ABSTRACT
Several Bacillus strains were isolated from “Ouled Yelass” hot spring soil, located in Setif city (Eastern Algeria). Three isolates of them coded (4RH), (14 RH), and (Set-oxy), were screened for their ability to inhibit the growth of some phytopathogenic fungi, such as Fusarium oxysporium, Botrytis cinerea, Aspergillus niger, Cladosporium cucumerinium and Alternaria alternata. The molecular identification of the strain (4RH) based on the 16S-DNA and gyrase-A genes sequences analysis, showed that it is closely related (99.9%) to the Bacillus amyloliquefaciens species. This bacterium was characterized by a high sporulation yield reaching 22±0.86 × 108 spores/ml and had important inhibition rates up to 80 and 70%, against F. oxysporium and B. cinerea, respectively. The bacterium (4RH) was able to produce cell wall degrading enzymes of cellulase and protease, in contrast to chitinase activity which was negative. Furthermore, it produced the three lipopeptides families, which are, iturins A (C14; C15), fengycin A (C14, C15, C16, C17, and C18), and surfactin (C12, C13, C14, C15, C16). Interestingly, LC-MS profiles of 80% acetonitrile fengycin extract of the (4RH) strain, showed the presence of some new molecular ions (MH+) with masses different, but near to conventional fengycin variants, which correspond to new variants described for the first time in this work. The isolate (4RH) produced 5 µg/ml of the phytohormone (IAA), on TGE medium and sidérophores with more than 10 mm of yellow-orange zones, on CAS medium. To conclude, B. amyloliquefaciens (4RH) strain possessed interesting biocontrol and biofertilization characteristics, in vitro, in addition to its high sporulation yield, which make it a potential agent for future biopesticide that could be efficient in the integrate pest management and organic agricultural production systems.
Key words: Bacillus, biocontrol, biofertilization, plant protection, hot spring, lipopeptides.
Chemical pesticides are used worldwide to protect agricultural crops against diverse enemies such as insects, weeds and pathogenic microbes (Strange, 2005). However, increasing use of such products had caused several negative impacts on their efficiency (development of pathogen resistance), on environment and on human health (Gerhardson, 2002). Bacillus-based bio-pesticides represent the most important class of microbial products commercially available, including bio-insecticides, bio-fungicides, and bio-fertilizers (Fravel, 2005). A Bacillus genus, belonging to a Bacilli class I of phylum Firmicute, encompasses Gram positive, aerobic, and endospores forming bacteria (Subhash, 2011). Members of Bacillus genus are classified among plant growth promoting bacteria, which stimulate or promote plant health and productivity. This is ensured thanks to diverse direct (Biofertilization) and indirect (Biocontrol) mechanisms (Lugtenberg and Kamilova, 2009; Abdalla et al., 2014).
Direct plant growth promotion is obtained in the absence of pathogens through several ways, including: (1) Production of phytohormones, such as auxins, cytokinins, and gibberellins (Santner et al., 2009); (2) Secretion of enzymes such as 1-aminocyclopropane-1- carboxylate deaminase which decrease ethylene levels; (3) Enhancing asymbiotic nitrogen fixation (Adesemoye et al., 2010); (4) Increasing the solubilization of phosphorus and other trace element (Jorquera et al., 2008); and (5) Synthesizing siderophores chelating soluble iron (Schwyn and Neilands, 1987). In the other side, PGPRs also can help plant growth indirectly by reducing or preventing the deleterious impacts of plant pathogens.
The biocontrol is established due to many mechanisms, including: (1) Producing antibiotics or other metabolites that inhibit pathogen growth; (2) Out-competing with phytopathogens for nutrients and niches; (3) Inducing plant defense against pathogen infection (Lugtenberg and Kamilova, 2009). Microorganisms isolated from hot springs have received considerable interest in recent years, especially because microorganisms co-habiting such environments are able to survive under stress conditions and may possess new phenotypes that can be exploited in diverse fields, that is, pharmaceutical, food, agriculture, and cosmetics (Sandrin et al., 1990). The main objective of the present study is to determine in vitro biocontrol and biofertilization features of Bacillus strains isolated from a hot spring soil of Ouled Yeless located in Setif city of Eastern Algeria.
Isolation of Bacillus genus bacteria
Bacillus strains were isolated from soil near the hot spring of Ouled Yalass (Eastern Algeria), using a procedure involving a heat treatment. Ouled Yeless hot spring is located in the municipality of Mezloug, daïra Aïn Arnat, at 23 km of Setif southwest. The water of this source is characterized by a temperature varying between 25 and 43°C and a SO4-Ca chemical face (Boudoukha and Athamena, 2012). Soil (1 g) was added to 250 ml of physiological water, after decimal dilutions were prepared until 10-6. Dilutions tubes were treated by heat at 80°C, during 12 min. 100 µl of spores’ suspension from each tube, were cultured on nutritive agar plates which were incubated then at 30°C for 24 to 72 h.
Selection of antifungal strains
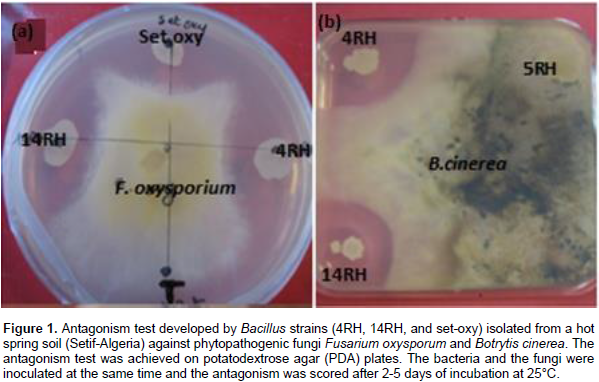
The selection of antifungal Bacillus strains was carried based on the dual culture antagonism test, on PDA plates. The phytopathogenic fungi used, were Fusarium oxysporium, Botrytis cinerea, Aspergillus niger, Cladosporium cucumerinium and Alternaria alternata. Mycelia inhibition rate was calculated as the reduction percentage of mycelia expansion compared to control plates without bacteria (Toure et al., 2004).
Identification of the strain (4 RH)
The total DNA was extracted from the bacterium (4RH) liquid cultures, by the wizard Genomic DNA purification kit (Promega), using the manufacturer's instructions. The primers used for the PCR amplification were the universal primers 16SP0 and 16SP6 for the 16S r-RNA gene (Almoneafy et al., 2012) and gyr-A.f and gyr-A.r for the gyrase-A gene (Izumi and Aranishi, 2004). The purification of the PCR products was achieved using the GFX PCR DNA and Gel Band Purification Kit instructions. The genes amplified were sequenced using the same primers cited earlier and the sequences obtained were corrected by the Bio-edit program. The sequences obtained were deposited in Genbank database and their corresponding accession numbers were established (Table 1). To identify the Bacillus isolate 4RH, the DNA sequences were compared to those previously published in Genbank using the BLASTN program.
Sporulation assessment
The Bacillus isolate 4RH was grown in the optimum liquid medium, prepared as described by Jacques et al. (1999), for 72 h of incubation, under a temperature of 30°C and an agitation of 180 rpm. The culture obtained was treated by heat chock at 80°C for 12 min, followed by immediate cooling to room temperature, in cooled water. Spores concentration was calculated on NA medium by counting colonies (Chen et al., 2000).
Production of antimicrobial compounds
Cell wall degrading enzymes
The enzymatic activities were assessed in a qualitative way through a halo formation on solid media containing, milk powder and carboxymethyl cellulose substrates to reveal protease and cellulase activities, respectively (Ariffin and Abdullah, 2006).
Lipopetides production
The lipopeptides were analyzed by mass spectrometry coupled to HPLC. The Bacillus strain 4RH was grown in agitated flasks (180 rpm) containing the optimum-medium, at 30°C for 72 h. Cultures were centrifuged at 15.000 g for 20 min. The resulting supernatants samples were analyzed by reverse phase HPLC coupled with single quad mass spectrometer (HPLC Waters Alliance 2695/diode array detector, coupled with Waters SQD mass analyzer), on a X- terra MS (Waters) 150×2.1 mm, 3.5 µm column as previously described by Nihorimbere et al. (2012). In this work, a single elution gradient allowing the simultaneous measurement of all three lipopeptides families was used. The water acidified with 0.1% formic acid (A) and acetonitril (ACN) acidified with 0.1% formic acid (B) were used as a mobile phase.
The flow rate was maintained at 0.5 mL min-1 and the column temperature at 40°C, with a gradient of 35 min (43 to 80% v/v ACN in 18 min; 100% v/v ACN for 9 min, and 43% v/v ACN in 8 min). Compounds were identified on the basis of their retention times compared to purified standards. In other hand, the identity of each homologue was confirmed on the basis of the masses detected in the SQD by setting electrospray ionization conditions in the MS as source temperature, 130°C; desolvation temperature, 250°C; nitrogen flow, 500 L/h; cone voltage, 70 V. The positive ion mode was used for analyzing all the three lipopetide families, because a higher signal/background ration was obtained compared to a negative ion recording. It is to signal that, the same LC-MS technique was used on fengycin extracts (80% acetonitrile), in order to reveal the presence of some new variants.
Production of indole 3 acetic acid (IAA)
The isolate 4RH was grown in TGE medium with agitation (160 rpm) at 30°C for 4 days. The indol 3 acetic acid production was assayed calorimetrically by using the Salkowski reagent (0.01 M FeCl3 in 36% H2SO4), as described by Benduzi et al. (2008). The test was achieved in duplicate.
Production of siderophores
The Bacillus isolates were streaked on chrome azurol S medium (CAS-medium) as described by Husen (2003) and siderophores production was indicated by the formation of yellow-orange halos around the colonies after plates incubation at room temperature, for 1 to 3 days.
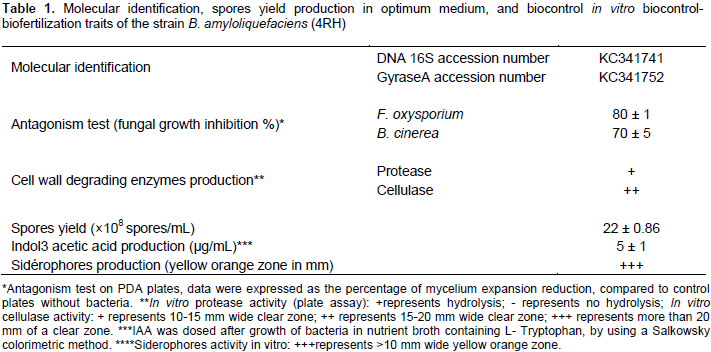
Twelve Bacillus strains were isolated from “Ouled Yalass” hot spring soil located in Setif city (Eastern Algeria). Three isolates of them coded (4RH), (14 RH), and (Set-oxy), were screened for their ability to inhibit the growth of some phytopathogenic fungi such as F. oxysporium, B. cinerea, A. niger, C. cucumerinium and A. alternata. The isolation of Bacillus species displaying antifungal activity, from a hot spring was investigated before by Ait Kaki et al. (2013). It is to note that in the present study, the best antifungal activity was established by the strain coded (4RH). In fact, it had important inhibition rates against F. oxysporium and B. cinerea, reaching 80 and 70%, respectively (Table 1 and Figure 1).
This is why, this isolate was chosen to complete our study, and was subjected to further molecular and in vitro biocontrol and biofertilization tests. The molecular identification of this strain based on the analysis of 16S-DNA showed that the bacterium was closely related to the Bacillus subtilis group, while gyrase-A gene sequencing specified more its identification and considered it as a Bacillus amyloliquefaciens. Access numbers provided by GenBank for 16S-DNA and gyrase-A genes were KC341741 and KC341752, respectively. The necessity to analyze gyrase A gene for determining the membership of bacterial isolates belonging to a Bacillus genus was investigated before by Ait Kaki et al. (2013) and Husen. (2003).
The presence of B. amyloliquefaciens strains in surrounding soil of hot spring was also investigated in the study of Ait Kaki et al. (2013). In fact, they isolated B. amyloliquefaciens (SEL, SEP and SI), Bacillus atrophaeus (6SEL), and Bacillus mojavensis (9SEL), from a hot spring of Oued Al-Athmanya. Reduced levels of nutrients in the environment or in a culture medium stimulate the sporulation process, exclusively in two bacterial species, that is, Clostridium and Bacillus (Jongsik et al., 2000). Spores have the advantage to survive for long periods without nutrients or water. This, make the production of bacteria belonging to the Bacillus genus, at the industrial level, realizable and profitable. In this work, the strain (4RH) was characterized by a high sporulation yield up to 22±0.86 X 108 spores/ml (Table 1), estimated after three days of incubation in optimum medium and under culture conditions, as described by Jacques et al. (1999). This sporulation yield is considered important, in comparison with the highest reported values estimated in a bioreactor culture conditions as established by Monteiro et al. (2010, 2014).
Antagonistic performances among Bacillus species had been widely noted in previous studies using diverse fungi (Gong et al., 2006). This inhibition phenomenon observed in Bacillus spp. results from one of the following mechanisms or their combination, that is, the production of antimicrobial molecules, the secretion of hydrolytic enzymes, and the competition for nutrients (Compant et al., 2005). In the present study, the bacterium (4RH) was able to produce cell wall degrading enzymes (cellulase and protease) and antibiotics of a cyclic lipopeptides class (C-LPs). The ability of Bacillus spp. isolated from hot springs to produce these enzymes was established previously by Lele and Deshmukh (2016) and Mohammad et al. (2017).
Cyclic lipopetides were mostly studied for their potent antagonistic activities against various phytopathogens, which constitute an important criterion, in using these bioactive molecules or their microorganism producer, in the field of a biological control of plant diseases. The world market of this class of molecules reached peak annual US revenue of >US$1 billion and there use has been approved in more than 70 countries (Ongena and Jacques, 2008). Members of the Bacillus genus are considered as efficient microbial factories for the large scale production of such type of bioactive molecules (Emmert et al., 1999; Roongsawang et al., 2011). In the present work, the analysis of the culture supernatant of the strain B. amyloliquefaciens (4RH), by the mass spectrometry coupled to the HPLC (LC-MS) had shown that this bacterium was able to produce the three C-LPs families, that is, iturins, fengycin, and surfactin (Figure 2).
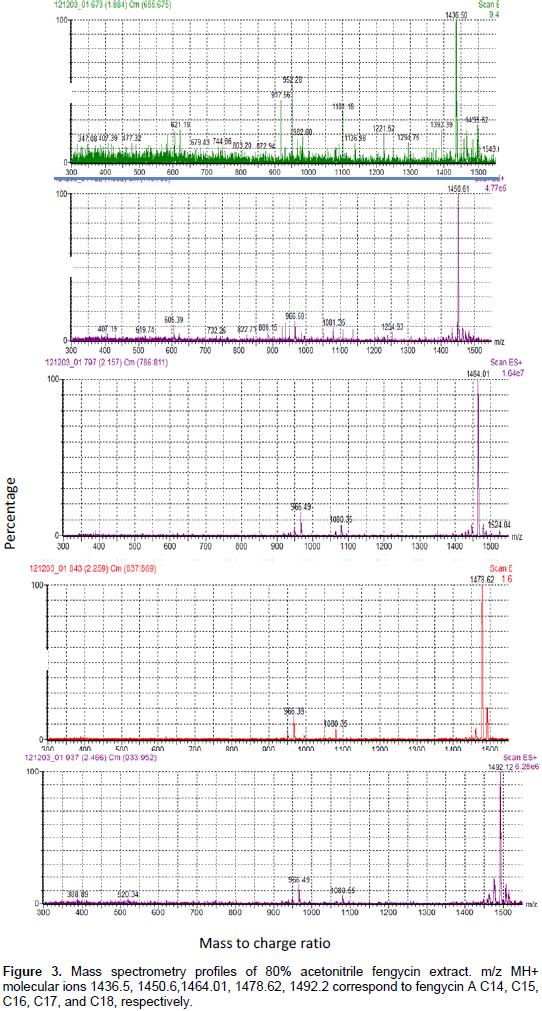
In fact, the HPLC profile had shown the apparition of different peaks at retention time ranges corresponding to C-LPs families, that is, 2 to 5 min for iturins family, 6 to 12 min for fengycin, and 22 to 26 min for surfactin. Furthermore, the integration of HPLC peaks gave masses which correspond to those of previously reported lipopeptides, which are, iturins A (C14, C15), fengycin A (C14, C15, C16, C17, C18), and surfactin (C12, C13, C14, C15, C16). It is important to signal that in the present work, the small peaks appearing at 6.8, 7.2, and 8.2 min (Figure 2) have masses near but different from previously reported fengycin molecules. The confirmation of this result was carried by analyzing (80%) acetonitrile fengycin extract with LC-ESI-MS technique. The interpretation of MS profiles obtained had shown the presence of molecular ions MH+ having the mass of previously reported fengycin A homologues (C14, C15, C16 C17 and C18), as mentioned in Figure 3.
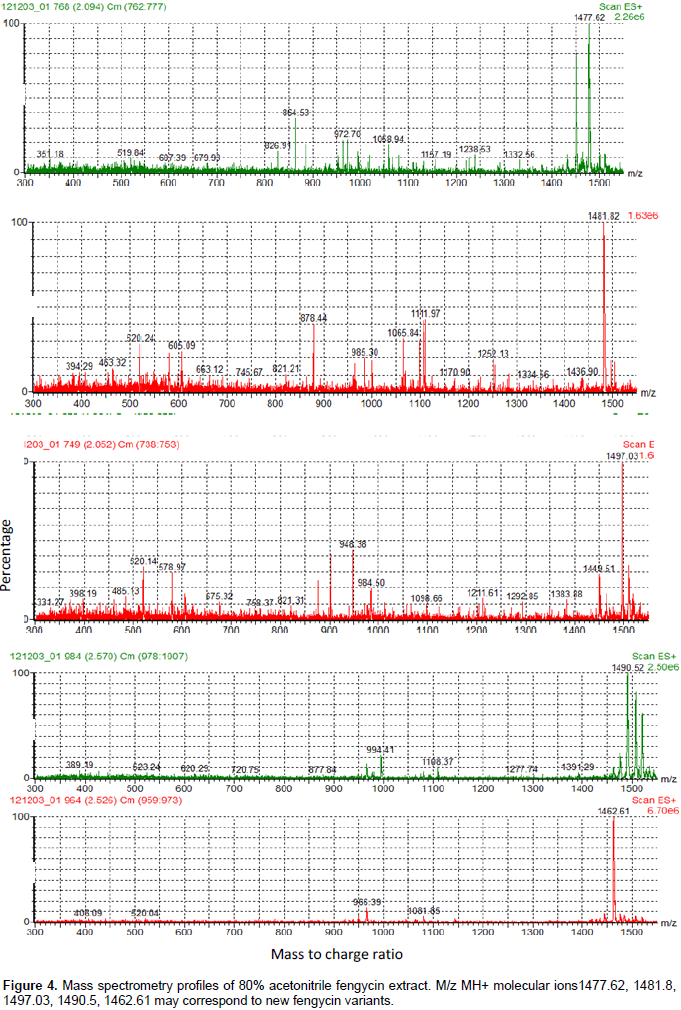
On the other hand, other molecular ions with masses different from known fengycin variants were present (Figure 4). New masses with –2D (MH+=1462.61; 1490.52) may correspond to the presence of an unsaturation in a fatty moiety, as previously sited by Nagorska et al. (2007). However, concerning the other new masses (1477.62, 1481.8, 1497.03), their further characterization with MS.MS analysis is required, to determine precisely the mutation carried in a peptide moiety, as conducted by De Faria et al. (2011) and Pathak et al. (2012). The strain B. amyloliquefaciens 4RH produced a low quantity of the phytohormone IAA, reaching 5 µg/ml (Table 1). Same results were obtained in the case of B. amyloliquefaciens strains isolated from a hot spring and a salt lake of Eastern Algeria, as described by Ait Kaki et al. (2013).
From the other side, the production of sidérophores, here, was important (Table 1 and Figure 5), with more than 10 mm of yellow-orange zones on CAS medium. It had been reported that auxins, including IAA have a positive effect on root growth and morphology; and that siderophores are responsible for the chelating of iron (Fe) depriving the phytopathogenic fungi of it (Benduzi et al., 2008). To conclude, B. amyloliquefaciens (4RH) strain possesses interesting biocontrol and biofertilization characteristics, in vitro, in addition to its high sporulation yield, which make of it a potential agent for future biopesticide that could be efficient in the integrate pest management and organic agricultural productions.
The authors have not declared any conflict of interests.
The authors are grateful to a fellowships European program Erasmus Mundus External Cooperation Window-consortium AVERROES.
REFERENCES
|
Almoneafy AA, Xie GL, Tian WX, Xu LH, Zhang GQ, Ibrahim M (2011) Characterization and evaluation of Bacillus isolates for their potential plant growth and biocontrol activities against tomato bacterial wilt. Afr. J. Biotechnol. 11:7193-7201.
|
|
|
|
Abdalla SA, Algam SAA, Ibrahim EA, El Naim AM (2014) In vitro Screening of Bacillus Isolates for Biological Control of Early Blight Disease of Tomato in in Shambat Soil. World J. Agric. Res. 2:47-50
Crossref
|
|
|
|
|
Adesemoye AO, Torbert HA, Kloepper JW (2010) Increased plant uptake of nitrogen from N-15-depleted fertilizer using plant growth-promoting rhizobacteria. Appl. Soil Ecol. 46:54-59.
Crossref
|
|
|
|
|
Ait Kaki A, Kacem Chaouche N, Ongena M, Kara Ali M, Dehimat L, Kahlat K, Thonart P (2013). In vitro and in vivo Characterization of Plant Growth Promoting Bacillus Strains Isolated from Extreme Environments of Eastern Algeria. Appl. Biochem. Biotech 172:1735-46.
Crossref
|
|
|
|
|
Ariffin, H Abdullah, N Umi Kalsom, MS Shirai, Y Hassan, MA (2006). Production and characterization of cellulase by Bacillus pumilus EB3. Int. J. Eng. Technol. 3:47-53.
|
|
|
|
|
Beneduzi A, Peres D, Da Costa PB, Zanettini MHB (2008) Genetic and phenotypic diversity of plant growth- promoting bacilli isolated from wheat fields in southern Brazil. Res. Microbiol. 159:244-25.
Crossref
|
|
|
|
|
Boudoukha A, Athamena M (2012) Caractérisation des eaux thermales de l'ensemble Sud Sétifien. Est algérien."Revue des sciences de l'eau 252:103-118.
Crossref
|
|
|
|
|
Chen Y, Fukuoka S, Makino S (2000). A novel spore peptidoglycan hydrolase of Bacillus cereus: Biochemical characterization and nucleotide sequence of the corresponding gene, sleL. J. Bacteriol. 182:1499-1506.
Crossref
|
|
|
|
|
Compant S, Duffy B, Nowak J, Clement C, Ait Barka E (2005) Use of plant growth-promoting bacteria for biocontrol of plant diseases: principles, mechanisms of action, and future prospects. Appl. Environ. Microbiol. 71:4951-4959.
Crossref
|
|
|
|
|
De Faria AF, Stéfani D, Vaz BG, Silva ÍS, Garcia JS, Eberlin MN, Grossman MJ, Alves OL, Durrant LR (2011). Purification and structural characterization of fengycin homologues produced by Bacillus subtilis LSFM-05 grown on raw glycerol. J. Ind. Microbiol. Biotechnol. 38:863-871.
Crossref
|
|
|
|
|
Driks A (2002). Overview: Development in bacteria: spore formation in Bacillus subtilis. Cell. Mol. Life. Sci. 59:389- 391.
Crossref
|
|
|
|
|
Emmert EAB, Handelsman J (1999). Biocontrol of plant disease: A (Gram-) positive perspective, FEMS Microbiol Lett 171:1-9.
Crossref
|
|
|
|
|
Fravel DR (2005). Commercialization and implementation of biocontrol. Annu Rev Phytopathol 43:337-59.
Crossref
|
|
|
|
|
Gerhardson B (2002). Biological substitutes for pesticides. Trends Biotechnol 20:338-343.
Crossref
|
|
|
|
|
Gong MW, Zhang J, Yang H, Lu X (2006). Study of the Antifungal Ability of Bacillus subtilis Strain PY-1 in vitro and Identification of its Antifungal Substance (Iturin A). Biochim. Biophys. Acta. 38:233-240.
Crossref
|
|
|
|
|
Husen E (2003). Screening of soil bacteria for plant growth promoting activities in vitro. Indones. J. Agric. Sci. 4:27-31.
Crossref
|
|
|
|
|
Izumi S, Aranishi F (2004). Relationship between gyrA mutations and quinolone resistance in Flavobacterium psychrophilum isolates. Appl Environ. Microb. 70:3968-3972.
Crossref
|
|
|
|
|
Jacques P, Hbid C, Destain J, Razafindralambo H, Paquot M, De Pauw E, Thonart P (1999). Optimumimization of biosurfactant lipopeptides production from Bacillus subtilisS499 by Plackett-Burman design. Appl Biochem Biotechnol 77:223-233.
Crossref
|
|
|
|
|
Jongsik C, Kyung SB (2000). Phylogenetic analysis of Bacillus subtilis and related taxa based on partial gyrA gene sequences. Antonie Van Leeuwenhoek. 78:123-127.
Crossref
|
|
|
|
|
Jorquera M, Martinez O, Maruyama F, Marschner P, Mora MDLL (2008). Current and future biotechnological applications of bacterial phytases and phytase-producing bacteria. Microbes Environ. 23:182-91.
Crossref
|
|
|
|
|
Lele OH, Deshmukh PV (2016). Isolation and characterization of thermophilic Bacillus sp. with extracellular enzymatic activities from hot spring of Ganeshpuri, Maharashtra, India. Int. J. Appl. Res. 2: 427-430.
|
|
|
|
|
Lugtenberg B, Kamilova F (2009). Plant growth promoting rhizobacteria. Annu Rev Microbiol 63:541-56.
Crossref
|
|
|
|
|
Mohammad BT, Al Daghistani HI, Jaouani A, Abdel-Latif S, Kennes C (2017). Isolation and Characterization of Thermophilic Bacteria from Jordanian Hot Springs: Bacillus licheniformis and Thermomonas hydrothermalis Isolates as Potential Producers of Thermostable Enzymes. Int. J. Microbiol. vol. 2017, Article ID 6943952, 12 pages, 2017.
Crossref
|
|
|
|
|
Monteiro SMS, Clemente JJ, Carrondo MJT, Cunha AE (2014). Enhanced Spore Production of Bacillus subtilis Grown in a Chemically Defined Medium. Adv. Microbiol 4:444-454.
Crossref
|
|
|
|
|
Monteiro SMS, Clemente JJ, Henriques AO, Gomes RG, Carrondoand MJT, Cunha AEA (2005). Procedure for High-Yield Spore Production by Bacillus subtilis. Biotechnol. Prog. 1026-1031.
|
|
|
|
|
Nagorska K, Bikowski M, Obuchowski L (2007). Multicellular behaviour and production of a wide variety of toxic substances support usage of Bacillus subtilis as a powerful biocontrol agent. Acta Biochimica 54:495.
|
|
|
|
|
Nihorimbere V, Cawoy H, Seyer A, Brunelle A, Thonart P, Ongena M (2012). Impact of rhizosphere factors on cyclic lipopeptides signature from the plant beneficial strain Bacillus amyloliquefaciens S499. FEMS Microbiol Ecol. 79:176-191.
Crossref
|
|
|
|
|
Ongena, M. and Jacques P (2008). Bacillus lipopeptides: versatile weapons for plant disease biocontrol. Trends Microbiol .16:115-25.
Crossref
|
|
|
|
|
Pathak KV, Keharia H, Gupta K, Thakur SS, Balaram P (2012). Lipopeptides from the Banyan Endophyte, Bacillus subtilis K1: mass spectrometric characterization of a library of Fengycins. J Am Soc Mass Spectrom. 23:1716-1728.
Crossref
|
|
|
|
|
Roongsawang N, Washio K, Morikawa M (2011). Diversity of nonribosomal peptide synthetases involved in the biosynthesis of lipopeptide biosurfactants Int. J. Mol. Sci. 12:141-172.
Crossref
|
|
|
|
|
Sandrin, C, Peypoux F, Michel G (1990). Production of surfactin and iturin A lipopeptides with surfactant and antifungal properties by Bacillus subtilis. Biotechnol. Appl. Biochem. 12:370-375.
|
|
|
|
|
Santner A, Calderon-Villalobos LIA, Estelle M (2009). Plant hormones are versatile chemical regulators of plant growth. Nat. Chem. Biol. 5:301-317.
Crossref
|
|
|
|
|
Schwyn B Neilands JB (1987). Universal chemical-assay for the detection and determination of siderophores. Anal. Biochem. 160:47-56.
Crossref
|
|
|
|
|
Strange RN (2005).Scott PR. disease: A threat to global food security. Annu. Rev. Phytopathol. 43:83-116.
Crossref
|
|
|
|
|
Subhash Y (2011). Diversity and phylogeny of plant growth promoting bacilli from moderately acidic soil. J. Basic Microbiol. 51:98-106.
Crossref
|
|
|
|
|
Toure Y, Ongena M, Jacques P, Guiro A, Thonart P (2004). Role of lipopeptides produced by Bacillus subtilis GA1 in the reduction of grey mould disease caused by Botrytis cinerea on apple. J. Appl. Microbiol. 96:1151-1160.
Crossref
|
|