ABSTRACT
Novel fluorous and non-fluorous surfactants have been synthesized and examined for their potential as microbicides compared with nonoxynol-9 (N-9) by testing their effects on Candida albicans (C. albicans) and Escherichia coli (E. coli). These compounds include nonionic surfactants consisting of F5-triethylene glycol (F5-TEG), F7-triethylene glycol (F7-TEG), C5-triethylene glycol (C5-TEG), C7-triethylene glycol (C7-TEG), and anionic surfactants consisting of F5-propane sultone (F5-PS), and F7-propane sultone (F7-PS). In this study, we investigated the possible effects of fluorous and non-fluorous surfactants on the growth of C. albicans and E. coli cultured in vitro, which were treated individually with different concentrations of these novel surfactants and nonoxynol-9 (N-9). Then, each sample was incubated for 3, 6, 24 and 48 h at 35°C for C. albicans and 37°C for E. coli. After incubation, C. albicans and E. coli colonies were evaluated compared with the control. N-9 and F5-PS had only small effects (25% growth inhibition or lower) on C. albicans but F7-PS, F7-TEG, F5-TEG, and C5-TEG notably inhibited C. albicans growth, and had potential to control their population. C. albicans cells treated with 10% F7-TEG, F5-TEG, or C5-TEG showed no growth; especially, C5-TEG gave the maximum growth inhibition of C. albicans. For E. coli, N-9 had no growth inhibition but F7-PS, F5-TEG, C5-TEG, C7-TEG, and F7-TEG inhibited E. coli growth. Interestingly, F7-TEG showed the maximum inhibition for E. coli starting at a concentration of 1%. Therefore, these surfactants might have potential for prevention or treatment of genital and urinary tract infection from C. albicans and E. coli.
Key words: Candida albicans, Escherichia coli, nonoxynol-9, fluorous surfactants, non-fluorous surfactants.
Since the 1950’s the nonionic surfactant, nonoxynol-9 (N-9), has been widely used as a contraceptive agent (Savle et al., 1999; Gandour, 2005). It immobilizes sperm in seconds after penetrating into sperm membranes and forming mixed micelles with their lipids and causes sperm membrane damage (Schill and Wolff, 1981; Wilborn et al., 1983; Doncel, 2006). While effective at killing sperm, many recent studies report that N-9 also damages epithelial cells and normal flora in vagina. N-9 disrupts normal flora such as Lactobacillus and creates imbalance
in the vagina (Klebanoff, 1992; McGroarty et al., 1992; Stafford et al., 1998; Patton et al., 1999; Gupta, 2005). The imbalance in vagina can enhance the growth of Candida and Escherichia coli, which often become serious pathogens in the vagina and urinary tract. In addition, with prolonged use N-9 can cause tiny abrasions inside the sensitive vaginal and anal walls, leading to increased risk of infections (Raymond et al., 2004; Gandour, 2005).
E. coli, a Gram negative bacterium, is commonly found in the lower intestine of warm-blooded animals. Virulent strains of E. coli can cause vaginal and urinary tract infections (McGroarty et al., 1994). Use of N-9 can increase the risk of E. coli colonization in vagina and increase rates of urinary tract infection by four times (McGroarty et al., 1994; Watts et al., 1999). Candida is yeast and the most common cause of opportunistic infection of the female reproductive tract. It is among the normal flora of skin, mouth, vagina, and intestinal tract. There are many species of Candida that can cause genital candidiasis or vulvovaginitis. The most important causative agent is Candida albicans (Dupont, 1995). Use of spermicides containing N-9 can increase the chance of Candida adhesion to epithelia, with consequent increase in the opportunity of genital tract infection (Gandour, 2005).
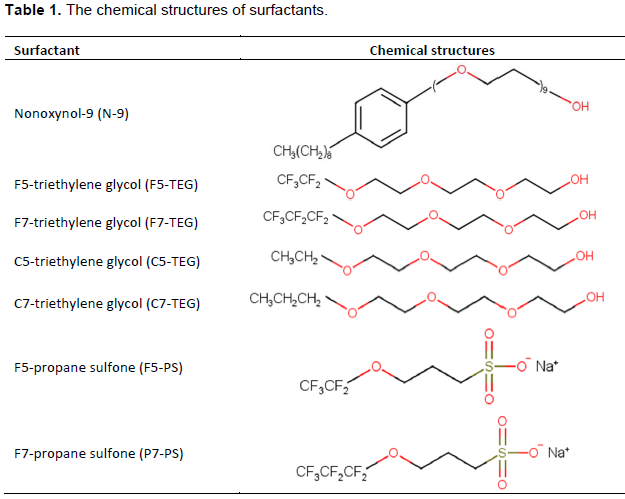
Due to adverse effects of N-9, novel fluorous and non-fluorous surfactants have been synthesized and their potentials as spermicides and microbicides are under investigation. These novel surfactants are able to kill human and mouse sperm, but show only little effect on HeLa cells. Additionally, they might have potential to control overgrowth of C. albicans and E. coli. The structures of the compounds reported here are shown in Table 1 along with their common names.
Here, we report the potential of new fluorous and non-fluorous surfactants as microbicides for C. albicans and E. coli.
Microbes and culture media
Candida albicans received from St. Luke’s Hospital (Bethlehem, PA) was cultured in Yeast Extract-Peptone-Dextrose (YPD) broth and YPD agar (Becton, Dickinson and Company, MD) and incubated at 35oC. E. coli (ATCC 25922) was cultured in Luria-Bertani (LB) broth and agar (Becton, Dickinson and Company, MD) and incubated at 37°C. All culture media were prepared as the instructions from the manufacture.
Surfactants
Non-ionic nonoxynol-9 (N-9) was procured from Sigma-Aldrich Inc., MO. Other surfactants, F5-triethylene glycol (F5-TEG), F7-triethylene glycol (F7-TEG), C5-triethylene glycol (C5-TEG), C5-triethylene glycol (C5-TEG), F5-propane sultone (F5-PS), and F7-propane sultone (F7-PS), were obtained from the Department of Chemistry, Lehigh University, Bethlehem, PA. The chemical structures of all surfactants are listed in the Table 1. The synthesis and properties of these compounds have been previously reported (Bean et al., 2011).
Microbicidal effects
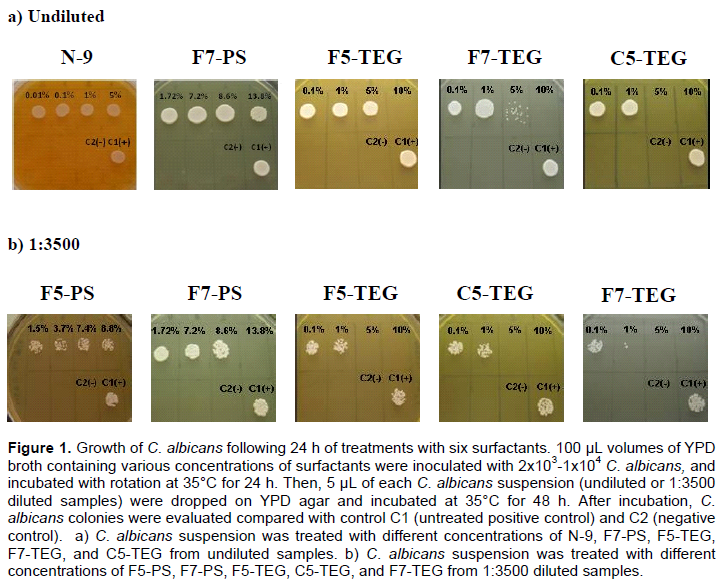
Several concentrations of each surfactant were made in YPD broth: 0.1, 1, and 5% non-ionic N-9, 0.1, 1, 5, and 10% non-ionic C5-TEG, non-ionic C7-TEG, non-ionic F5-TEG, and non-ionic F7-TEG, 1.5, 3.7, 7.4, and 8.8% anionic F5-PS, and 1.72, 7.2, 8.6 and 13.8% anionic F7-PS. Positive control C1 (98 µL YPD broth and 2 µL C. albicans suspension) and negative control C2 (100 µL YPD broth) were prepared. Yeast cells were suspended in 0.85% NaCl solution and cell densities were estimated by absorbance. Cell density was adjusted with spectrophotometer by adding sufficient sterile saline to increase the transmittance to that produced by a 0.5 McFarland standard at 530 nm wavelength. This procedure yielded a yeast stock suspension of 1-5x106 cells per mL. The stock suspension was diluted by 1:50 in YPD broth medium containing each surfactant, which results in 2x104 to 1x105 cells per mL. Tubes containing these treated cultures were incubated with rotation at 35°C for 3, 6, 24, and 48 h. After incubation, 5 µL of each C. albicans suspension were dropped on YPD agar. C. albicans suspensions were undiluted and diluted to 1:1000 and 1:3500 before plating on YPD agar. Each C. albicans-plated YPD agar was incubated at 35°C for 24 and 48 h. After incubation, C. albicans colonies were evaluated compared with control. Results were documented and shown in Figure 1.
For E. coli investigations, similar concentrations of each of the above surfactants were made in LB broth positive control C1 (98 µL LB broth and 2 µL E. coli suspension) and C3 (49 µL LB broth and 49 µL sterile distilled water) and negative control C2 (100 µL LB broth) were prepared. E. coli was suspended in 0.85% NaCl solution and adjusted with saline to give a turbidity equivalent to the McFarland 0.5 standard (around 1x108 cells/mL). The stock suspension was diluted by 1:50 in LB broth containing each surfactant to give a final organism density of 2x104-1x105 cells per mL. Tubes were cultured with rotation at 37°C for 3, 6, 24 and 48 h. After incubation, 5 µL of each E. coli suspension was dropped on LB agar. E. coli suspensions were undiluted and diluted to 1:3500 before plating on LB agar. Each E. coli-plated LB agar was incubated at 37°C for 24 and 48 h. After incubation, E. coli colonies were evaluated and compared with control. Results were documented and shown in Figure 1.
Effects of fluorous and non-fluorous surfactants on C. albicans
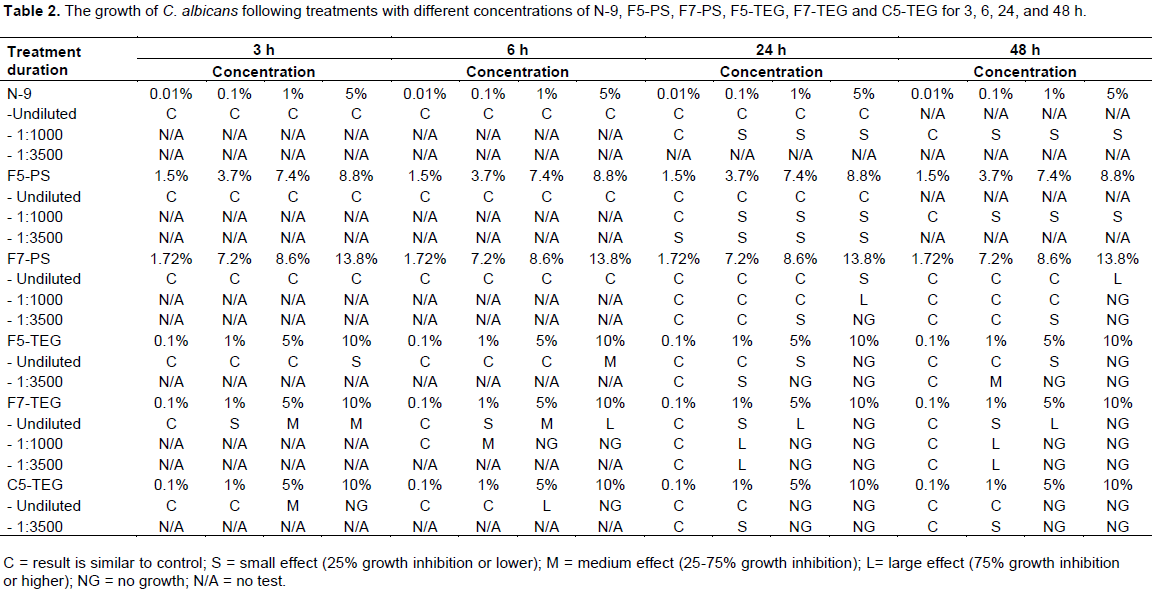
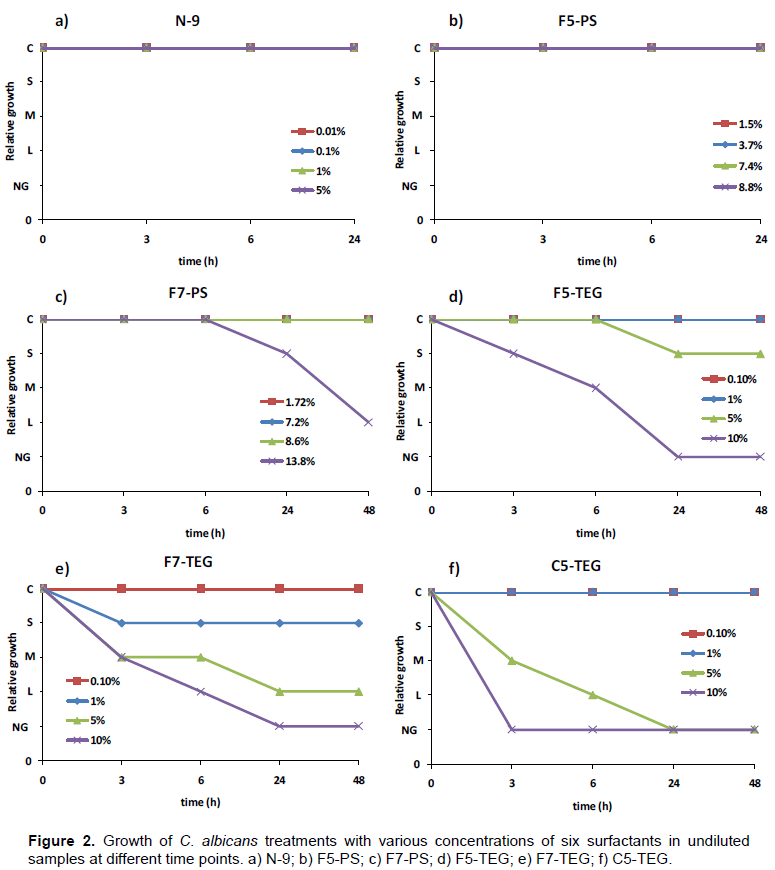
The effects on growth of C. albicans treated with various concentrations of six surfactants were determined. A sampling of results is shown in Figure 1a for undiluted samples and Figure 1b for dilution at 1:3500 at 24 h and a detailed summary for all treatments is presented in Table 2. There was no effect of all concentrations of N-9 on C. albicans of undiluted samples at 3, 6, and 24 h (Figure 1a, 2a). At dilution 1:1000, N-9 showed a little effect in 0.1, 1, and 5% at 24 h and 48 h (Table 2). F5-PS did not show growth-inhibition effect on C. albicans in undiluted samples at any time periods as shown in Figure 2b. However, Candida growth was slightly inhibited at 3.7, 7.4, and 8.8% of F5-PS in the sample dilution of 1:1000 (at 24 and 48 h) and 1:3500 at 24 h (Figure 1b and Table 2). F7-PS at 13.8% concentration showed small effect (25% growth inhibition or lower) on C. albicans in undiluted samples at 24 h (Figure 1a), but it had a large effect (75% growth inhibition or higher) on C. albicans at 48 h (Figure 2c). Moreover, 13.8% of F7-PS showed large effect (75% growth inhibition or higher) on C. albicans at 24 h, and no growth at 48 h for diluted samples of 1:1000 and we found that it inhibited C. albicans growth effectively at 24 and 48 h for diluted samples of 1:3500 (Figure 1b at 24 h and Table 2). At 10% concentration of F5-TEG, the results of undiluted samples revealed that there were small (25% growth inhibition or lower) and medium (25-75% growth inhibition) effects at 3 and 6 h, respectively and F5-TEG killed all C. albicans following 24 (Figure 1a) and 48 h of treatment (Figure 2d and Table 2). At sample dilution of 1:3500, 5 and 10% of F5-TEG inhibited Candida growth significantly at 24 (Figure 1b) and 48 h (Table2).The growth of C. albicans cells treated with 1 ,5 and 10% of F7- TEG was decreased in undiluted samples at 3 and 6 h (Figure 2e and Table 2). At 24 and 48 h, C. albicans growth was dramatically decreased at 5% and completely inhibited at 10% (Figure 1a and 2e). For sample dilution of 1:1000, 1% concentration of F7-TEG inhibited growth of C. albicans cells, which were significantly killed in 5% and 10% at 6, 24 and 48 h (Table 2). The similar results were shown for sample dilution of 1:3500 at 24 (Figure 1b) and 48 h (Table 2). When C. albicans was treated with C5-TEG, 5% of C5-TEG decreased cell growth in undiluted samples at 3 and 6 h and showed no growth in the concentration of 10% (Figure 2f and Table 2). Additionally, at 24 and 48 h, 5% and 10% of C5-TEG completely inhibited C. albicans growth in undiluted (Figure 1a, 2f and Table 2) and 1:3500 samples (Figure 1b and Table 2). Based on the experimental results, N-9, F5-PS, and F7-PS showed little growth inhibition (25% inhibition or lower) ability at the same concentration. On the other hand, F5-TEG, F7-TEG, and C5-TEG revealed striking growth inhibition of C. albicans.
Effects of fluorous and non-fluorous surfactants on E. coli
E. coli cells were treated with various concentrations
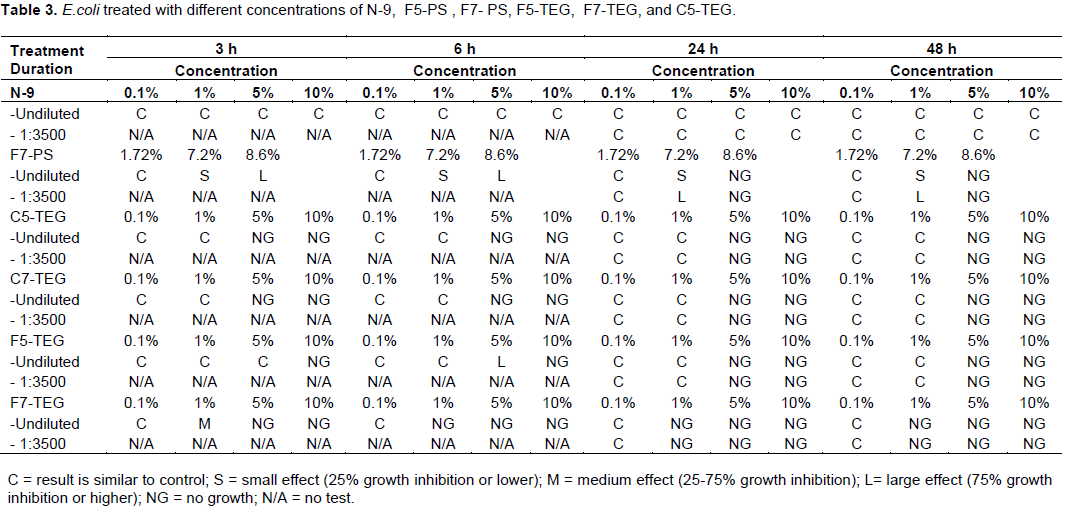
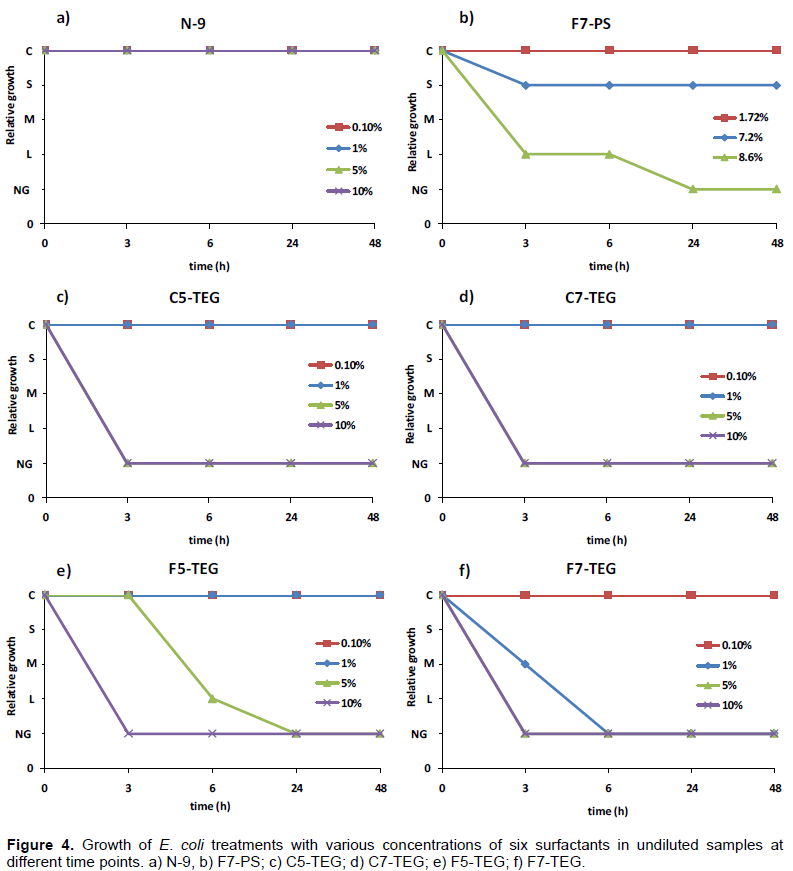
of different surfactants. The results for all surfactants are listed in Table 3, and examples of plates are shown in Figure 3. There was no growth inhibition effect of N-9 on E. coli at all concentrations (Figure 3, 4a and Table 3). At undiluted samples, E. coli treated with F7-PS showed large effects (75% inhibition or higher) at 3 and 6 h, and no growth in concentration of 8.6% at 24 and 48 h (Figure 3a, 4b and Table 3). Similar results of 1:3000 diluted samples treated with 8.6% of F7-PS were found (Figure 3b and Table 3). In addition, we found that 5, 10%, or higher concentration of C5-TEG and C7-TEG inhibited E. coli growth effectively at 3, 6, 24 and 48 h (Figure 3, 4c, 4d and Table 3). 10% of F5-TEG showed E. coli growth inhibition at 3, 6, 24, and 48 h in undiluted treatments (Figure 3a, 4e and Table 3). Moreover, at
24 and 48 h, 5% and higher concentration of F5-TEG completely inhibited E. coli in all samples (Figure 3, 4e and Table3). In concentration of 1, 5 and 10% of F7-TEG dramatically decreased E. coli growth at 6, 24 and 48 h (Figure 3, 4f and Table 3). All F7-TEG treatments produced similar effects on E. coli in undiluted (Figure 4f) and diluted (1:3500) samples (Figure 3 and Table 3). Therefore, F7- TEG was observed to be the most potent surfactant for E. coli growth inhibition. Based on the results, N-9 showed no effect on E. coli, but F7-PS, F5-TEG, C5-TEG, C7-TEG, and F7-TEG showed effective inhibition on E. coli growth. Interestingly, F7-TEG showed the excellent inhibition growth of E. coli starting at low concentration from 1%.
Based on the results, there was no effect of all concentrations of N-9 on C. albicans and E. coli similar to several reports. Uropathogenic bacteria including E. coli, Proteus mirabilis, Enterococcus faecalis and Staphylococcus species have been found growing at concentration of 25% or higher of N-9 (McGroarty et al., 1994). Watts et al. (1999) studied the effects of N-9 on E. coli and found that the number of women increased the colonization of E. coli in vagina after using N-9 (Watts, et al., 1999). Their results are consistent with many previous researches both in vivo and in vitro studies (Fihn et al., 1985; Foxman and Frerichs, 1985; Watts et al., 1999). After N-9 insertion into vagina without using diaphragm, E. coli colonization increase has been reported in several studies (Rosenstein et al., 1998; Watts, et al., 1999). These lead to increase rates of bacteriuria or urinary tract infection (Percival-Smith et al., 1983; Fihn et al., 1996). C. albicans, another organism that causes vaginal infection, can survive at high concentration of N-9 (McGroarty et al., 1994). Candida colonization in vagina has been found after using N-9 and also causes vaginal burning, vaginal itching, and vulvar burning (Schreiber et al., 2006). Moreover, N-9 has been found to increase the adhesion of Candida species to human epithelial cell leading to increase in the risk of a serious fungal infection (Gandour, 2005). Additionally, many studies have determined that N-9 increases the risk of infection and also causes vaginal inflammation and ulceration which increase the risk of HIV-1 infection in females (Kreiss et al., 1992; Fichorova et al., 2001; Van Damme et al., 2002; Howett and Kuhl, 2005). Increased rates of vaginal ulceration have been found in the use of the vaginal contraceptive sponge, which has high concentration of N-9 (Kreiss et al., 1992; Watts et al., 1999). Vulvar itching, pain, burning and abnormal discharge have been found after using N-9 (d'Oro et al., 1994; McGroarty et al., 1994). It has been shown that N-9 kills the natural vaginal flora including Lactobacillus causing disturbance of the normal acidic vaginal pH and leading to vaginal infection and urinary tract infection (Hooton et al., 1991; Klebanoff, 1992; McGroarty et al., 1992; Stafford et al., 1998; Patton et al., 1999; Watts et al., 1999; Handley et al., 2002; Brzezinski et al., 2004; Gupta, 2005; Zhou et al., 2010; Ravel et al., 2011).
In this study, C. albicans and E. coli were treated with different concentrations of N-9 and various new surfactants at different periods of times. As mentioned above, N-9 was ineffective on C. albicans and E. coli growth inhibition. All surfactants except F5-PS showed the effective effects on C. albicans growth inhibition. N-9 and F5-PS revealed the low level of inhibition on C. albicans. In E. coli, all surfactants showed effects on growth inhibition except N-9. Based upon the results, most of our novel surfactants have potential to inhibit growth of E. coli and C. albicans. 0.1% N-9 kills human sperm effectively (data not shown), but cannot inhibit growth of both microorganisms. There is a research showing that N-9 is toxic to HeLa cells and Lactobacillus (Gupta, 2005). The five novel surfactants can kill sperm, but show only little effect on HeLa cells (Bean et al., 2011). From the experimental results, some novel surfactants, F5-TEG (12%) and F7-TEG (10%), killed sperm and controlled E. coli and C. albicans population, but revealed very small effect on HeLa cells (Bean et al., 2011). Therefore, they might be developed to be used as contraceptive agents that have an ability to protect E. coli and C. albicans infections in vagina and urinary tract and also gentle to epithelium cells. Another surfactant, C5-TEG (12%) killed sperm and inhibited growth of E. coli and C. albicans effectively. From its ability, it might be used as spermicide and microbicide; however, it needs to be tested on HeLa cells. Based on effects on both microorganisms, F5-TEG, F7-TEG, C5-TEG, and C7-TEG were able to inhibit microbial growth better than N-9, F5-PS, and F7-PS.
The structure differences between anionic surfactants (F5-PS and F7-PS) and non-ionic surfactants (F5-TEG, F7-TEG, C5-TEG, and C7-TEG) may be the major point resulting in different effects on C. albicans and E. coli growth inhibition. Non-ionic surfactants gave the better results on growth inhibition for both microorganisms. The dissociation in water may relate to the inhibiting activity of the surfactants. It might involve the surface electrostatic potential of membranes in which the hydrophobic portion of these surfactants is inserted as a consequence of the hydrophobic effect (Vieira and Carmona-Ribeiro, 2006; Vieira et al., 2008). Non-ionic surfactants may insert into cell membranes without charge relationship on the membranes, but anionic surfactant insertion relates to charges on the membranes. However, the mechanism of both anionic and non-ionic surfactants on microorganisms is still not clearly understood.
Non-fluorous TEG provided the excellent inhibiting effects on C. albicans. On the other hand, fluorous TEG showed the large effect (75% inhibition or higher) on E. coli. Based upon the results, surfactant toxicity is not depended on only the chemical structure of the surfactants but also on the nature of cell membranes, which are different in diverse species. The C. albicans membrane has differences in chemical composition and physical properties from the membrane of E. coli (Vieira et al., 2008). Moreover, F7-TEG gave better inhibition effects than F5-TEG for both microorganisms. These show that the amount of fluorine in surfactants is important for inhibiting growth activity. The effect of fluorous surfactant on microbial growth is positively correlated with the number of fluorine in surfactant molecule. Nevertheless, in E. coli, the amount of hydrocarbon in non-fluorous TEG surfactants shows no difference in growth inhibition.
Our novel surfactants inhibit growth of C. albicans and E. coli including sperm killing. Based on experimental results, these surfactants might be used as antifungal and antibacterial agents. Moreover, they showed the potential to be developed as contraceptive agents, which might substitute the use of N-9. Therefore, non-ionic and anionic fluorous surfactants may have practical values as fungistatic/bacteriostatic or fungicidal/bactericidal agents and might be useful as vaginal microbicides and spermicides. However, the effects of these surfactants on normal flora need to be determined and their mechanisms on C. albicans and E. coli will be studied to better understand. In the future, these compounds might be useful for treating genital candidiasis and urinary tract infection in patients.
The author(s) did not declare any conflict of interest.
REFERENCES
|
Bean B, Flowers RA, Venditti JJ, Singh R (2011). Spermicidal and microbicidal compositions. US 2011/0237676 A1. USA
|
|
|
|
Brzezinski A, Stern T, Arbel R, Rahav G, Benita S (2004). Efficacy of a novel pH-buffering tampon in preserving the acidic vaginal pH during menstruation. Int. J. Gynaecol. Obstet. 85(3): 298-300.
Crossref
|
|
|
|
|
Doncel GF (2006). Exploiting common targets in human fertilization and HIV infection: development of novel contraceptive microbicides. Hum. Reprod. Update 12(2): 103-117.
Crossref
|
|
|
|
|
d'Oro LC, Parazzini F, Naldi L, La Vecchia C (1994). Barrier methods of contraception, spermicides, and sexually transmitted diseases: a review. Genitourin Med. 70(6): 410-417.
Crossref
|
|
|
|
|
Dupont PF (1995). Candida albicans, the opportunist. A cellular and molecular perspective. J. Am. Podiatr. Med. Assoc. 85(2): 104-115.
Crossref
|
|
|
|
|
Fichorova RN, Tucker LD, Anderson DJ (2001). The molecular basis of nonoxynol-9-induced vaginal inflammation and its possible relevance to human immunodeficiency virus type 1 transmission. J. Infect. Dis. 184(4): 418-428.
Crossref
|
|
|
|
|
Fihn SD, Boyko EJ, Normand EH, Chen CL, Grafton JR, Hunt M, Yarbro P, Scholes D, Stergachis A (1996). Association between use of spermicide-coated condoms and Escherichia coli urinary tract infection in young women. Am. J. Epidemiol. 144(5): 512-520.
Crossref
|
|
|
|
|
Fihn SD, Latham RH, Roberts P, Running K, Stamm WE (1985). Association between diaphragm use and urinary tract infection. JAMA 254(2): 240-245.
Crossref
|
|
|
|
|
Foxman B, Frerichs RR (1985). Epidemiology of urinary tract infection: I. Diaphragm use and sexual intercourse. Am. J. Public Health 75(11): 1308-1313.
Crossref
|
|
|
|
|
Gandour RD (2005). Toward a design of affordable, topical microbicides: acylcarnitine analogues. Curr. Pharm. Des. 11(29): 3757-3767.
Crossref
|
|
|
|
|
Gupta G (2005). Microbicidal spermicide or spermicidal microbicide? Eur. J. Contracept. Reprod. Health Care 10(4): 212-218.
Crossref
|
|
|
|
|
Handley MA, Reingold AL, Shiboski S, Padian NS (2002). Incidence of acute urinary tract infection in young women and use of male condoms with and without nonoxynol-9 spermicides. Epidemiology 13(4): 431-436.
Crossref
|
|
|
|
|
Hooton TM, Fennell CL, Clark AM, Stamm WE (1991). Nonoxynol-9: differential antibacterial activity and enhancement of bacterial adherence to vaginal epithelial cells. J. Infect. Dis. 164(6): 1216-1219.
Crossref
|
|
|
|
|
Howett MK, Kuhl JP (2005). Microbicides for prevention of transmission of sexually transmitted diseases. Curr. Pharm. Des. 11(29): 3731-3746.
Crossref
|
|
|
|
|
Klebanoff SJ (1992). Effects of the spermicidal agent nonoxynol-9 on vaginal microbial flora. J. Infect. Dis. 165(1): 19-25.
Crossref
|
|
|
|
|
Kreiss J, Ngugi E, Holmes K, Ndinya-Achola J, Waiyaki P, Roberts PL, Ruminjo I, Sajabi R, Kimata J, Fleming TR (1992). Efficacy of nonoxynol 9 contraceptive sponge use in preventing heterosexual acquisition of HIV in Nairobi prostitutes. JAMA 268(4): 477-482.
Crossref
|
|
|
|
|
McGroarty JA, Reid G, Bruce AW (1994). The influence of nonoxynol-9-containing spermicides on urogenital infection. J. Urol. 152(3): 831-833.
|
|
|
|
|
McGroarty JA, Tomeczek L, Pond DG, Reid G, Bruce AW (1992). Hydrogen peroxide production by Lactobacillus species: correlation with susceptibility to the spermicidal compound nonoxynol-9. J. Infect. Dis. 165(6): 1142-1144.
Crossref
|
|
|
|
|
Patton DL, Kidder GG, Sweeney YC, Rabe LK, Hillier SL (1999). Effects of multiple applications of benzalkonium chloride and nonoxynol 9 on the vaginal epithelium in the pigtailed macaque (Macaca nemestrina). Am. J. Obstet. Gynecol. 180(5):1080-1087.
Crossref
|
|
|
|
|
Percival-Smith R, Bartlett KH, Chow AW (1983). Vaginal colonization of Escherichia coli and its relation to contraceptive methods. Contraception 27(5): 497-504.
Crossref
|
|
|
|
|
Ravel J, Gajer P, Abdo Z, Schneider GM, Koenig SS, McCulle SL, Karlebach S, Gorle R, Russell J, Tacket CO, Brotman RM, Davis CC, Ault K, Peralta L, Forney LJ (2011). Vaginal microbiome of reproductive-age women. Proc. Natl. Acad. Sci. USA. 108 (Suppl. 1):4680-4687.
Crossref
|
|
|
|
|
Raymond EG, Chen PL, Luoto J (2004). Contraceptive effectiveness and safety of five nonoxynol-9 spermicides: a randomized trial. Obstet. Gynecol. 103(3):430-439.
Crossref
|
|
|
|
|
Rosenstein IJ, Stafford MK, Kitchen VS, Ward H, Weber JN, Taylor-Robinson D (1998). Effect on normal vaginal flora of three intravaginal microbicidal agents potentially active against human immunodeficiency virus type 1. J. Infect. Dis. 177(5): 1386-1390.
Crossref
|
|
|
|
|
Savle PS, Doncel GF, Bryant SD, Hubieki MP, Robinette RG, Gandour RD (1999). Acylcarnitine analogues as topical, microbicidal spermicides. Bioorg. Med. Chem. Lett. 9(17): 2545-2548.
Crossref
|
|
|
|
|
Schill WB, Wolff HH (1981). Ultrastructure of human spermatozoa in the presence of the spermicide nonoxinol-9 and a vaginal contraceptive containing nonoxinol-9. Andrologia 13(1): 42-49.
Crossref
|
|
|
|
|
Schreiber CA, Meyn LA, Creinin MD, Barnhart KT, Hillier SL (2006). Effects of long-term use of nonoxynol-9 on vaginal flora. Obstet. Gynecol. 107(1): 136-143.
Crossref
|
|
|
|
|
Stafford MK, Ward H, Flanagan A, Rosenstein IJ, Taylor-Robinson D, Smith JR, Weber J, Kitchen VS (1998). Safety study of nonoxynol-9 as a vaginal microbicide: evidence of adverse effects. J. Acquir. Immune Defic. Syndr. Hum. Retrovirol. 17(4): 327-331.
Crossref
|
|
|
|
|
Van Damme L, Ramjee G, Alary M, Vuylsteke B, Chandeying V, Rees H, Sirivongrangson P, Mukenge-Tshibaka L, Ettiegne-Traore V, Uaheowitchai C, Karim SS, Masse B, Perriens J, Laga M (2002). Effectiveness of COL-1492, a nonoxynol-9 vaginal gel, on HIV-1 transmission in female sex workers: a randomised controlled trial. Lancet 360(9338): 971-977.
Crossref
|
|
|
|
|
Vieira DB, Carmona-Ribeiro AM (2006). Cationic lipids and surfactants as antifungal agents: mode of action. J. Antimicrob. Chemother. 58(4): 760-767.
Crossref
|
|
|
|
|
Vieira OV, Hartmann DO, Cardoso CM, Oberdoerfer D, Baptista M, Santos MA, Almeida L, Ramalho-Santos J, Vaz WL (2008). Surfactants as microbicides and contraceptive agents: a systematic in vitro study. PLoS ONE 3(8): e2913.
Crossref
|
|
|
|
|
Watts DH, Rabe L, Krohn MA, Aura J, Hillier SL (1999). The effects of three nonoxynol-9 preparations on vaginal flora and epithelium. J. Infect. Dis. 180(2):426-437.
Crossref
|
|
|
|
|
Wilborn WH, Hahn DW, McGuire JJ (1983). Scanning electron microscopy of human spermatozoa after incubation with the spermicide nonoxynol-9. Fertil. Steril. 39(5):717-719.
Crossref
|
|
|
|
|
Zhou X, Hansmann MA, Davis CC, Suzuki H, Brown CJ, Schutte U, Pierson JD, Forney LJ (2010). The vaginal bacterial communities of Japanese women resemble those of women in other racial groups. FEMS Immunol. Med. Microbiol. 58(2):169-181.
Crossref
|
|