ABSTRACT
This cross-sectional and descriptive study was carried out to determine the socio-demographic, and clinical profiles of mycologically diagnosed pityriasis versicolor (PV) among outpatients of the Department of Dermatology, Teaching Hospital of Yopougon (Abidjan, Côte d’Ivoire) between July, 2012 and February, 2013. Among 1578 patients screened for various consultations, 200 presented with lesions suggestive of PV, reflecting a rate of 12.7% (CI95% = 11.1 to 14.4). These 200 patients were enrolled regardless of gender or age and sampled using the cellophane tape method for identification of Malassezia yeast. Percentage of patients receiving mycological diagnose was 10.8% (CI95% = 9.4 to 12.4). The average age of the patients was 24.8 years (3 to 56 years; SD = 9.6). Women (58.5%) and patients between 20 and 30 years (54.4%) were most commonly affected. Education level (p = 0.0013), lesion type (p = 0.0005) and use of cosmetic products (p < 0.0001) were associated with the occurrence of PV. Cosmetic products with depigmenting effects were among the most commonly used by affected patients (52.2%). Lesions were mostly located on the back (38.8%) and the chest (20.5%). There were no associated symptoms (43.9%) observed and affected patients (33.3%) experienced high sweating. A better understanding of the risk factors associated with this condition and biological confirmation of suspicious cases would allow for more effective therapeutic management.
Key words: Pityriasis versicolor, contributing factors, Malassezia sp., Côte d’Ivoire.
Pityriasis versicolor (PV) is a benign, superficial, cutaneous fungal infection of the stratum corneum (Pramanik et al., 2014). It is a common, non-contagious mycosis caused by commensal skin yeasts of the genus Malassezia. The most well-known of these is Malassezia furfur, although other species, especially Malassezia globosa, Malassezia sympodialis and Malassezia restricta, have also been isolated from patient lesions (Crespo et al., 2000). Malassezia spp. yeasts are lipophilic yeasts that induce multiple scaly macules or patches. The lesions induced are polymorphous in terms of color, size, and topographic location (Ben Salah et al., 2010). Lesion color varies from pale to red, yellow, brown, or dark brown, thus the name “versicolor”.
Some of the lesions are lighter in color than normal skin (El Ghotamy, 1981). Pityriasis versicolor occurs worldwide; however, higher frequencies are found in tropical and sub-tropical areas (Gupta et al., 2003), where rates are as high as 50% (Gupta et al., 2002). Heat and humidity as well as sweating are some of the factors contributing to its occurrence (Bastide, 2001; Pramanik et al., 2014). Far from being a life-threatening disease, PV is mostly an aesthetic concern. There is little available information on PV in Côte d’Ivoire. The aim of this study was to determine the socio-demographic and clinical profiles of mycologically diagnosed PV in outpatients at the department of dermatology of the Teaching Hospital, Yopougon (Abidjan, Côte d’Ivoire).
Study type and area
This cross-sectional and descriptive study of mycological diagnosis of PV was carried out from July 2012 to February 2013 (8 months). Patients were selected from the Department of Dermatology of the Teaching Hospital of Yopougon and mycological investigations were conducted at the Laboratory of Parasitology of the Diagnosis and Research Centre on AIDS and Other Infectious Diseases (CeDReS). Both sites are located in Abidjan in Côte d’Ivoire. Located on the coast, Abidjan has a humid, equatorial-type climate with two rainy seasons (April to July and October to November) and two dry seasons (December to March and August to September). The temperature varies from 25°C to 30°C, and the relative humidity varies from 80% to 90%.
Study population
The methodology used for this study involved the screening of all patients received in the dermatology department during the study period, regardless of the reason for consultation. Then, a clinical examination was done by a consulting physician in order to select patients presenting with skin lesions suggestive of PV (hypochromic, hyperchromic or achromic macules), regardless of the age or gender of the patient. From this clinical examination, patients who provided informed consent and who had not received any specific treatment for at least two weeks before the medical consultation were enrolled. Finally, enrolled patients were interviewed and their skin was sampled. In order to maintain confidentiality, an identifying number was given to each patient.
Sampling protocol
Sampling was performed using the cellophane tape method. After removing the fat and disinfecting the area around the suspected lesion with ether, lesions were sampled by applying a piece of transparent adhesive cellophane. Samples were immediately placed on a glass slide that had been previously labeled, and the slides were brought to the CeDReS for microscopic examinations.
Mycological examination
Slides underwent direct examination under an optical microscope using a low power field (×10) and then a high power field (×40) to identify specific elements of the pathogenic agent, such as the presence of clusters of thick-walled, round yeasts (2 to 6 μm in diameter) and budding cells with or without short and curved pseudohyphae.
Statistical analysis
Statistical analyses of data were conducted using EPI Info 6.04 software. The Chi-squared (χ2) and Fisher’s exact tests were performed, and a p < 0.05 was considered to be statistically significant.
A total of 1578 patients were received at the department of dermatology for various consultations during the study period. Two hundred of them presented with lesions suggestive of PV, reflecting a rate of 12.7% (CI95% = 11.1 to 14.4). Among these 200 patients, mycological analysis confirmed 171 cases for a mycological positivity rate of 85.5% (CI95% = 80.1 to 89.9). The frequency among the total number of patients received in the department during the study period was therefore estimated at 10.8% (CI95% = 9.4 to 12.4).
While the majority of subjects were women (sex ratio = 0.71), sex was not a contributing factor to the occurrence of PV (p = 0.55). The ages of the patients varied from 3 to 56 years old, with an average of 24.8 years (SD = 9.6). The majority of patients affected were between the ages of 20 and 30 years old (54.4%); however, age was not a contributing factor to the presence of PV (p= 0.44). Patients with high levels of education were affected most frequently (36.8%) (Table 1).
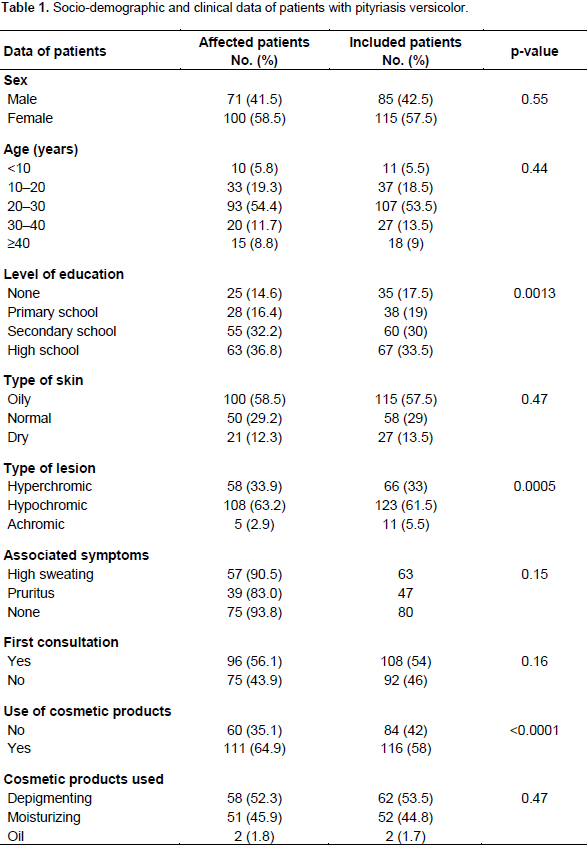
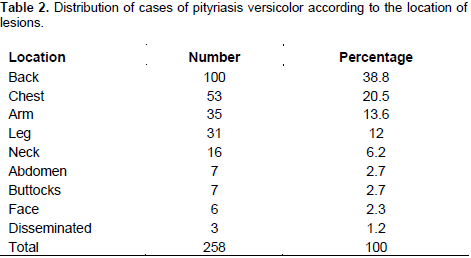
Clinically, the majority of patients affected by PV had oily skin (58.5%) and hypochromic lesions (63.2%). Lesion type was significantly associated with the diagnosis of mycosis (p < 0.0005). Most of affected patients had no associated symptoms (43.9%) followed by those expressed a high sweating (33.3%). In addition, most of the patients affected (56.1%) were enrolled at their first consultation. The use of cosmetic products was significantly associated with the occurrence of PV (p<0.0001). Depigmenting products were most commonly used by the patients affected (52.2%) (Table 1). Lesions were observed at different locations (Table 2) with the primary locations being the back (38.8%) and the chest (20.5%).
Pityriasis versicolor is a benign mycosis that is particularly widespread in tropical and sub-tropical areas (Gupta et al., 2003). Abidjan is characterized by temperatures ranging from 25 to 30°C and high humidity (80 to 90%). This tropical and humid climate, which causes high rates of sweating, could explain the high prevalence of PV in the city. The biological prevalence obtained through the cellophane tape method showed that there was an overestimation of the disease prevalence at the clinical level. A similar trend was reported in Brazil, where 6/10 patients with suggestive lesions were actually affected by PV (Silva-Rocha et al., 2017). That could be explained by the previous use of specific treatments not reported by the patients or by the persistence of lesion discoloration even after healing (Gupta and Foley, 2015).
It is therefore essential to combine mycological analysis with the relatively simple clinical diagnosis for appropriate management of the disease. A diagnosis of PV can be confirmed by microscopy using skin scrapings from theborders of lesions in a KOH mount or by obtaining samples using the cellophane tape method (Gupta and Foley, 2015), as in this study. The two techniques are similarly reliable (Hassab-El-Naby et al., 2010). Mycological culture of Malassezia offers the advantage of isolating the species concerned but is a difficult technique which requires special media (Sabouraud and Dixon’s media with added olive oil in both) (Sharma et al., 2016). In addition, similar prevalences have been reported after comparing culture- and microscopy-based techniques (Sivakumar et al., 2009; Petry et al., 2011).
Therefore, microscopy remains a sensitive easily implemented method for the diagnosis of PV. The rate of positive cases detected mycologically among the total number of patients received in the department during the study period (10.8%) is higher than those reported in several studies from Brazil on the epidemiology of dermatomycosis in hospital settings, which reported rates of 4.5% (Sylva-Rocha et al., 2016), 9.5% (Calado et al., 2011) and 2.1% (Di Chiacchio et al., 2014). In contrast, a higher prevalence (37.31%) was reported in Burkina Faso (Zida et al., 2015) among prisoners of Ouagadougou.
Pityriasis versicolor is a disease presenting mostly aesthetic concerns. It is generally well known that women express greater aesthetic concern than men, leading them to consult dermatologists more (Duhard et al., 2006). This is consistent with our group of predominantly female affected patients. In addition, more women than men use cosmetic products for hydration or depigmentation purposes. In this study, we did not find an association between the use of depigmentation products and the occurrence of the disease; however, the use of cosmetic products associated with humidity and heat appears to be a contributing factor to the spread of these fungi.
This is likely due to the relationship between the lipophilic character of these yeasts and post-pubertal hormonal stimulation, which is associated with increased stimulation of sebaceous gland activity in regions with a high production of sebum, promoting a favorable environment for the proliferation of Malassezia spp. (Di Chiacchio et al., 2014; Heidrich et al., 2015). Pityriasis versicolor is frequently reported among patients with high levels of education (Belem et al., 2001; Ghosh et al., 2008; Morais et al., 2010), with rates ranging from 29.09% to 38.5%. In this study, this parameter was significantly associated with the occurrence of PV (p = 0.0013). This could be caused by greater aesthetic concerns in this category of patients, making them to consult dermatologists more.
The genus Malassezia includes yeast-like fungi that are part of the normal human skin microbiota (Heidrich et al., 2015). Almost all Malassezia species are obligate lipid-dependent yeasts, influencing their distribution in sebum rich areas of the skin such as the scalp, face, and trunk and their avoidance of areas without hair (e.g. the palms and soles of the feet) (Peyrefitte, 1997; SFD, 2005). The face is the most common location reported in most studies (Miskeen and Shroff 1984; Faergemann 1999; De Oliveira and Steiner 2002; Jena et al., 2005; Silva-Rocha et al., 2017), though the neck and trunk were the common locations reported in Burkina Faso (Zida et al., 2015).
In this study, the back was the most common location of mycosis, followed by the chest. The predominance of PV on the face could be explained by the presence of a larger quantity (400 to 900 cm2) of large sebaceous glands in the mediofacial region (forehead, nose and chin) than on the chest and upper back (60 to 80 cm2) (Peyrefitte, 1997; SFD, 2005). Pityriasis versicolor is usually characterized by dyschromic lesions (Faergemann 1999). The high frequency of hypochromic lesions in our study may have been caused by relationship of Malassezia yeasts and the pigmentary changes of skin. Indeed, azelaic acid and several tryptophan metabolites produced by Malassezia yeasts can interfere with melanization and are believed to play important roles in the skin pigmentation changes seen in PV (Ashbee, 2007).
Pityriasis versicolor is relatively frequent in hospital settings in Abidjan. The main contributing factors noted in this study are heat and high humidity, which are associated with tendencies to sweat and use cosmetic products. Investigations into the use of these products by women, especially those with a depigmenting effect, in terms of the occurrence of this mycosis should be conducted in the future. Furthermore, the biological confirmation of this disease as well as a better understanding of its risk factors should enable a reduction in the number of cases owing to effective therapeutic management and better prevention.
The authors have not declared any conflict of interests.
The authors thank the authorities and staff of the Department of Dermatology, Teaching Hospital, Yopougon, Abidjan, Côte d’Ivoire, as well as those of the Department of Parasitology–Mycology, Diagnosis and Research Centre on AIDS and Other Infectious Diseases (CeDReS) (Abidjan, Côte d’Ivoire) for their availability and help in carrying out this work.
REFERENCES
|
Ashbee HR (2007). Update on the genus Malassezia. Med. Mycol. 45:287-303.
Crossref
|
|
|
|
Bastide JM (2001). "Malassezioses. Infectious diseases" In: Encycl Med Chir. Paris: Elsevier SAS; 2001: 1-18 [8-603-A-10].
|
|
|
|
|
Belem LF, Lima EO, Andrade DA, Filho PAV, Guerra MFL, Carvalho MFFP, Vargas VES (2001). Estudo epidemiologico da Pitiriase versicolor no estado da Paraiba, Brasil. Rev. Bras. Anal. Clin. 33:63-67.
|
|
|
|
|
Ben Salah I, Makni F, Cheikhrouhou F, Neji S, Sellami H, Ayadi A (2010). Malassezia species: Pathology, isolation and identification media. J. Mycol. Med. 20:53-60.
Crossref
|
|
|
|
|
Calado NB, de Sousa Junior FC, Diniz MG, Fernandes AC, Cardoso FJ, Zaror LC, Ferreira MA, Milan EP (2011). A 7-year survey of superficial and cutaneous mycoses in a public hospital in Natal, Northeast Brazil. Braz. J. Microbiol. 42:1296-1299.
Crossref
|
|
|
|
|
Crespo Erchiga V, Ojeda Martos A, Vera Casano A, Crespo Erchiga A, Sanchez Fajardo F (2000). Malassezia globosa as the causative agent of pityriasis versicolor. Br. J. Dermatol. 143:799-803.
Crossref
|
|
|
|
|
De Oliveira JMVT, Steiner D (2002). Pityriasis Versicolor. An. Bras. Dermatol. 77:611-618.
Crossref
|
|
|
|
|
Di Chiacchio NMC, Humaire CR, Silva CS, Fernandes LH, Dos Reis AL (2014). Superficial mycoses at the Hospital do Servidor Público Municipal de São Paulo between 2005 and 2011. An. Bras. Dermatol. 60:147-150.
Crossref
|
|
|
|
|
Duhard E, Coudière P, Voisard JJ, Allaert FA (2006). Management of presumed onychomycosis in private practice. Ann. Dermatol. Venereol. 133:11-15.
Crossref
|
|
|
|
|
El Gothamy Z (1981). Amino acid metabolism of Malassezia furfur. Ann. Parasitol. Hum. Comp. 56:359-361.
Crossref
|
|
|
|
|
Faergemann J (1999). Pityrosporum species as a cause of allergy and infection. Allergy. 54:413-419.
Crossref
|
|
|
|
|
Ghosh SK, Dey SK, Saha I (2008). Pityriasis versicolor: a clinicomycological and epidemiological study from a tertiary care hospital. Indian J. Dermatol. 53(4):182-185.
Crossref
|
|
|
|
|
Gupta AK, Foley KA (2015). Antifungal Treatment for Pityriasis Versicolor. J. Fungi. 1:13-29.
Crossref
|
|
|
|
|
Gupta AK, Bluhm R, Summerbell R (2002). Pityriasis versicolor review. Journal of the European Academy of Dermatology and Venereology. 16:19-33.
Crossref
|
|
|
|
|
Gupta AK, Ryder JE, Nicol K, Cooper EA (2003). Superficial fungal infections: An update on pityriasis versicolor, seborrheic dermatitis, tinea capitis, and onychomycosis. Clin. Dermatol. 21:417-425.
Crossref
|
|
|
|
|
Hassab-El-Naby HMM, Salem ASM, Abdo HM, Hassan HM (2010). Comparative study for the reliability of cellophane tape and standard KOH mount in diagnosis of pityriasis versicolor. Global J. Dermatol. Venereol.17(2):29-34.
|
|
|
|
|
Heidrich D, Daboit TC, Stopiglia CD, Magagnin CM, Vetoratto G, Amaro TG, Scroferneker ML (2015). Sixteen years of pityriasis versicolor in metropolitan area of Porto Alegre, Southern Brazil. Rev. Inst. Med. Trop. Sao Paulo. 57:277-280.
Crossref
|
|
|
|
|
Jena DK, Sengupta S, Dwari BC, Ram MK (2005). Pityriasis versicolor in the pediatric age group. Indian J. Dermatol. Venereol. Leprol. 71:259-261.
Crossref
|
|
|
|
|
Miskeen AK, Shroff SHJ (1984). Pityriasis versicolor in children. Indian J. Dermatol. Venereol. Leprol. 50:144-146.
|
|
|
|
|
Morais PM, Cunha MD, Frota MZ (2010). Clinical aspects of patients with pityriasis versicolor seen at a referral center for tropical dermatology in Manaus, Amazonas, Brazil. Anais brasileiros de dermatologia. 85(6):797-803.
Crossref
|
|
|
|
|
Petry V, Tanhausen F, Weiss L, Milan T, Mezzari A, Weber MB (2011). Identification of Malassezia yeast species isolated from patients with pityriasis versicolor. An. Bras. Dermatol. 86:803-806.
Crossref
|
|
|
|
|
Peyrefitte G (1997). " Biology of the skin" 3è éd. Paris: SIMEP. P 48.
|
|
|
|
|
Pramanik SB, Bandopadhyay M, Nandi A (Mitra) (2014). A study of prevalence of different species of malassezia causing pityriasis versicolor in different age groups in a tertiary care hospital in Kolkata. IOSR J. Dental Med. Sci. 13(70):88-92.
|
|
|
|
|
Sharma Y, Jain S, Chandra K, Munegowda KCA (2016). Clinico-epidemiologic evaluation of pityriasis versicolor from a government Hospital, India: Conventional methods-still a thumbs up? Indian J. Med. Sci. 68:63-66.
Crossref
|
|
|
|
|
Silva-Rocha WP, De Azevedo MF, Chaves GM (2017). Epidemiology and fungal species distribution of superficial mycoses in Northeast Brazil. J. Mycol. Med. 27:57-64.
Crossref
|
|
|
|
|
Sivakumar N, Karthikeyan A, Vivek A, Santhamani MD (2009). Prevalence of etiological agents in superficial mycoses with reference to dermatophytoses and pityriasis versicolor. Internet J. Microbiol. 7:13-18.
|
|
|
|
|
Société Française de Dermatologie (2005). Understanding the Skin. Ann. Dermatol. Venereol. 132:8S3-8S104.
|
|
|
|
|
Zida A, Barro-Traoré F, Dera M, Bazié Z, Niamba P, Guiguemdé TR (2015). Epidemiological and etiological aspects of superficial fungal infections among prison inmates in Ouagadougou, Burkina Faso. J. Mycol. Med. 25:e73-e79.
Crossref
|
|