Full Length Research Paper
ABSTRACT
Anemia is a public health problem and has significant adverse health consequences in HIV-infected persons. In Burkina Faso, there are little data on anemia in HIV-1 infected children. This study aimed to describe the prevalence of anemia and its associated factors in HIV-infected children before and after their highly active antiretroviral therapy (HAART). This was a retrospective study that involved a cohort of 151 HIV-1 infected children on HAART at the pediatric service of Saint Camille Hospital, from January 2018 to October 2018. Data were collected before and after their HAART initiation and analyzed using SPSS version 21.0. The prevalence of anemia was 81.46% before treatment and 48.34% 12 months after initiation of treatment. There was a significant association between gender, BMI, TB, and CD4 counts before HAART initiation While, after HAART initiation, only gender, age, and CD4 T cell count were significantly associated with anemia. Children with a CD4 count <200 Cell/µl had a risk of developing anemia before HAART initiation but no longer had a risk of developing anemia 1 year after HAART initiation [OR=0.35 (0.14-0.89); p=0.028 vs. OR=1.54 (0.67-3.51); p=0.300].This study showed that antiretroviral treatment contributes strongly to improving the hemoglobin level in persons living with HIV.
Key words: HIV, anemia, prevalence, highly active antiretroviral therapy (HAART), Children, Burkina Faso.
INTRODUCTION
The number of children (0-14 years) infected with HIV was estimated to be 1.7 million [1.2 million- 2.2 million] in 2020 (UNAIDS, 2021). HIV infection causes several complications, including anemia (Cao et al., 2022). Anemia defined as reduced hemoglobin levels of red blood cells may carry less oxygen to skeletal muscle and impair physical performance (Tsai et al., 2019). Anemia is a risk factor for death in HIV-1 infected individuals (Harding et
al., 2020). In Burkina Faso, the prevalence of anemia in children aged 6-59 months was 87.0% in 2020 (Muriuki et al., 2020). In children, depending on the hemoglobin level, anemia can be considered severe (Hb level<7 g/dL), moderate (Hb level [7-9.9] g/dL), and mild (Hb level [10-11] g/dL). According to WHO, Africa represented the continent with the highest rate of anemia in children aged 6-59 months (60.2%), followed by Southeast Asia (49.0%) (WHO, 2021a).
In Africa, anemia remains the most frequently observed hematologic abnormality and an independent predictor of disease progression in people living with HIV, particularly in children and most people infected with HIV-1 are anemic (Abioye et al., 2020; Duffy et al., 2020). However, the risk of anemia is reduced after the initiation of highly active antiretroviral therapy (HAART) (Yesuf et al., 2019). Indeed, studies have shown that antiretroviral therapy helps to improve anemia and reduce mortality in children (Haider et al., 2019; Duffy et al., 2020). Moreover, antiretroviral treatment (ART) is known to profoundly suppress viral replication. It increases CD4 cell count, delays disease progression and death; patients on highly active antiretroviral therapy commonly suffer from side effects of the drugs (Geletaw et al., 2017; Mouhari-Toure et al., 2018). Each antiretroviral (ARV) drug is associated with specific adverse effects and among the ARV drugs, zidovudine (AZT) remains the most widely used drug, resulting in myelosuppression (Leroi et al., 2017) and has been associated with anemia (Tamir et al., 2018).
Studies have identified factors associated with anemia in HIV-infected children such as age, low CD4 percentage, WHO clinical stage, and Iron deficiency (Bisong et al., 2017; Lai et al., 2018; Huibers et al., 2020). A study in Uganda showed that in patients who started or continued ARV therapy, CD4 counts increased significantly over 18 months of follow-up, and the improvement did not differ by baseline ferritin level or anemia status (Ezeamama et al., 2019).
In Burkina Faso, there are little data on the effect of ARV treatment on anemia in HIV-1 infected children as well as the associated factors. However, having information on this would help in the better therapeutic and clinical management of children. So, the objective of this study was to describe the prevalence and associated factors of anemia in HIV-infected children before and after their HAART.
MATERIALS AND METHODS
Study population and data collection
This was a retrospective study whose data were collected from the records of HIV-1 children on HAART at the pediatric ward of Saint Camille Hospital in Ouagadougou, Burkina Faso during the period from January 2018 to October 2018. This study involved 151 HIV-1 infected children. Data were collected before and 12 months after initiation of HAART and included: socio-demographic, clinical, hemoglobin (Hb), and immunologic characteristics. Hemoglobin and CD4 T cell counts were determined using the CELL-DYN Ruby (Abbott, Illinois, USA) and the BD FACSCOUNT (Becton Dickenson, California, USA) respectively. To ensure good quality results, the data were collected through a questionnaire form and incomplete data were eliminated during processing.
Definition of variables and data analysis
Based on hemoglobin level, anemia was classified as severe (Hb level<7 g/dL), moderate (Hb level [7-9.9] g/dL), and mild (Hb level [10-11] g/dL). Body mass index (BMI) was classified into normal body weight (18.5 - 24.9), underweight (<18.5), and overweight (≥25) according to WHO, and the calculation was based on the formula BMI= (weight in Kg/height in m2) (WHO, 2021b). Bivariate analysis and multivariate logistic regression were performed using IBM SPSS version 21.0 software and any value was considered statistically significant for p<0.05. Odds ratios with confidence intervals (95% CI) were used to determine the association between anemia and potential risk factors.
RESULTS
General characteristics of the study population
The socio-demographic and clinical characteristics of the study population at the time of initiation of ARV treatment are shown in Table 1. Female children were more represented (54.3%) than males (45.7%) with a sex ratio of 0.84 and the mean age was 10.04±3.7 years. The most represented age group was 5-11 years (58.28%). The majority of HIV-1 infected children (70.86%) were underweight at the start of ARV treatment. Approximately, 64.9% of the children were in WHO clinical stages I and II and 25.83% of them had a CD4 count below 200 cells/µl (Table 1).
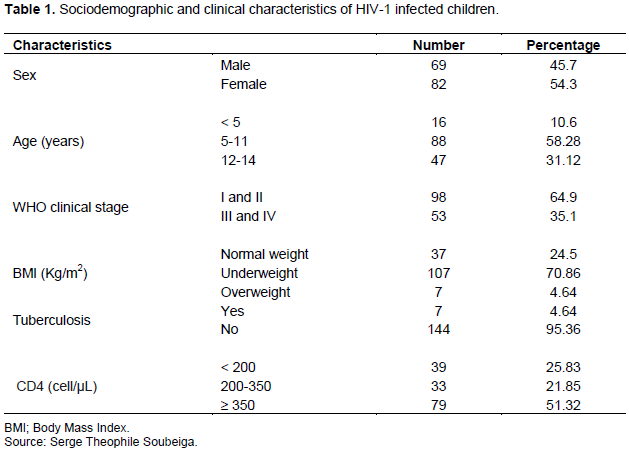
Prevalence of anemia before and after HAART initiation
Figure 1 shows the severity of anemia in HIV-1 infected children before and after initiation of HAART. The prevalence of anemia was 81.46% (123/151) before treatment and 48.34% (73/151) 12 months after initiation of treatment. About 5.96% of the children had severe anemia, 50.33% had moderate anemia and 25.16% had mild anemia before initiation of HAART (Figure 1). Among the children with anemia before starting ARV treatment, 97.10% (67/69) were male and 68.29% (56/82) were female. This study shows that all children under 5 years of age (100%) and 86.92% of those who were underweight developed anemia. Also, anemia was found in all HIV-1 infected children with CD4 T cell count between 200 and 350 cells/µl (Table 2). In contrast, 12 months after initiation of HAART, the prevalence of anemia decreased overall. It was 63.49% (40/63) in male children and 37.5% (33/88) in female children. The age group of 5-11 years was the most anemic (60.23%).
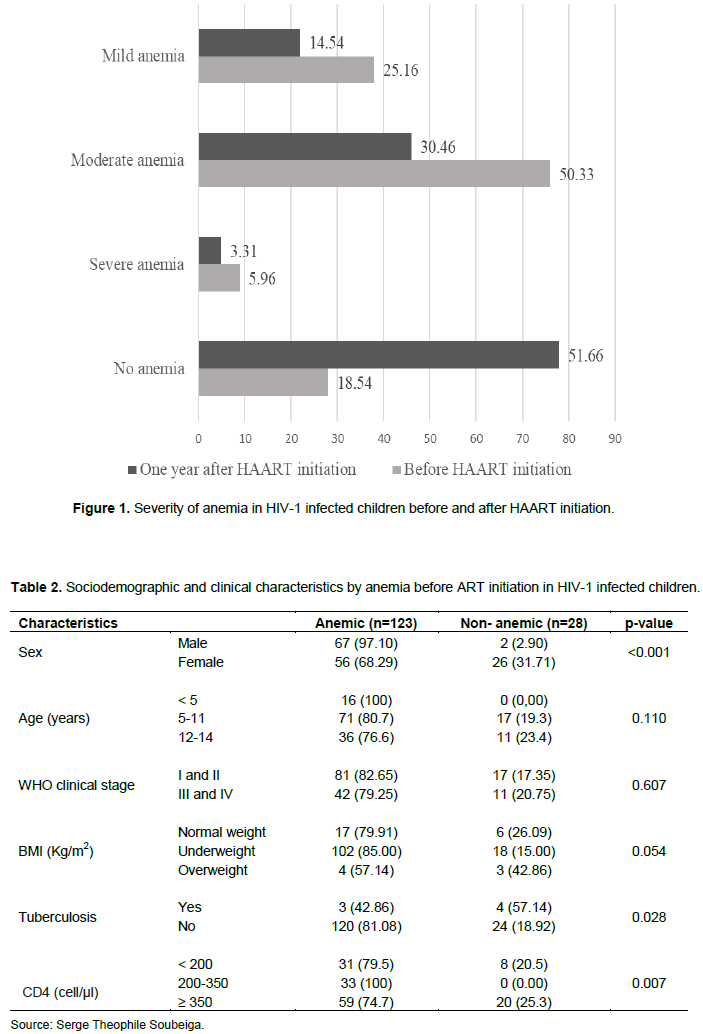
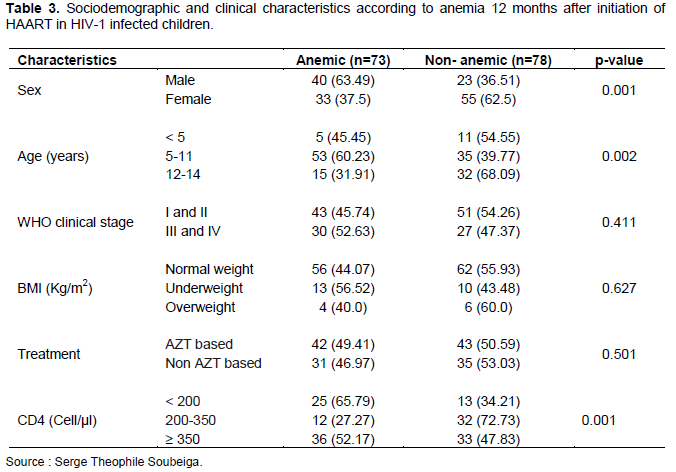
Similarly, 46.97% (31/68) of HIV-1 infected children in whom treatment was not AZT-based developed anemia (Table 3).
Factors associated with anemia before and after HAART
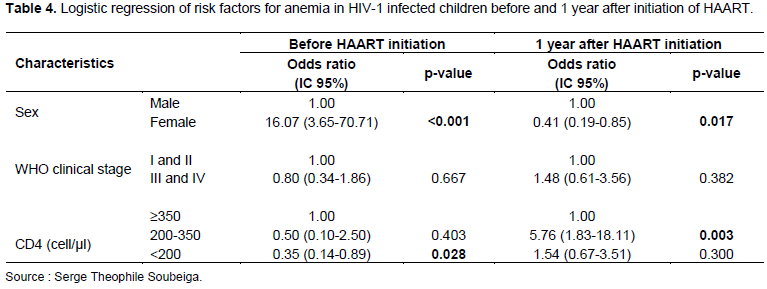
Analysis of the data showed a significant association between gender, BMI, TB, and CD4 counts before HAART initiation, but there was no association between age, WHO clinical stage, and anemia (Table 2). After HAART initiation, gender, age, and CD4 T cell count were significantly associated with anemia. However, there was no association between WHO clinical stage, BMI, ARV treatment, and anemia (Table 3). Female children had a 16-fold [OR=16.07 (3.65-70.71; p<0.001] risk of developing anemia before HAART initiation but this risk significantly decreased 1 year after HAART [OR=0.41 (0.19-0.85), p=0.017]. Children with a CD4 count <200 Cell/µl had a risk of developing anemia before HAART initiation but no longer had a risk of developing anemia 1 year after HAART [OR=0.35 (0.14-0.89); p=0.028 vs. OR=1.54 (0.67-3.51); p=0.300] (Table 4).
DISCUSSION
This study showed a high prevalence (81.46%) of anemia in HIV-1 infected children before initiation of HAART, which is lower than the prevalence of 87.0% found in children in 2020 from Burkina Faso in a study conducted in African children but higher than the prevalence of anemia found other countries (70.0% in Kenya; 49.7% in Uganda; 60.1% in Gambia) (Muriuki et al., 2020). This high prevalence of anemia confirms that the situation remains concerning in Central and Western Africa. Indeed, children in developing countries have a high prevalence of anemia due to poverty, a high burden of infectious diseases whose inflammation is a primary contributor to anemia (Mantadakis et al., 2020). In this study, the prevalence of anemia decreased 12 months after treatment initiation. It decreased from 81.46 to 43.34%, that is, a reduction of 33.90% in one year. This prevalence is nevertheless higher than that found in several countries: 11.4% in Southern Ethiopia (Fenta et al., 2020); 54.2% in Nigeria (Ahumareze et al., 2016) but remains lower than 49.6% found in Cameroon (Bate et al., 2016) and 88% in Mozambique (Duffy et al., 2020). These differences could be due to the methodologies used, the heterogeneity of the populations, and the nutritional status of the children. Indeed, a study conducted in Sub-Saharan Africa among children aged 6-59 months revealed a difference in the prevalence of anemia between countries. West Africa had the highest prevalence (70-88%), followed by Central Africa (63-67%) and East Africa (38-69%) (Tesema et al., 2021). In this study, the decrease in anemia after 1 year of HAART truly demonstrates the involvement of ARV therapy in improving hemoglobin levels. Indeed, studies have shown that ARV therapy contributes to improved anemia and reduced mortality in children (Haider et al., 2019). This is confirmed by the decrease in the rate of severe anemia (5.96% before HAART vs 3.31% after HAART). Our results are in agreement with other studies (Geletaw et al., 2017; Beletew et al., 2020). Authors have shown the reduction of anemia 6 months, 12 months, 18 months, 24 months, and 30 months after AZT-based ART treatment (Getaneh et al., 2021). While our study found a higher rate of anemia in children on HAART with AZT (64.2%) than in those on non-AZT HAART (51.11%). The factor that may have contributed to the observed high prevalence of anemia is the possible effect of AZT. Side effects such as myelotoxicity, mitochondrial toxicity, myopathy, and incidence of anemia associated with AZT use in people living with HIV have been reported in several studies (Tamir et al., 2018). AZT is known to be associated with life-threatening hematological toxicity like anemia due to early long-term higher-dose therapy. AZT also causes bone marrow suppression, which causes anemia (Getaneh et al., 2021).
In this study, the under-5 years' age group was most affected by anemia before HAART initiation. This confirmed that children under 5 years of age in developing countries are more vulnerable to severe anemia (Kejo et al., 2018) certainly due to malnutrition, malaria, and opportunistic infections (Bate et al., 2016; Wagnew et al., 2019). The 5-11-year-old was most affected 1 year after HAART treatment in contrast to the under 5-year-old. This was also found in a study conducted in Cameroon (Bate et al., 2016). There was an association between gender, BMI, tuberculosis, and CD4 T-cell count with anemia before initiation of HAART in children. Indeed, opportunistic infections and immune system failure are factors that can promote anemia. One study reported that tuberculosis and malaria were associated with anemia in HIV-1 infected children (Huibers et al., 2020). In terms of BMI, anemia was found most in underweight HIV-1 infected children. Indeed, the hemoglobin level is an important biological parameter that, when it is reduced, can affect growth, especially in children (Ibrahim et al., 2017).
Children with CD4 counts < 200 Cell/µL and 200-350 Cell/µl had a 0.35- and 5.76-fold risk of developing anemia before initiation and 1 year after HAART, respectively, as a study in Ethiopia showed that immunocompromised children were at higher risk of developing anemia than those with normal immunity (Techane et al., 2020). Tuberculosis, zidovudine-based drugs, severe immunosuppression, and undernutrition have remained predictors of anemia among children on antiretroviral therapy (Atalell et al., 2018; Techane et al., 2020).
In general, HAART reduces the incidence of anemia by suppressing viral replication and increasing CD4 cell counts (Beletew et al., 2020; Getaneh et al., 2021). In addition to HAART, all HIV-infected children received Cotrimoxazole prophylaxis to prevent other opportunistic infections. This treatment could contribute to the reduction of anemia in children. Indeed, cotrimoxazole may have a direct impact on erythropoiesis by reducing the number of red blood cells (Bouyou Akotet et al., 2018). This study showed important results but has presented some limits. Outside of tuberculosis, there was no existing data on other diseases that could influence the hemoglobin level of children before their treatment and we did not identify the exact cause of anemia. This study confirmed with many authors that antiretroviral treatment contributes strongly to improving the hemoglobin level in persons living with HIV. However, there is the need to enhance the management of HIV-infected children to restore their immune system, monitor their nutritional status, and prevent opportunistic infections.
Ethics approval
This study received approval from the Burkina Faso Health Research Ethics Committee (deliberation N° 2014-7-084). Anonymity and confidentiality were respected and the results were used to improve the clinical management of the children included in the study.
CONFLICT OF INTERESTS
The authors have not declared any conflict of interests.
ACKNOWLEDGMENT
The authors appreciate the personnel of the Pediatric Service of the Saint Camille Hospital of Ouagadougou for their support in the data collection.
REFERENCES
|
Abioye AI, Andersen CT, Sudfeld CR, Fawzi WW (2020). Anemia, Iron Status, and HIV: A Systematic Review of the Evidence. Advances in Nutrition (Bethesda, Md.) 11(5):1334-1363. |
|
|
Ahumareze RE, Jean R, Agatha D, Agatha W, Elizabeth D, Yetunde B, Olufemi A (2016). Prevalence of Anaemia and the Relationship between Haemoglobin Concentration and CD4 Count in HIV Positive Children on Highly Active Antiretroviral Therapy (HAART) in Lagos, Nigeria. Current Pediatric Research 20(1&2):29-36. |
|
|
Atalell KA, Birhan Tebeje N, Ekubagewargies DT (2018). Survival and predictors of mortality among children co-infected with tuberculosis and human immunodeficiency virus at University of Gondar Comprehensive Specialized Hospital, Northwest Ethiopia. A retrospective follow-up study. PloS ONE 13(5):e0197145. |
|
|
Bate A, Kimbi HK, Lum E, Lehman LG, Onyoh EF, Ndip LM, Bessong PO (2016). Malaria infection and anaemia in HIV-infected children in Mutengene, Southwest Cameroon: a cross sectional study. BMC Infectious Diseases 16(1):523-523. |
|
|
Beletew B, Mengesha A, Ahmed M, Fitwi A, Wudu M (2020). Determinants of Anemia among HIV-Positive Children on Highly Active Antiretroviral Therapy Attending Hospitals of North Wollo Zone, Amhara Region, Ethiopia, 2019: A Case-Control Study. Anemia Article ID 3720572. |
|
|
Bisong EM, Okpa HO, Ogbonna UK, Enang OE, Monjok E (2017). The prevalence and risk factors associated with anaemia among HIV patients attending clinic at the University of Calabar Teaching Hospital, Calabar, Nigeria. Global Journal of Pure and Applied Sciences 23(1):187-192. |
|
|
Bouyou Akotet MK, Koumba Lengongo JV, Ondounda M, Kendjo E, Mongo Delis A, Essomeyo Mebale M, Okome Nkoumo M (2018). Burden of asymptomatic malaria, anemia and relationship with cotrimoxazole use and CD4 cell count among HIV1-infected adults living in Gabon, Central Africa. Pathogens and Global Health 112(2):63-71. |
|
|
Cao G, Wang Y, Wu Y, Jing W, Liu J, Liu M (2022). Prevalence of anemia among people living with HIV: A systematic review and meta-analysis. EClinical Medicine 44:101283. |
|
|
Duffy C, Kenga DB, Gebretsadik T, Maússe FE, Manjate A, Zaqueu E, Moon TD (2020). Multiple Concurrent Illnesses Associated with Anemia in HIV-Infected and HIV-Exposed Uninfected Children Aged 6-59 Months, Hospitalized in Mozambique. The American Journal of Tropical Medicine and Hygiene 102(3):605-612. |
|
|
Ezeamama AE, Sikorskii A, Bajwa RK, Tuke R, Kyeyune RB, Fenton JI, Fawzi WW (2019). Evolution of Anemia Types During Antiretroviral Therapy-Implications for Treatment Outcomes and Quality of Life Among HIV-Infected Adults. Nutrients 11(4):755. |
|
|
Fenta DA, Nuru MM, Yemane T, Asres Y, Wube TB (2020). Anemia and related factors among highly active antiretroviral therapy experienced children in Hawassa comprehensive specialized hospital, southern Ethiopia: emphasis on patient management. Drug, Healthcare and Patient Safety 12:49. |
|
|
Geletaw T, Tadesse MZ, & Demisse AG (2017). Hematologic abnormalities and associated factors among HIV infected children pre- and post-antiretroviral treatment, North West Ethiopia. Journal of Blood Medicine 8: 99-105. |
|
|
Getaneh Z, Wale W, Chanie B, Temesgen E, Abebe M, Walie M, Lemma M (2021). Magnitude and associated factors of anemia among AZT-based HAART experienced adult HIV patients at University of Gondar Comprehensive Specialized Referral Hospital, Northwest, Ethiopia, 2019: a retrospective cohort study. BMC Infectious Diseases 21(1):1-12. |
|
|
Haider BA, Spiegelman D, Hertzmark E, Sando D, Duggan C, Makubi A, Fawzi WW (2019). Anemia, Iron Deficiency, and Iron Supplementation in Relation to Mortality among HIV-Infected Patients Receiving Highly Active Antiretroviral Therapy in Tanzania. The American Journal of Tropical Medicine And Hygiene 100(6):1512-1520. |
|
|
Harding B, Whitney B, Nance R, Ruderman S, Crane H, Burkholder G, Moore RD, Mathews WC, Eron JJ, Hunt PW, Volberding P, Rodriguez B, Mayer KH, Saag MS, Kitahata MM, Heckbert SR, Delaney, JAC (2020). Anemia risk factors among people living with HIV across the United States in the current treatment era: a clinical cohort study. BMC Infectious Diseases 20(1):1-8. |
|
|
Huibers MHW, Bates I, McKew S, Allain TJ, Coupland SE, Phiri C, Calis JC (2020). Severe anemia complicating HIV in Malawi; Multiple co-existing aetiologies are associated with high mortality. PloS One 15(2):e0218695-e0218695. |
|
|
Ibrahim A, Atef A, Magdy RI, Farag MA (2017). Iron therapy and anthropometry: A case-control study among iron deficient preschool children. Egyptian Pediatric Association Gazette 65(3):95-100. |
|
|
Kejo D, Petrucka PM, Martin H, Kimanya ME, Mosha TC (2018). Prevalence and predictors of anemia among children under 5 years of age in Arusha District, Tanzania. Pediatric Health, Medicine and Therapeutics 9:9. |
|
|
Lai J, Chen Y, Liu Y, Yuan J, Lin J, Huang A, Ye HH (2019). Prevalence and risk factors of anaemia in hospitalised HIV-infected patients in southeast China: a retrospective study. Epidemiology and Infection 147p. |
|
|
Leroi C, Balestre E, Messou E, Minga A, Sawadogo A, Drabo J, Maiga M, Zannou M, Seydi M, Dabis F, Jaquet A (2017). Incidence of Severe Neutropenia in HIV-Infected People Starting Antiretroviral Therapy in West Africa. PloS One 12(1):e0170753. |
|
|
Mantadakis E, Chatzimichael E, Zikidou P (2020). Iron deficiency anemia in children residing in high and low-income countries: risk factors, prevention, diagnosis and therapy. Mediterranean Journal of Hematology and Infectious Diseases 12(1). |
|
|
Mouhari-Toure A, Mahamadou G, Kombate K, Akakpo AS, Teclessou JN, Singo APP (2018). Morbidity and mortality in HIV-infected children on antiretroviral therapy in Togo. Médecine et Santé Tropicales 28(1):54-60. |
|
|
Muriuki JM, Mentzer AJ, Webb EL, Morovat A, Kimita W, Ndungu FM, Macharia AW, Crane RJ, Berkley JA, Lule SA, Cutland C, Sirima SB, Diarra A, Tiono AB, Bejon P, Madhi SA, Hill AVS, Prentice AM, Suchdev PS, Elliott AM, Williams TN, Atkinson SH (2020). Estimating the burden of iron deficiency among African children. BMC Medicine 18(1):1-14. |
|
|
Tamir Z, Alemu J, Tsegaye A (2018). Anemia among HIV Infected Individuals Taking ART with and without Zidovudine at Addis Ababa, Ethiopia. Ethiopian Journal of Health Sciences 28(1):73-82. |
|
|
Techane MA, Anlay DZ, Tesfaye E, Agegnehu CD (2020). Incidence and Predictors of Anemia Among Children on Antiretroviral Therapy at the University of Gondar Comprehensive Specialized Hospital, Northwest Ethiopia, 2007-2017: A Retrospective Follow-Up Study. HIV/AIDS (Auckland, N.Z.) 12:951-962. |
|
|
Tesema GA, Worku MG, Tessema ZT, Teshale AB, Alem AZ, Yeshaw Y, Liyew AM (2021). Prevalence and determinants of severity levels of anemia among children aged 6-59 months in sub-Saharan Africa: A multilevel ordinal logistic regression analysis. PloS One 16(4):e0249978-e0249978. |
|
|
Tsai KZ, Lai SW, Hsieh CJ, Lin CS, Lin YP, Tsai SC, Chung PS, Lin YK, Lin TC, Ho CL, Han CL, Kwon Y, Hsieh CB, Lin GM (2019). Association between mild anemia and physical fitness in a military male cohort: The CHIEF study. Scientific Reports 9(1):1-7. |
|
|
UNAIDS (2021). Fiche d'information - Dernières statistiques sur l'état de l'épidémie de sida. (Accessed Nov 05, 2021). |
|
|
Wagnew F, Eshetie S, Alebel A, Tesema C, Kibret GD, Gebrie A, Abajobir AA (2019). Burden of anemia and its association with HAART in HIV infected children in Ethiopia: a systematic review and meta-analysis. BMC Infectious Diseases 19(1):1032. |
|
|
World Health Organization (WHO) (2021a). Nutrition - Body mass index - BMI. (Accessed Feb 28, 2022). Retrieved from |
|
|
World Health Organization (WHO) (2021b). Anaemia in women and children. (Accessed Feb 28, 2022). Retrieved from |
|
Copyright © 2024 Author(s) retain the copyright of this article.
This article is published under the terms of the Creative Commons Attribution License 4.0