ABSTRACT
Susceptibility of Streptococcus pneumoniae (pneumococci) to oxacillin and ceftriaxone was compared using disk diffusion and E-test methods. A total of 206 children attending Gertrude’s Children’s Hospital (GCH) were recruited. Sterile Copan Flocked Swabs were used to obtain nasopharyngeal swabs. Samples were inoculated on gentamicin blood agar and initial identification was done on the basis of colony morphology. Optochin test was performed to definitively identify the isolates as pneumococci. Antibiotic testing was done using disk diffusion and E-test methods on Muller Hinton agar enriched with 5% defibrinated sheep blood. A total of 42 (20%) isolates were recovered from the samples. Based on disk diffusion method, 74 and 40% of the isolates were resistant to oxacillin and ceftriaxone while; on the basis of E-test, 45 and 14% of the isolates were resistant to oxacillin and ceftriaxone, respectively. About 40% (n=17) of isolates that had zone diameters ≥ 20 mm which is considered susceptible to oxacillin by disk diffusion, had MICs ≤0.06 µg/ml correspondingly susceptible by E-test. Most isolates deemed susceptible to (≤0.5 µg/ml) ceftriaxone by E-test (72%, n=30) also exhibited susceptibility (≥27mm) to the antimicrobial agent by disk diffusion. E-test presents more sensitive results compared to disk diffusion. Isolates that exhibit resistance to penicillin and ceftriaxone by disk diffusion should be confirmed by E-test before being reported as resistant.
Key words: E-test, disk diffusion, antibiotic resistance, ceftriaxone and oxacillin.
Pneumococcus (Streptococcus pneumoniae) kills over 1 million children across the world every year (Köksal et al., 2017). Remarkably, more than 50% of these fatalities occur in Africa (Pagan, 2011). To reverse this trend, the World Health Organization (WHO) recommends two major interventions: inclusion of higher valence conjugate vaccines in national immunization programs and judicious use of antibiotic agents (WHO, 2011). Kenya introduced the 10 valent pneumococcal conjugate vaccine (PCV-10) in her national immunization program (KEPI) in 2011 (Ojal et al., 2019). The uptake of the vaccine overtime surpassed the recommended threshold and cases of pneumococcal infections due to the vaccine serotypes reduced remarkably (Hammitt et al., 2014). Ironically, pneumococcal disease has continuously remained one of the leading etiologies of child morbidity and mortality in Kenya (Heath et al., 2018). As a result, appropriate use of antibiotics seems to be the only other promising alternative (Esposito and Principi, 2013).
Information on susceptibility and resistance profiles to relevant antibiotics is paramount if their effective use is to be achieved (Reeve et al., 2015). This can only be made possible by use of reliable, accurate, precise and affordable laboratory methods. Often, the choice of the method used is determined by its reproducibility, accuracy, cost, practicality among others (OIE., 2012). Antibiotics in the class of macrolides are used to treat pneumococcal infections but mostly among persons above the age of five years. For pediatrics, the recommended agents are mainly beta-lactams like ampicillin (Gamache, 2019). For children aged between 2 days to 5 years, cephalosporins like cefotaxime or ceftriaxone can be administered as alternative drug therapy and as a single therapy (Gamache, 2019). While this antibiotic plan has been effective among pediatrics over-time, cases of penicillin resistance have been on the rise lately (Daniel, 2020). Unfortunately, studies have reported that most penicillin resistant pneumococci have also exhibited high resistance to fallback alternatives like cephalosporins (Kim et al., 2016). Considering the fact that ceftriaxone (cephalosporin) is one of the agents used in cases where penicillins have failed, it is important to understand its invitro effectiveness against pneumococci.
Laboratory assays such as broth dilution, agar dilution, disk diffusion and molecular based methods are used to profile susceptibility of bacteria to several antibiotic agents (OIE., 2012). The broth dilution method determines the lowest concentration of an antibiotic agent that hinders growth of bacteria (Clinical and Laboratory Standards Institute (CLSI), 2008). The test is done using varying antimicrobial concentrations against optimal bacterial inocula. The point between the lowest minimum inhibitory concentration (MIC) and the next point is recorded. As such, broth dilution method may not always signify total values and may therefore be prone to errors. The technique is not flexible to the changing surveillance needs because dilution panels are often predesigned and available commercially (Yakoob, 2014). Therefore, utilization of reference organisms for quality checks is fundamental to ensure reproducibility and accuracy of results. Procurement of test panels and relevant equipment for broth dilution assays may be costly and therefore the test may not be viable for some laboratories especially in the developing world (Turnidge and John, 2015).
In agar dilution method, gradually changing serial dilutions of the antibiotic agent is integrated in a preferred culture medium. Bacterial inoculum is thereafter applied on the surface of the media plate (Wiegand et al., 2008).
Except for organisms that exhibit swarming, this method represents the most reliable and reproducible results (Griffin et al., 2000). However, the technique may be labor intensive and expensive especially if not automated. The ability to accurately determine endpoints and purity of the inocula makes it partially inappropriate. The disk diffusion (Kirby Bauer) method is relatively affordable and effective (Shields et al., 2018). According to Hudzicki (2016), the method measures capacity of antimicrobials to inhibit growth of bacteria under optimal conditions. The radius of the zone around an antibiotic disk in which the bacterium has not grown is directly proportional to the efficacy of the antibiotic agent against that bacterium. While the manual measurement of the inhibition zone may present a platform for inaccurate results, automated methods of measurement are now available (EUCAST, 2015). The technique is easy to perform, provides relatively reproducible results and is generally less costly.
E-test is a gradient based technique that utilizes both dilution and diffusion capacities of the antimicrobial agent of interest (Romney and Hindler, 2015). The point at which the lower part of the growth intersects with the ellipse strip represents the minimum inhibition concentration (MIC) value (Bremmer et al., 2017). The test provides a substitute method to detecting even low levels of resistance for a range of bacteria (Amy, 2016). However, it may be slightly expensive given the high cost of purchasing commercially available gradient strips. Molecular based methods like polymerase chain reaction (PCR) and gene expert detect the presence of resistant genes (Dunne et al., 2017). The techniques are highly rapid and obviously less labor intensive. Unfortunately, the level of expertise, quality of reagents and equipment required make molecular based methods unpopular for economically emerging regions.
Against this backdrop, most laboratories in the developing world routinely use disk diffusion and e-test methods for antimicrobial susceptibility testing (AST). Therefore, continuous surveillance and quality checks to establish reproducibility and reliability of results obtained using these methods may be fundamental to provision of effective clinical management. This study compared sensitivity levels of the disk diffusion and e-test methods using oxacillin and ceftriaxone antibiotic agents against Streptococcus pneumoniae isolates.
Subject recruitment
Samples were collected between February 2017 and February 2018 from children below the age of five years seeking care at Gertrude’s Childrens hospital (GCH). The participants were clinically diagnosed of a variant of pneumococcal disease by the resident clinician. Biological mothers or legal guardians gave voluntary written assent permitting the recruitment of their children to the study. Any child who had a known history of an immunosuppressive condition and those who had used any antibiotic agent two weeks prior to collection of the sample were excluded.
Research design and sampling technique
The study used cross-sectional descriptive design. Study participants were studied within a specified period of time and findings inferred to the entire population of interest. Purposive sampling method was used to recruit subjects because the researchers had pre-determined rationale that prospective participants required to satisfy before being recruited.
Ethical considerations
Ethics approval for the study was given by Kenyatta University Ethics Review Committee (KU/ERC/APPROVAL/VOL. 1 (12)). The research permit was given by the National Commission of Science Technology and Innovation (NACOSTI/P/17/65428/15801). The study was further approved by the Gertrude’s Childrens Hospital Research Committee to be conducted at the outpatient clinic of GCH (GCH/ERB/VOL.MMXVII/121). A written informed assent was given by parents or legal guardians to prospective subjects before recruitment.
Sample size determination
To determine the minimum sample size, the formula for Fisher et al. (1991)was used, with a prevalence rate of 17% (Githii et al., 2013).
Where:
n= Desired minimal sample size (where population is ≥10,000);
z= Standard normal deviation = 1.96;
p= Prevalence rate;
m= the desired degree of accuracy @ 95% confidence level= 0.05 and;
n=1.962 x 0.17(1-0.17) / 0.052=217
Sample size (n) = 217
Since the target population is ≤10000, the value of n was further adjusted as follows:
nf= n/1+ {n/N}
Where:
nf= Desired minimum sample size (where population is ≤10,000)
n= Calculated sample size N= Total population nf= 217÷
[1+ (217/5,000)] nf= 207 subjects
Sample collection and processing
Nasopharyngeal swabs were collected by use of Copan Flocked Swabs (FLOQSwabs®) according to (CDC, 2015). Collected samples were immediately suspended in Amies Transport Media and kept in a cool box with ice until transportation to laboratory within three hours of collection. At the laboratory, samples were immediately streaked on 5% sheep blood agar (BA) containing 5.5 Pg/ml of gentamicin (GBA) and incubated in anaerobic conditions at 37°C for 24-48 h. Gentamicin was added to prevent growth of bacteria other than Streptococcus pneumoniae. Initial identification of the pneumococcus was on the basis of colony morphology as follows: α-hemolysis, draughtsman’s appearance and a mixture of either large or small gray-mucoid colonies. Initially identified isolates were further cultured on blood agar in anaerobic conditions at favorable conditions. Optochin test was done on each sample that had initially demonstrated features akin to pneumococcus for definitive identification. Fresh cultures were thereafter obtained from the BA plates, inoculated in brain heart infusion agar (BHI) enriched with 5% defibrinated sheep blood and stored at -80°C.
Disk diffusion (Kirby-Bauer) method
The stored isolates were thawed for a period of 30-45 min at room temperature in a sterile room. Colony suspension equivalent to 0.5 McFarland standard was prepared and inoculated evenly on Mueller Hinton agar enriched with 5% sheep blood. A sterile antibiotic disk dispenser (ADD) was used to dispense ceftriaxone (10 µg) and oxacillin (1 µg) impregnated disks onto a 100 mm petri dish. The preparations were incubated in anaerobic conditions at 37°C for 24-48 h. A linear caliber was used to measure the diameter of the zone of inhibition including the diameter of the disk which was 6 mm. The results were interpreted based on Clinical and Laboratory Standards Institute (CLSI) guidelines (CLSI, 2017). S. pneumoniae ATCC 49619 strain was used as part of quality control (QC).
E-test method
The stored isolates were thawed and fresh bacterial colony suspension equivalent to 0.5 McFarland standard was prepared, which was lawned evenly on Mueller Hinton agar enriched with 5% sheep blood. A sterile forceps was then used to place E-test strips (bioMe’rieux) containing gradually increasing concentrations of oxacillin and cetriaxone antibiotics on the opposite sides of each plate. The point on the epsilometer strip where the antibiotic agent no longer inhibited growth of the test organism was considered as the minimum inhibitory concentration (MIC).
Data analysis
Data were entered and analysed using SPSS version 22. Descriptive statistics and bivariate analysis were done to compare sensitivity levels of the pneumococci to oxacillin and ceftriaxone using disk diffusion and E-test methods. The analysis was done at 95% CI.
Sensitivity of pneumococcus to oxacillin and ceftriaxone by disk diffusion method
A total of 42 pneumococci isolates were recovered from the samples and thereafter subjected to the study antibiotic agents using both E-test and disk diffusion methods. Based on disk diffusion findings, 74% (n=31) and 40% (n=17) of the isolates were resistant to oxacillin and ceftriaxone respectively. On the other hand, 55% (n=23) of the isolates were susceptible to ceftriaxone while 19% (n=8) were susceptible to oxacillin by disk diffusion as shown in Table 1.
E-test MICs (µg/ml) for pneumococci to oxacillin and cetriaxone
The minimum inhibitory concentration (MIC) results obtained from E-test showed varied sensitivity patterns for the pneumococcal isolates. More isolates exhibited resistance to oxacillin (45%, n=19) while 14% (n=6) were resistant to ceftriaxone. Isolates susceptible to oxacillin and ceftriaxone were 41% (n=17) and 72% (n=30) respectively as shown in Table 2. The distribution of MICs of the isolates on oxacillin compared to the respective zone diameters showed at ≤17 mm, 45% were resistant at ≥2.0 MIC. Further, 10% (n=4) and 5% (n=2) were intermediately susceptible at 0.12-1 and ≤ 0.06 MICs respectively. Interestingly, at ≥20 mm cut off, only 40% of the isolates were susceptible at ≤ 0.06 MIC as shown in Table 3. The minimum inhibitory concentration (MIC) for ceftriaxone compared with disk diffusion results showed that at ≤24 mm cut-off point for disk diffusion, only 14% (n=6) of the isolates were resistant at ≥2.0 MIC. Further, at ≥27 mm 72% (n=30) of the isolates were susceptible to oxacillin at ≤ 0.5 MIC. Finally, at 25-26 mm point, 12% (n=5) and 2% (n=1) were intermediately susceptible to the antibiotic at 1 and ≤ 0.5 MIC respectively as shown in Table 4.
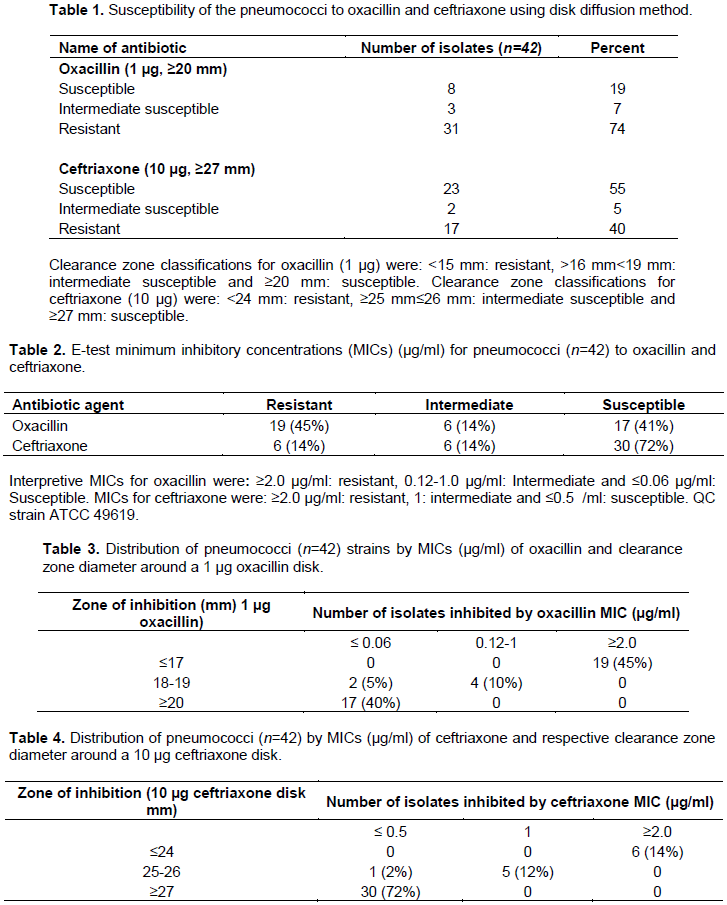
Understanding sensitivity levels of disk diffusion and e-test methods of antimicrobial resistance is a vital step in the clinical management infections. This study compares sensitivity of the two methods in determining antibiotic susceptibility of clinically important S. pneumoniae isolates. Based on disk diffusion, 74% (n=31) and 40% (n=17) of the isolates were classified as resistant to oxacillin and ceftriaxone respectively while 55% (n=23) and 19% (n=8) were considered susceptible to ceftriaxone and oxacillin respectively (Table 1). The disk diffusion results indicate that more isolates were sensitive to ceftriaxone compared to oxacillin. However, it is imperative to note that ceftriaxone was tested at a higher concentration (10 µg) compared to oxacillin (1µg) and it is a broad spectrum cephalosporin with extended half-life. It may therefore not be unreasonable if one attributed these results to the reasons stated above. Further, the results demonstrate almost clear-cut coherence with that of Waqas (2019), which reported efficacy of 84.7% for ceftriaxone and 71.4% for penicillin.
The results obtained on e-test for both antimicrobial agents show that 45% (n=19) and 14% (n=6) of the isolates were resistant to oxacillin and ceftriaxone, respectively; while 40% (n=17) and 72% (n=30) were susceptible to oxacillin and ceftriaxone, respectively (Table 2). The pattern of e-test susceptibility results was consistent with disk diffusion results based on the number of isolates that were sensitive or resistant to the antimicrobial agents tested. Overall, ceftriaxone showed better efficacy compared to oxacillin in both methods. Previous studies have reported discrepancies in the results from e-test and disk diffusion methods (Erfani et al., 2011). Notable is that the number of isolates resistant to both antibiotic agents was lower when using e-test method. This is a crucial comparator indicator of the two methods which qualifies e-test as being relatively superior to disk diffusion. Manoharan et al. (2003)reported that e-test presented minor to no errors when used to assay for AMR by Haemophilus Influenzae to chloramphenicol and ampicillin agents, respectively. This additional evidence enhances possibility of concluding that e-test method may be a little more sensitive than disk diffusion A comparison of the distribution of MICs with the corresponding zone diameters for the test antimicrobial agents revealed that 40% (n=17) of isolates with > 20 mm zone diameter for oxacillin had a corresponding MIC ≤ 0.06 µg/ml while 45% (n=19) had MIC≥2.0 µg/ml. On the contrary, 72% (n=30) isolates subjected to ceftriaxone had a corresponding MIC ≤ 0.5 µg/ml with fewer isolates (14%, n=6) having MIC ≥2.0 µg/ml. The findings demonstrate a higher efficacy of ceftriaxone against the pneumococcal isolates with possible resistance to Oxacillin exhibited by majority of the isolates. According to the CLSI, MIC (e-test) testing should be performed on all Streptococcus pneumoniae isolates showing oxacillin zones ≤ 19 mm before reporting as resistant (Horna et al., 2016). E-test and disk diffusion methods are relatively easy to perform; present reproducible and reliable results and do not require high technical expertise. Unfortunately, although fast and consistent, procurement of e-test strips is relatively costly and may not constitute a viable option for routine assaying of AMR especially in low-income countries.
E-test method presents more sensitive results as compared to disk diffusion in terms of quantitative classification of the level of resistance. However, both methods are non-laborious, reliable techniques which can be adopted in resource limited settings.
Pneumococci isolates that exhibit resistance to ceftriaxone by disk diffusion method may be confirmed by e-test method before being reported as resistant.
The authors have not declared any conflict of interests.
The authors appreciate Dr. Richard Kagia of Kabarak University for keenly reviewing the contents of this paper.
This work was partially funded by the National Commission on Science Technology and Innovation (NACOSTI/ RCD/ST & I/7th CALL/PhD/148).
REFERENCES
|
Amy LL (2016). In Clinical Microbiology Procedures Handbook pp. 5.3.1-5.3.9.
Crossref
|
|
|
|
Bremmer DN, Balada-Llasat JM, Goff DA, Bauer KA (2017). Ceftriaxone Etest non-susceptible methicillin susceptible Staphylococcus aureus time-kill responses. Diagnostic Microbiology and Infectious Disease 88(2):192-194.
Crossref
|
|
|
|
|
Centers for disease control & preventition (CDC) (2015). Nasopharyngeal Swab Procedure. Yukon Communicable Disease Control.
View
|
|
|
|
|
Clinical & Laboratory Standards Institute (CLSI) (2017). Performance Standards for Antimicrobial Susceptibility Testing (27 th). Wayne, USA-Clinical and Laboratory Standards Barbara L. Zimmer, PhD Institute, 950 West Valley Road, Suite 2500, Wayne, Pennsylvania 19087 USA.
|
|
|
|
|
Clinical and Laboratory Standards Institute (CLSI) (2008). Reference method for broth dilution. Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts. Approved Standard 3rd Ed 28(14):0-13.
|
|
|
|
|
Daniel M (2020). Resistance of Streptococcus pneumoniae to beta-lactam antibiotics. Clinical Infectious Diseases 50(37):1997-2002.
View
|
|
|
|
|
Dunne WM, Jaillard M, Rochas O, Van-Belkum A (2017). Microbial genomics and antimicrobial susceptibility testing. Expert Review of Molecular Diagnostics.
Crossref
|
|
|
|
|
Erfani Y, Rasti A, Mirsalehian A, Mirafshar SM, Ownegh V (2011). E-test versus disk diffusion method in determining multidrug resistant strains of Escherichia coli in urinary tract infection. African Journal of Microbiology Research 5(6):608-611.
|
|
|
|
|
Esposito S, Principi N (2013). Pharmacotherapy for pneumococcal infections: An update. Expert Opinion on Pharmacotherapy.
Crossref
|
|
|
|
|
Fisher AA, Laing EJ, Stoeckel EJ, Townsend WJ (1991). Handbook for family planning operations research design 2nd Ed.
Crossref
|
|
|
|
|
Gamache J (2019). Bacterial Pneumonia Medication. Medscape.
View Retreived on 10th January, 2021.
|
|
|
|
|
Griffin SG, Markham JL, Leach DN (2000). An agar dilution method for the determination of the minimum inhibitory concentration of essential oils. Journal of Essential Oil Research 12(2):249-255.
Crossref
|
|
|
|
|
Githii S, Revathi G, Muigai A, Kariuki S (2013). Carriage rate and serotypes of Streptococcus pneumoniae amongst children in thika hospital, Kenya. African Journal of Laboratory Medicine 2:1.
Crossref
|
|
|
|
|
Hammitt LL, Akech DO, Morpeth SC, Karani A, Kihuha N, Nyongesa S, Tahren B, Mumbo E, Kamau T, Sharif SK, Scott JA (2014). Population effect of 10-valent pneumococcal conjugate vaccine on nasopharyngeal carriage of Streptococcus pneumoniae and non-typeable Haemophilus influenzae in Kilifi, Kenya: Findings from cross-sectional carriage studies. The Lancet Global Health 2(7):e397-e405.
Crossref
|
|
|
|
|
Heath CJ, Nayakwadi SM, King CH, Malhotra I, Mutuku F, Mukoko D, LaBeaud AD (2018). Nasopharyngeal carriage of Streptococcus pneumoniae in children in Coastal Kenya. American Journal of Tropical Medicine and Hygiene 98(4):1046-1050.
Crossref
|
|
|
|
|
Horna G, Molero ML, Benites L, Roman S, Carbajal L, Mercado E, Maria EC, Rito Z, Eduardo C, Roger H, Wilda S, Francisco C, Andy S, Isabel R, Alex V, Ochoa TJ (2016). Oxacillin disk diffusion testing for the prediction of penicillin resistance in Streptococcus pneumoniae. Revista Panamericana de Salud Publica/Pan American Journal of Public Health 40(1):57-63.
|
|
|
|
|
Hudzicki J (2009). Kirby-Bauer Disk Diffusion Susceptibility Test Protocol. Ameican Society for Microbiology pp.1-23.
View
|
|
|
|
|
Kim L, McGee L, Tomczyk S, Beall B (2016). Biological and epidemiological features of antibiotic-resistant Streptococcus pneumoniae in pre- and post-conjugate vaccine eras: A United States perspective. Clinical Microbiology Reviews.
Crossref
|
|
|
|
|
Köksal Ä°, Åženol E, Çilli A, Alıcı DE (2017). The pilgrimage and the burden of pneumococcal disease in adults. Tuberkuloz ve Toraks.
Crossref
|
|
|
|
|
Manoharan A, Pai R, Shankar V, Thomas K, Lalitha MK (2003). Comparison of disc diffusion & E test methods with agar dilution for antimicrobial susceptibility testing of Haemophilus influenzae. Indian Journal of Medical Research 117:81-87.
|
|
|
|
|
Office International des Epizooties (OIE) (2012). Laboratory Methodologies for Bacterial Antimicrobial Susceptibility Testing. OIE Terrestrial Manual 1-11.
View
|
|
|
|
|
Ojal J, Griffiths U, Hammitt LL, Adetifa I, Akech D, Tabu C, Flasche S (2019). Sustaining pneumococcal vaccination after transitioning from Gavi support: a modelling and cost-effectiveness study in Kenya. The Lancet Global Health 7(5):e644-e654.
Crossref
|
|
|
|
|
Pagan R (2011). Pneumococcal conjugate vaccines as a probe for better understanding pneumococcal respiratory infections. Paediatric Respiratory Reviews 12:S43-S44.
Crossref
|
|
|
|
|
Reeve SM, Lombardo MN, Anderson AC (2015). Understanding the structural mechanisms of antibiotic resistance sets the platform for new discovery. Future Microbiology.
Crossref
|
|
|
|
|
Romney MH, Hindler JA (2015). Susceptibility Test Methods: Fastidious Bacteria. Manual of Clinical Microbiology, 11th Edition, Wiley online library.
Crossref
|
|
|
|
|
Shields RK, Clancy CJ, Pasculle AW, Press EG, Haidar G, Hao B, Chen L, Kreiswirth BN, Nguyen MH (2018). Verification of Ceftazidime-Avibactam and Ceftolozane-Tazobactam Susceptibility Testing Methods against Carbapenem-Resistant Enterobacteriaceae and Pseudomonas aeruginosa. Journal of Clinical Microbiology 56: 2.
Crossref
|
|
|
|
|
Turnidge JD, Jorgensen J (2015). Susceptibility Test Methods: Dilution and Disk Diffusion Methods. Warnock (Ed.), Manual of Clinical Microbiology 11th Edition.
Crossref
|
|
|
|
|
The European Committee on Antimicrobial Susceptibility Testing (EUCAST) (2015). EUCAST disk diffusion method for antimicrobial susceptibility testing.
|
|
|
|
|
Waqas M (2019). Comparative Efficacy of Ceftriaxone Versus Penicillin in the Treatment of Children with Severe Community-Acquired Pneumonia (CAP). Biomedical Journal of Scientific & Technical Research 14:4.
Crossref
|
|
|
|
|
Wiegand I, Hilpert K, Hancock RE (2008). Agar and broth dilution methods to determine the minimal inhibitory concentration (MIC) of antimicrobial substances. Nature Protocols 3(2):163-175.
Crossref
|
|
|
|
|
World Health Organization (WHO) (2011). Immunization, Vaccines and Biologicals, Pneumococcal disease.
View. Retrieved on 09th January, 2021
|
|
|
|
|
Yakoob MY (2014). Comparison of microdilution and standard agar dilution method for determining Penicillin resistance among Streptococcus pneumoniae. Journal of Nepal Health Research Council 12(27):112-115.
|
|