ABSTRACT
Studies have shown that Klebsiella oxytoca is a major cause of infections in humans. This study was designed to determine the prevalence and antibiotic resistance pattern of extended spectrum beta-lactamase (ESBL)-producing K. oxytoca from urine samples of patients visiting private laboratories in Abakaliki, Ebonyi State. A total of 150 mid-steam urine samples of patients visiting three main private laboratories in Abakaliki, Ebonyi State were analyzed for the presence of K. oxytoca using standard bacteriological identification methods. Out of the 150 urine samples cultured, seven (7) were phenotypically identified as K. oxytoca. Isolated K. oxytoca were screened for ESBL production by antibiotic susceptibility test using second and third generation cephalosporins, and double disc synergy test. Susceptibility of ESBL-positive K. oxytoca to various classes of antibiotics was done on Mueller-Hinton agar (Oxoid, England) by Kirby Bauer disk diffusion technique. Results showed that 3 (42.8%) out of the seven (7) K. oxytoca isolates were ESBL-producers. All the ESBL-producers were completely resistant (100%) to ofloxacin, gentamicin, cefoxitin, sulphamethoxazole, ciprofloxacin, nitrofurantoin and ertapenem. The average multiple antibiotic resistance index (MARI) of the K. oxytoca isolates was one (1) and this explains their high multi-drug resistance trait. This study revealed that ESBL-producing K. oxytoca isolates exhibited complete resistance to all antibiotics tested against them. The multi-drug resistant traits expressed by these K. oxytoca isolates in our study area could lead to grave public health consequences if not curtailed.
Key words: Klebsiella oxytoca, extended spectrum beta-lactamase (ESBL), antibiotics, multi-drug resistance, urine.
K. oxytoca is a
gram-negative, rod-shaped, lactose-fermenting, non-motile, aerobic rod-shaped bacterium. Since it was isolated in the late nineteenth century, it has become a known human pathogen. Infections caused by
K.
oxytoca could lead to a variety of diseases such as urinary tract infections (UTIs), liver abscess, pneumonia, meningitis, blood stream infection, infection of the heart and valves, and prostate infection (Paterson and Bonomo, 2005). ESBL is a rapidly evolving group of β-lactamases which have the ability to hydrolyze third-generation cephalosporins and aztreonam but are inhibited by clavulanic acid (Philippon et al., 1989). ESBL-producing
Klebsiella spp. have been established since the 1980s as a major cause of nosocomial infections. Interestingly, in the late 1990s, some community-acquired pathogens which commonly cause UTIs and diarrhea were also found to be ESBL-producers (Paterson and Bonomo, 2005). ESBLs are usually encoded by genes located on large plasmids, and these plasmids sometimes carry genes for resistance to other antimicrobial agents including trimethoprim, tetracycline, sulphonamides, aminoglycosides, and chloramphenicol (Paterson, 2000). Some recent studies have shown fluoroquinolone resistance mediated by co-transfer of the
qnr determinant on ESBL-producing plasmids (Mammeri et al., 2005). As a result, multidrug resistance trait is now a frequent characteristic of ESBL-producing enterobacteria. Thus, ESBL-producing bacteria pose a major problem in clinical therapeutics. Throughout bacterial history, resistance to β-lactamases has become a useful trait ever since the clinical application of β-lactam antibiotics in the treatment of bacterial infections. These drugs exhibit a Darwinian selection; thus killing susceptible bacteria and allowing the resistant strains to survive. Resistance to β-lactamase may be intrinsic to a particular species, as observed in enterococci, which have intrinsically insensitive penicillin binding proteins (PBPs). Alternately, it may be acquired via spontaneous mutation or DNA transfer. The frequent causes of resistance in Gram-positive cocci such as pneumococci and methicillin-resistant
Staphylococcus aureus (MRSA) are alterations in the normal PBPs or the acquisition of additional β-lactam–insensitive PBPs. This study focuses on the isolation, phenotypic characterization, and determination of the antibiotic resistance patterns of
K. oxytoca in urine samples of patients visiting private laboratories in Abakaliki metropolis.
Study area
This research was conducted in three main private laboratories namely; Best diagnostics, comprehensive medical and research laboratory (Lab A), New life medical laboratory (Lab B), and City of david medical laboratory (Lab C), all in Abakaliki metropolis, the state capital of Ebonyi State. Ebonyi State is located in the South Eastern part of Nigeria. It shares boundary with Enugu, Cross River, Abia, and Benue states. It is between longitude 7°30' and latitude 60°45' E.
Sample collection
A total of 150 mid-stream urine samples (50 urine samples from each of the three laboratories) were collected using sterile universal containers which have been labeled with the patients’ information. The patients were given instructions on how to collect the urine samples. The collected urine samples were then immediately transported to the Laboratory unit of Applied Microbiology Department, Ebonyi State University, Abakaliki for bacteriological analysis.
Isolation and phenotypic identification of the isolates
The urine samples were aseptically streaked on MacConkey agar, cysteine lactose electrolyte deficient (CLED) agar, and incubated at 37°C for 24 h. After incubation, the plates were observed for typical Klebsiella growth on MacConkey agar (red or pink colonies) and CLED (yellow to whitish blue mucoid colonies). These suspected K. oxytoca isolates were further characterized using standard microbiology techniques such as Gram-staining, catalase test, motility test, and other biochemical tests which include indole test, citrate utilization test, oxidase test, methyl red test, Voges-Proskauer test, and sugar fermentation test (Cheesbrough, 2004).
Screening of bacterial isolates for ESBL production
Screening the K. oxytoca isolates for ESBL production was done by observing their sensitivities to 2nd and 3rd generation cephalosporins; such as ceftazidime (30 g), cefotaxime (30 g), cefepime (30 g), and aztreonam (3). These antibiotics were aseptically placed at a distance of 30 mm apart on Mueller-Hinton agar (Oxoid, UK) plate that was previously inoculated with standardized inocula of the test bacterium using a sterile swab stick in order to get a confluent growth. The plates were allowed to stand for about 30 min for pre-diffusion of the antibiotics and after which was incubated for 18 to 24 h at 37°C. After the incubation time, the zones of inhibition were measured in millimeter using a metre rule and results were interpreted according to clinical and laboratory standard institute (CLSI) chart. ESBL production was suspected if any of the test bacteria showed reduced susceptibility or is resistant to any of the antibiotics used for the screening studies according to the CLSI guidelines (CLSI, 2014).
Phenotypic screening/confirmation of ESBL production by isolated K. oxytoca using double disk synergy test (DDST)
The K. oxytoca isolates that showed reduced susceptibility to any of the 2nd and 3rd generation cephalosporins were phenotypically confirmed for ESBL production using the double disc synergy test (Iroha et al., 2009). DDST was performed as a standard disc diffusion assay on Mueller-Hinton (MH) agar (Oxoid, UK) plates in
line with CLSI criteria (CLSI, 2014). Sterile swab sticks were dipped into bacterial suspension(s) standardized to 0.5 McFarland turbidity standards, and was inoculated on MH agar plates. Antibiotic disc of amoxicillin/clavulanic acid (20/10 µg) was placed at the center of the MH agar plate and antibiotic discs containing cefotaxime (30 µg) and ceftazidime (30 µg) each was placed at a distance of 15 mm (center to enter) from the central disc, amoxicillin/clavulanic acid (20/10 µg) and the plates was incubated at 37°C for 18 to 24 h. ESBL production was suspected phenotypically when the zones of inhibition of the cephalosporins (cefotaxime 30 µg and ceftazidime 30 µg) increased in the presence of amoxicillin/clavulanic acid disk (20/10 µg). A≥ 5mm increase in the inhibition zone diameter for either of the cephalosporins (cefotaxime and ceftazidime) tested in combination with amoxycillin-clavulanic acid versus its zone when tested alone confirmed ESBL production phenotypically (Iroha et al., 2009).
Antibiotics susceptibility test
Antibiotic susceptibility test was carried out on all the K. oxytoca isolates using Kirby Bauer disc diffusion method. The standardized test organisms were aseptically swabbed on Mueller-Hinton agar plates using sterile swab sticks and each of the antibiotic discs (Oxoid, UK) was aseptically placed on the swabbed MH agar plates using sterile forceps. The MH plates were allowed for 15 minutes on the work bench for pre-diffusion of the antibiotics. The plates were then incubated at 37°C for 18 to 24 h. The inhibition zone diameter (IZD) produced by the test antibiotics on the test isolates were measured in millimeter (mm) using a ruler and interpreted according to the standard breakpoints of the Clinical Laboratory Standard Institute (CLSI) in order to classify them as resistant (R), intermediate (I), or susceptible (S) (CLSI, 2014).
Multiple antibiotic resistance (mar) index calculation
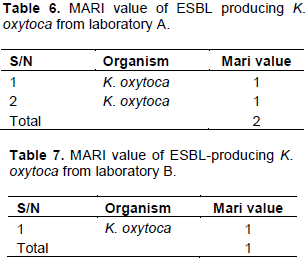
The K. oxytoca isolates were tested against seven different antibiotics to determine the prevalence of multiple antibiotic resistance traits among isolates. Multiple antibiotic resistance (MAR) index was calculated as a/b; where ‘a’ denotes the number of antibiotics to which the isolates showed resistance, and ‘b’ denotes the total number of antibiotics tested against the isolates.
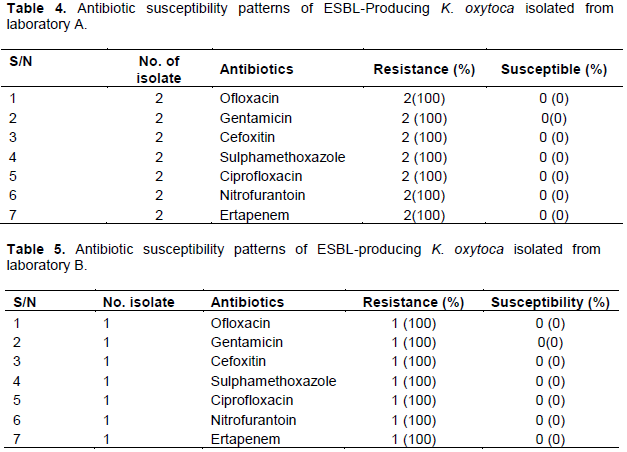
In our study, 50 urine samples were each collected from three different private laboratories (Best Diagnostics, Comprehensive Medical and Research Laboratory (Lab A), New Life Medical Laboratory (Lab B), City of David Medical Laboratories (Lab C)), and later analyzed. Out of a total of 150 urine samples analyzed, seven (Lab A = 4, Lab B = 2, Lab C = 1) were positive for K. oxytoca (Table 1). Results showed that 3 (42.8 %) out of the seven (7) K. oxytoca isolates were phenotypically identified as ESBL-producers using disc diffusion technique (Tables 2 and 3).
The widespread application of beta-lactam antibiotics in most healthcare institutions today and communities unarguably established problems which have led to increased mortality, morbidity and cost of health care (Blomberg et al., 2005). Understanding the antibiotic resistance profiles of urinary tract bacteria is very pertinent not only in helping clinicians in the prescription of appropriate antibiotics but also for evidence-based recommendations especially in empirical antibiotic treatment of UTI (Blomberg et al., 2005). Results showed that 3 (42.8%) out of the seven (7) K. oxytoca isolates were phenotypically identified as ESBL-producers. This is a serious cause of concern as many clinicians revert to fluoroquinolones for the treatment of infections caused by Gram-negative pathogens (Paterson, 2007). In this study, all the ESBL-producers were found to be completely resistant (100%) to ofloxacin, gentamicin, cefoxitin, sulphamethoxazole, ciprofloxacin, nitrofurantoin, and ertapenem (Tables 4 and 5). The observed resistance (100%) to ciprofloxacin by ESBL-producing K. oxytoca isolates in our study does not completely agree with the study conducted by Akujobi and Ewuru (2010) who reported 37.6% resistance to ciprofloxacin among ESBL-producers. Also, our findings are in great consonance with that of Sasirekha et al. (2010) who reported a high resistance frequency value of 68% by Enterobacteriaceae to ciprofloxacin. Interestingly, aminoglycosides have been recorded to have good antibacterial activity against gram-negative organisms of clinical importance. This study revealed that all the K. oxytoca isolates were completely resistance to gentamicin. Our study contradicts the findings of Sasirekha et al. (2010) who reported that 41.8% of the Klebsiella isolates in their study were resistant to gentamicin. This study is in concord with that of Al-Zarouni et al. (2008), who reported that over 90% of ESBL isolates in their study exhibited resistance to cephalosporin and aztreonam. Interestingly, Haque and Salam (2010), Sasirekha et al. (2010), and Ullah et al. (2009) reported antibiotic resistance frequencies of 59% and 55.5% to gentamicin for similar isolates in India and Bangladesh. These differences might possibly be due to the selective pressure on aminoglycosides as a result of increased use of gentamicin in various regions (Miller and Sabatelli, 1997). Chowdhury et al. (1994) reported in Bangladesh that 65 to 92% of Enterobacteriaceae isolated from urine samples were mostly resistant to frequently used antibiotics such as tetracycline, ampicillin, and co-trimoxazole. Recent findings indicated that bacteria harbouring multiple antibiotic resistance genes have suddenly become increasingly prevalent (Perez et al., 2007). In our study, all the K. oxytoca isolates were completely resistant (100%) to ofloxacin. The result of this study is in total agreement with other studies by Sasirekha et al. (2010) and Ullah et al. (2009), where resistance frequencies to antibiotics were reported to be 75 to 98% among Enterobacteriaceae. Also, third generation cephalosporins have been used for treatment of Gram-negative bacterial infections (Samaha-Kfoury and Araj, 2003). In this present study, all the K. oxytoca isolates were completely resistance to cefoxitin. Our study is also in concord with the work done by Sasirekha et al. (2010) in India where 85% of Klebsiella isolates were resistant to cefoxitin. In this study, the ESBL- producing isolates exhibited high resistance profiles. This agrees with the report of Okesola and Fowotade (2012) in Western Nigeria, where 70% Klebsiella isolates among the Enterobactericeae isolates were recorded among outpatients. The high rate of antibiotic resistance obtained from this study might possibly be attributed to poor antibiotic policy, irrational use of third generation cephalosprins (Shobha et al., 2007), and the emergence of antibiotic-resistant bacteria in hospitals. However, it has been reported that Kebsiella spp. were more resistant than E. coli which may be due to the fact that Klebsiella spp. harbour some virulence factors such as polysaccharide capsules, production of endotoxin, and carbapenemases, which make it more resistant than E. coli (Lin et al., 2011). Thomson (2001), in his findings, reported more resistance to cefotaxime and ceftazidime. This could probably signify overuse and irrational use of third-generation cephalosprins. In the present study, 3 (42.8%) out of the seven (7) K. oxytoca isolates were ESBL-producers. This study is similar to the findings of Livrelli et al. (1996) and Lal et al. (2007) who reported Klebsiella spp. as the leading bacteria that produce ESBLs. The high prevalence of ESBLs in Klebsiella spp. is of serious concern as infections caused by this bacterium is very common and antibiotic resistance exhibited by this bacterium may be due to the presence of the capsule that gives extra level of protection to the cells, presence of multi-drug resistance efflux pump, and their ability to acquire and disseminate resistance plasmids (Chaudhary and Aggarwal, 2004; Yushaꞌu et al., 2010). Also, the average MARI value of the ESBL- producing K. oxytoca isolates was one (1) (Tables 6 and 7). This result shows that the organisms expressed high resistance frequencies (100%).
In West Africa, especially Nigeria, β-lactam antibiotics are the most commonly prescribed drugs against gram-negative aerobic bacilli infections according to literature. The selective pressure exerted by the overuse of these β-lactam antibiotics especially in the treatment of some life-threatening infections, most likely result in strains developing ESBL enzymes. Results obtained from our study showed that the K. oxytoca isolates obtained from urine samples of patients were multi-drug resistant as they were resistant to at least two different classes of antibiotics. Our study also revealed the presence of ESBL-producing K. oxytoca strains in the urine samples of patients. High frequency of resistance exhibited by these K. oxytoca isolates against second and third generation cephalosporins indicates that very soon, these antibiotics may no longer be effective in the treatment of infections caused by these Enterobacteria (K. oxytoca) isolated from urine samples of patieints in Abakaliki. This is a serious public health problem and if not properly addressed, could metastasize into serious consequences. Although this study showed low prevalence of ESBL-producing K. oxytoca isolates from urine samples of patients, however, it is paramount to properly monitor antibiotic resistance in Klebsiella spp. so as to avert serious public health problem. Thus, proper antibiotics screening should be incorporated before antibiotics administration in clinical practice in order to detect bacteria that harbour multi-drug resistance traits and curtail the abuse of antibiotics.
The authors have not declared conflict of interests.
REFERENCES
|
Akujobi CN, Ewuru CP (2010). Detection of Extended Spectrum Beta-Lactamases in Gram Negative Bacilli from Clinical Specimens in a Teaching in South Eastern Nigeria. Nigerian Medical Journal 51:141-146.
|
|
|
|
Al-Zarouni M, Senok A, Rashid F, Al-Jesmi SM, Panigrahi D (2008). Prevalence and Antimicrobial Susceptibility Pattern of Extended-Spectrum Beta-Lactamase-Producing Enterobacteriaceae in the United Arab Emirates. Medical Principles and Practice 17(1):32-36.
Crossref
|
|
|
|
|
Blomberg B, Jureen R, Manji KP (2005). High Rate of Fatal Pediatric Septicemia caused by Gram-Negative Bacteria with Extended-Spectrum Beta-Lactamases in Dares Salaam, Tanzania. Journal of Clinical Microbiology 43:745-749.
Crossref
|
|
|
|
|
Chaudhary U, Aggarwal R (2004). Extended Spectrum Beta Lactamases (ESBL) An Emerging Threat to Clinical Theraputics. Indian Journal of Medical Microbiology 22(2):75-80.
|
|
|
|
|
Cheesbrough M (2004). District Laboratory Practice in Tropical Countries, Part Two, 2ndEdition. Cambridge University Press, UK, pp. 134-180.
|
|
|
|
|
Chowdhury MA, Yamanaka H, Miyoshi S, Aziz KM, Shinoda S (1994). Ecology of Vibrio mimicus in Aquatic Environments. Applied and Environmental Microbiology 55(8):2073-2078.
|
|
|
|
|
CLSI (2014). 24th informational supplement. Clinical and Laboratory Standards Institute (2014). M100-S24 Performance standards for antimicrobial susceptibility testing.
|
|
|
|
|
Haque R, Salam M (2010). Detection of ESBL Producing Nosocomial Gram Negative Bacteria from a Tertiary Care Hospital in Bangladesh. Pakistan Journal of Medical Sciences 26(4):887-891.
|
|
|
|
|
Iroha IR, Adikwu MU, Esimone CO, Abinu I, Amadi ES (2009). Exetended Spectrum Beta-Lactamase (ESBL) in Escherichia coli isolated from a Tertiary Hospital in Enugu State, Nigeria. Pakistan Journal of Medical Sciences 25(2):279-282.
|
|
|
|
|
Lal P, Kapil A, Das B, Sood S (2007). Occurrence of TEM and SHV Genes in Extended Spectrum Beta Lactamases (ESBLs) Producing Klebsiella species isolated from a Tertiary Care Hospital. Indian Journal of Medical Research 125:173-178.
|
|
|
|
|
Lin Y, Lu M, Hui-Ling C, Tang H, Liu H, Chen C, Liu K, Lin C, Chiou C, Chiang M, Chen C, Yi C, Lai Y (2011). Assessment of hypermucoviscosity as a Virulence Factor for Experimental Klebsiella pneumoniae Infections: Comparative Virulence Analysis with hypermucoviscosity-Negative Strain. Bio Med Central Microbiology 11:50.
Crossref
|
|
|
|
|
Livrelli V, Champs CD, Martino PD, Michaud AD, Forestier C, Joly B (1996). Adhesive Properties and Antibiotic Resistance of Klebsiella, Enterobacter and Serratia Clinical Isolates Involved in Nosocomial Infections. Journal of Clinical Microbiology 34(8):1963-1969.
|
|
|
|
|
Mammeri H, Van De LM, Poirel L, Martinez-Martinez L, Nordmann P (2005). Emergence of plasmid-mediated quinolone resistance in Escherichia coli in Europe. Antimicrobial Agents Chemotherapy 49:71-76.
Crossref
|
|
|
|
|
Miller GH, Sabatelli FJ (1997). The Most Frequent Aminoglycoside Resistance Mechanisms Changes with Time and Geographic Area: a Reflection of Aminoglycoside Usage Patterns? Aminoglycoside Resistance Study Groups. Clinical Infectious Diseases 24(1):46-62.
Crossref
|
|
|
|
|
Okesola AO, Fowotade A (2012). Extended-Spectrum Beta-Lactamase Production among Clinical Isolates of Escherichia coli. International Research Journal of Microbiology 3(4):140-143.
|
|
|
|
|
Paterson DL (2000). Recommendation for treatment of severe infections caused by Enterobacteriaceae producing extended-spectrum β-lactamases (ESBLs). Clinical Microbiology Infection 6:460-463.
Crossref
|
|
|
|
|
Paterson DL, Bonomo RA (2005). Extended-spectrum β-lactamases: A clinical update. Clinical Microbiology Review 18:657-686.
Crossref
|
|
|
|
|
Paterson DL (2007). Recommendation for Treatment of Severe Infections caused by EnterobacteriaceaeProducing Extended-Spectrum β-Lactamases (ESBLs). Clinical Microbiology and Infection 6:460-463.
Crossref
|
|
|
|
|
Perez F, Enridimiani A, Hujer K, Bonomo RA (2007). The continuing challenge of ESBL. Current Opinion in Pharmacology 7(5):459-469.
Crossref
|
|
|
|
|
Philippon A, Labia R, Jacoby G (1989). Extended-spectrum beta-lactamases. Antimicrobial Agents Chemotherapy 33:1131-1136.
Crossref
|
|
|
|
|
Samaha-Kfoury JN, Araj GF (2003). Recent Developments in β Lactamases and Extended Spectrum β-lactamases. Beirut Medical Journal 327(22):1209-1213.
Crossref
|
|
|
|
|
Sasirekha B, Manasa R, Ramya P, Sneha R (2010). Frequency and Antimicrobial Sensitivity Pattern of Extended Spectrum β- Lactamases-Producing Escherichia coli and Klebsiella pneumonia Isolated In A Tertiary Care Hospital, Al Ameen. Journal of Medical Science 3(4):265-271.
|
|
|
|
|
Shobha KL, Gowrish RS, Sugandhi R, Sreeja CK (2007). Prevalence of Extended Spectrum β-Lactamases in Urinary Isolates of Escherichia coli, Klebsiella and Citrobacter Species and their Antimicrobial Susceptibility Pattern in tertiary care hospital. Indian Journal for the Practicing Doctor 3(6):01-02.
|
|
|
|
|
Thomson KS (2001). Controversies about Extended-Spectrum and AmpC β-Lactamases. Emerging Infectious Diseases 7:333-336.
Crossref
|
|
|
|
|
Ullah F, Malik SA, Ahmed J (2009). Antimicrobial Susceptibility Pattern and ESBL Prevalence in Klebsiella pneumoniae from Urinary Tract Infections in the North. West of Pakistan. African Journal of Microbiology 3(11):670- 680.
|
|
|
|
|
Yushaꞌu M, Kumurya AS, Suleiman L (2010). Prevalence of Extended spectrum β- lactamases among Enterobacteriaceae in Murtala Mohammed specialist hospital, Kano. Journal of Pure and Applied Sciences 3(1):169-172.
Crossref
|
|