition and treatment options (PDF). who.int. World Health Organization. Archived (PDF). Retrieved 25 March 2016.
|
|
|
|
World Health Organization (WHO) (2017). Retrieved 2017-11-09. Global tuberculosis report".
|
|
|
|
Zumla A, Mario R, Richard H, Fordham C (2013). Tuberculosis. The New England Journal of Medicine 368:745-55.
Crossref
|