ABSTRACT
Traditional medicinal plants claim many traditional uses across different parts of the world. In Ethiopia, it remains the main source of treatment for the majority of human population and livestock. The aim of this study was to evaluate antibacterial activity of crude extracts of some traditional medicinal plants commonly used for traditional human medication in selected localities of Jimma Zone, Southwest Ethiopia. Data on ethno-botanical information and traditional uses of medicinal plants were gathered using semi-structured interview questionnaire involving a total of 30 experienced respondents of the study area. Candidate traditional medicinal plants were collected from Sigmo District of Jimma Zone, South western Ethiopia, labeled, processed, and extracted in accordance with standard procedures and the plant samples were deposited at Herbarium of Jimma University, Ethiopia. Antibacterial activities and minimum inhibitor concentration (MIC) of petroleum ether, chloroform and methanol extracts of leaves and stems of three frequently used plants [Kosteletzkya begonifolia, Leucas martinicensis, and Ranunculus multifidus] were evaluated against Staphylococcus aureus DSM 7346, Pseudomonas aeruginosa DSM 1117, Escherichia coli ATCC 25722 and Salmonella typhimurium ATCC 13311. Phytochemical constituents of the extracts were determined following standard analytical procedures. Results revealed that leaves were the most frequently used parts of the three medicinal plants. They are usually used for the treatment of tooth ache and gastro-intestinal ailments. The highest antimicrobial activities were observed in petroleum ether extract of K. begonifolia stems against S. aureus [Inhibition Zone (IZ), 28.3-30 mm], P. aeruginosa (IZ: 27-28.67 mm), E. coli (IZ: 28.3-31 mm) and S. typhimurium (IZ, 28-30.3)]. The extract displayed activity significantly closer to that of the control antibiotics, ciprofloxacin (IZ, 30-35 mm). Likewise, chloroform extracts of leaves of R. multifidus and methanol extracts of L. martinicensis displayed strong activities against S. aureus (IZ, 26.67±0.8 mm) and E. coli (IZ 26.67±3.3 mm), respectively. The lowest MIC observed in the current study was 5.6 mg/mL and recorded for both petroleum and chloroform extracts of leaves of R. multifidus, L. martinicensis, and K. begonifolia against S. aureus. The observed antibacterial activities could be accounted to combinations of phytochemical compounds isolated from the test plants including alkaloids, tannins, flavonoids, terpenoids and cardiac glycosides. Leaves of the three traditional medicinal plants evaluated in the current study displayed promising antibacterial activities against bacterial test strain. However, the highest activity was observed in petroleum ether extract of stems of K. begonifolia against all test strains with Inhibition Zone (IZ) diameter ranging between 28-31 mm. Further toxicity and pharmacokinetic study are recommended.
Keywords: Medicinal plant, MIC, plant extract, Ethiopia, Jimma, S. aureus, P. aeruginosa, E. coli
Plants represent a rich source of antimicrobial agents and have been used medicinally in different parts of the world. Plant based traditional medicine plays an essential role in human medication, with significant numbers of world population relying on traditional medicines for their primary health care (Owolabi et al., 2007). In spite of the great advances achieved in modern medicine, thousands of rural communities in developing countries still dependent on folklore medicine to cure diseases mainly because of economic and cultural factors (Kamatenesi and Oryem-Origa, 2007). However, such plants should be investigated for better understanding of their properties, safety and efficacy to develop alternative antimicrobial drugs (Khulbe and Sati, 2009). Accordingly, the utilization of plants for the production of natural or recombinant compounds of commercial interest has gained increasing attention over the past decades (Canter et al., 2005). Furthermore, in the era of high pressure from emergence of microbial drug resistance and limited therapeutic efficacy of many of the available drugs, search for potent antibacterial drugs with new modes of action should be given emphasis. Local medicinal plants are potential source of novel antimicrobial agents and anti-Quorum sensing substances (Bacha et al., 2016).
According to Abebe (2011), traditional remedies are the most important and sometimes the only source of therapeutics for nearly 80% of the population and 95% of traditional medicinal preparations in Ethiopia. Despite the use of traditional medicine over many centuries, relatively small numbers of plant species have been studied for possible medical applications and the source of information is largely limited to indigenous societies (Cunningham, 1993).
Ethiopia is a country known for its rich plant biodiversity and tradition use of plant based drugs for curing or treating of many human and animal diseases. Reports indicate that more than 35,000 plant species are being used for medicinal purposes all over the world (Lewington, 1993). In Ethiopia alone, 800 plant species are estimated to be in use for traditional medication (Tesema et al., 2002). Likewise, WHO estimates that majority of the population in developing countries, including 90% of African population; rely on traditional medicinal plants for their healthcare (WHO, 2002).
Sigmo District is located in Jimma zone, Oromia Regional state, Ethiopia. Though large part of the district is covered with forest and could serve as potential source of traditional medicinal plants, scientific information on the availability and practice of the use of traditional medicinal plants are lacking. On the other hand, food borne infections have been one of the major public health concerns in many parts of the world, including the study site, accounting for considerably high cases of illnesses. Among the etiological agents, Pseudomonas aeruginosa, Escherichia coli, Staphylococcus aureus and Salmonella species are the major ones (Branham HYPERLINK "http://onlinelibrary.wiley.com/doi/10.1111/j.1745-4565.2006.00052.x/full"et alHYPERLINK http://onlinelibrary.wiley.com/doi/10.1111/j.1745-4565.2006.00052.x/full., 2005; Dabassa and Bacha, 2012). The current study was designed to evaluate the antibacterial activities of the frequently used traditional medicinal plants in the study area. Accordingly, three of the traditional medicinal plants labeled as Kosteletzkya begonifolia (Ulbr.) Ulbr, Leucas martinicensis (Jacq.) R.Br. and Ranunculus multifidus), were characterized for their antibacterial activities.
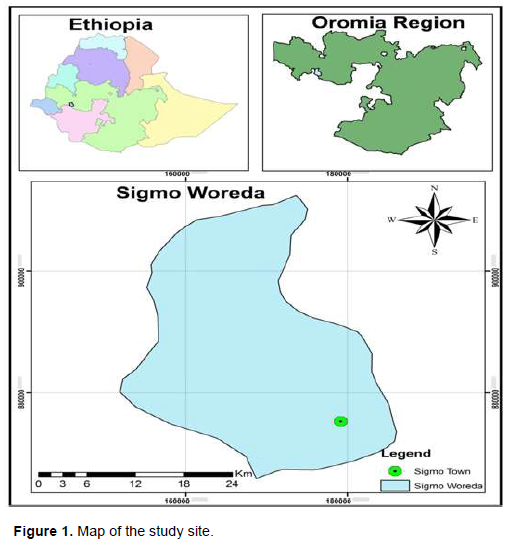
Ethno-botanical survey was conducted in Sigmo District, Jimma Zone, Southwest Ethiopia. The district was located at an altitude ranging from 2080 to 2490 masl and located at 7° 54’ 641”, N and 36° 06’ 092”, E latitude and longitude, respectively (Figure 1). According to the 2005 census conducted by Central Statistical Agency (CSA) of Ethiopia (CSA, 2005), this district has an estimated total population of 99,998, with male and female share of 50,355 and 49,643, respectively.
Collection of ethno-botanical data
Traditional Medical Practitioners (TMP's) and experienced elders were the main informants during collection of ethno-botanical information of the used traditional medicinal plants in the current study. A total 30 informants (11 TMP's and 19 other community members) were interviewed using semi-structured interview questionnaire. Accordingly, data on the plant parts being used, preparation techniques and administration of plants for management of common food and water borne diseases were collected. To ease the interview processes and get the detailed information without any language barrier, both interview and focus group discussions were conducted using local language having translated content of the interview questions into local language (Afan Oromo).
Collection and preparation of plant material
Having established the commonly used plants in the study area, the stems and leaves of three dominant plant species were collected using plastic bags and transported to Botanical Science Laboratory of Jimma University for identification. Having identified and labeled with the necessary botanical information, voucher specimens were deposited at herbarium of Jimma University, Department of Biology. The collected plant materials (stems and leaves) were dried under shade and grinded to appropriate size using mortar and pestle prior to extraction.
Plant extraction
Soxhlet extraction method was used for plant matter extraction (stem and leave). Briefly, 50 g of powdered plant material was weighed and packed into thimble placed in a glass. A reflux condenser and a round bottom flask with 500 ml solvent (starting with petroleum ether) was fitted above and below the thimble holder, respectively. Then, continuous extraction was carried for a period of at least 6 h until the extract becomes colorless. The solvent was removed from each extract by Rotary Evaporator under reduced pressure and temperature (£ 40°C). The concentrated crude extract was then added to 50 ml beaker and the remaining solvent was evaporated under reduced pressure followed by storage in a refrigerator (+4°C). The extraction was continued in the same way using same volume of two other solvents separately (chloroform and methanol) (Mulat et al., 2015).
Antibacterial activity assay
The antibacterial activity assay was evaluated using agar disc diffusion and micro-dilution broth assay techniques as described in Mackeen et al. (1997) and Komuraiah et al. (2009), respectively.
Agar disc diffusion assay
Four bacterial strains, including S. aureus DSM 7246, Salmonella typhimurium ATCC 13311, P. aeruginosa DSM 1117 and E. coli ATCC 25722, were used for the assay. The bacterial strains were activated overnight at 37°C prior to testing. Test solutions were prepared by dissolving 0.2, 0.1 and 0.05 g of plant extracts in dimethyl sulfoxide (DMSO) to achieve final stock concentrations of 200, 100 and 50 mg/mL, respectively. Sterile Whatman filter paper discs (6 mm diameter) were evenly placed on Mueller Hinton agar plate surface previously swabbed with an overnight activated culture of the test strains whose turbidity was adjusted to 0.5 McFarland standards. Then, 30 μL each of the test sample was loaded onto duplicate discs. Standard disc of ciprofloxacin (10 μg/disc) and paper disc loaded with 30 μL of DMSO were used as positive and negative controls, respectively.
The plates were then inverted and incubated for 18-24 h at 37°C. After incubation, clear zones around the discs (zones of growth inhibition) were measured and expressed as Means ± SD (mm) of two experiments.
Determination of minimum inhibitory concentration (MIC)
MIC of the crude extracts was determined as described by Komuraiah et al., (2009). Accordingly, extract that exhibited observable activity during antibacterial activity assay was further diluted in nutrient broth (to a concentration of 50 mg/mL, 16.6 mg/mL, 5.66 mg/mL, 1.8 mg/mL, 0.6 µg/mL and 0.2 µg/mL). Then, 0.1 mL (100 μl) of standardized inoculum (1 - 2 × 108 CFU/mL) was added to each test tube. The tubes were incubated aerobically at 37°C for 24 h. Control tubes were included in each test. The lowest concentration of extract that produced no visible bacterial growth (no turbidity) when compared to the control tubes was regarded as MIC.
Determination of minimum bactericidal concentration (MBC)
MBC was determined by sub-culturing the test dilutions onto a fresh agar plate (without extract) and incubated for 24 h. The highest dilution that yielded no single bacterial colony was taken as MBC.
Phytochemical screening
A small portion of the powdered plant extracts were used for phytochemical analysis (tested for the presence of plant secondary metabolites including alkaloids, tannins, glycosides, saponins, flavor-noids, steroids and terpenoids) following standard methods described earlier by Trease and Evans (1998).
Data obtained from both survey and experimental activities were analyzed using SPSS software (version 16:00 and Microsoft excel). Values were mean of duplicate experiments and statistical significance within in a category was evaluated using % coefficient of variation with values > 10% considered statistically significant. For mean separation, statistical significances were considered at p<0.05.
Socio-demographic characteristics of respondents
A total of 30 purposively sampled population responded to the questionnaires designed to gather ethno-botanical information and practices associated with traditional medicinal plants of the study area. All of the respondents were familiar with arrays of medicinal plants and have been using 5 to10 types of traditional medicinal plant for more than 5 years mainly for self medication (Tables 1 and 2). The use of traditional medicine has been practiced mostly by elderly males aged between 40-60 years and had more of religious than formal education.
Ethno-botanical description
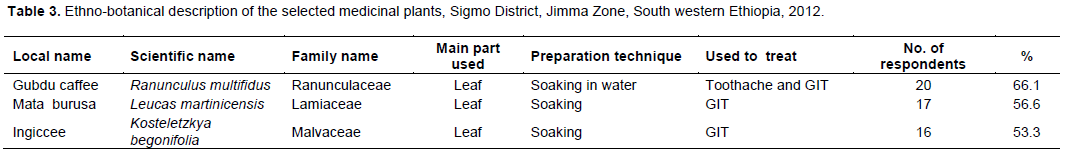
Among ten traditional medicinal plants rated for their frequency of use, three (R. multifidus, L. martinicensis, and K. begonifolia) were found the most preferred and frequently used traditional medicinal plants of the study site (Figure 2). Leaves of the three plants have been used to treat ailment of the gastrointestinal tracts (GIT) and oral cavity after simple soaking of the leaves in water and filtration (Table 3).
Antibacterial activities of leaves and stems extracts
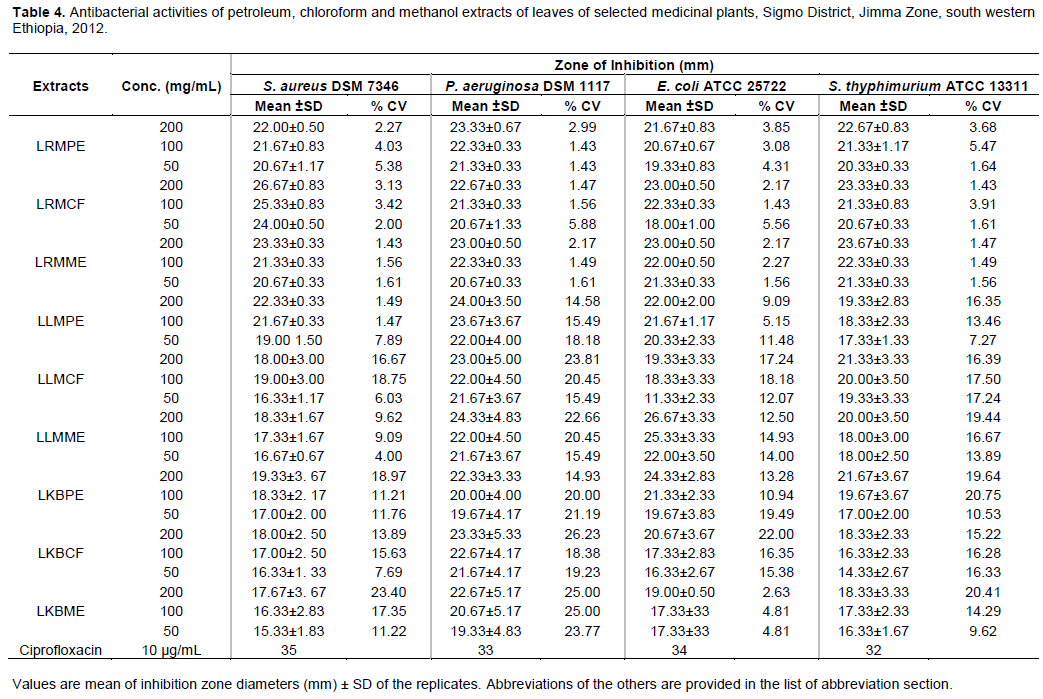
Mean inhibition zone diameters of duplicate experiments for the three different concentrations of extracts (200, 100 and 50 mg/mL) are as summarized below (Tables 4, and 5). Accordingly, all the tested leave extracts displayed observable activities with mean inhibition zone diameter (IZ) ranging between 14.33 ± 2.33 to 26.67 ± 3.33 mm. Strong inhibitions were recorded for methanol and chloroform extracts of L. martinicensis and R. multifidus against E. coli (26.67 ± 3.33) and S. aureus (26.67 ± 3.33 mm) at 200 mg/mL concentration, respectively. Relatively, similar activity was observed even at 100 mg/mL concentration of petroleum ether extract of L. martinicensis against P. aeruginosa (24.00 ± 3.50). The least activity was encountered in chloroform extract of K.begonifolia against S. typhimurium (14.33 ± 2.33) both at 50 and 30 µg/ml (Table 4).
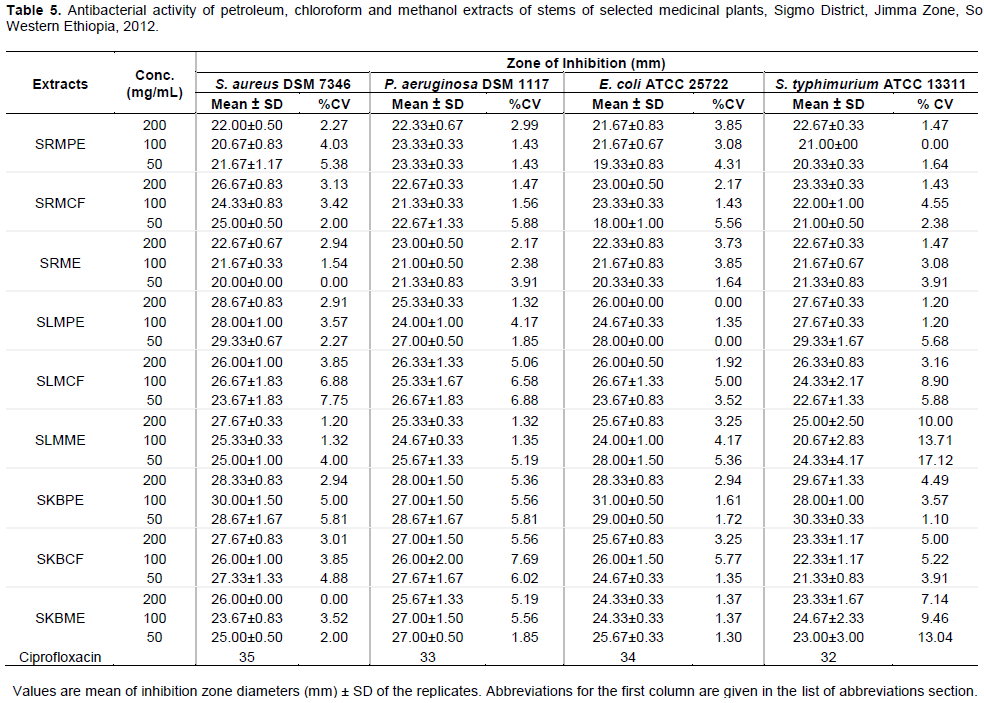
Like that of leaves, petroleum, chloroform and methanol extracts of stems of the three plants also showed variable antibacterial activities against the standard bacterial strains. However, exceptional activity comparable to the activity of the control commercial antibiotic Ciprofloxacin (IZ, 32-35 mm) were recorded for petroleum ether extract of stems of K. begonifolia against E. coli (31±0.5mm) and S. aureus (30.00 ± 1.50) at 100 mg/ml concentration of the extracts; and S.typhimurium (30.33 ± 0.33) at even lower concentration (50 mg/mL). The least activity was observed in methanol extract of stem of L. martinicensis against S. typhimurium (20.67±2.83mm) (Table 4).
MIC and MBC of crude extracts of leaves and stems
Petroleum ether and chloroform extracts of leaves of the three selected plants (R. multifidus, L. martinicensis and K. begonifolia) displayed the lowest MIC and MBC values of 5.6 and 16.6 mg/mL against S. aureus, respectively.
Whereas, all methanol extracts of the same leaves showed similar values for MIC and MBC (16.6 mg/mL each) against S. aureus. For Gram negative test strains, MIC and MBC values recorded for all extracts (petroleum ether, chloroform and methanol) were 16.6 mg/mL and 50 mg/mL, respectively (Table 5).
In the same way, petroleum ether extract of stems of R. multifidus, L. martinicensis, K. begonifolia, and chloroform extract of L. martinicensis showed lowest MIC value of 5.6 mg/mL against S. aureus. However, chloroform and methanol extract of stems of R. multifidus and methanol extract of stems of L. martinicensis showed the lowest MIC value of 16.6 mg/mL against S. aureus (Table 5). Furthermore, petroleum ether extracts of L. martinicensis, and K. begonifolia showed the lowest MIC value of 5.6 mg/mL against P. aeruginosa (Table 5).
Phytochemical screening
Phytochemical tests were conducted on all petroleum ether, chloroform and methanol extracts and the findings were summarized and presented below (Tables 6). All the plant extracts displayed the presence of many bioactive phytochemical compounds mainly alkaloids, tannins, flavonoid, saponins, steroids and cardiac glycosides (Table 6) except for absence of cardiac glycosides in Ranunculus multifidus.
In most developing countries where people are living under poor hygienic conditions, the possibility of contracting infectious diseases could be high. For several reasons, including low access to modern health care systems, high cost of the available commercial drugs and trust developed over long years of traditional practices, large proportion of a population in rural community prefer using traditional medicinal plants in order to manage infectious diseases of different sorts (Giday et al., 2003; Bacha et al., 2016). The current study area is not exceptional. Extensive review made on the status of use of traditional medicinal plants in different parts of Africa (Sofowora, 1993) also indicated that medicinal plants have been widely used among African communities to treat different types of bacterial diseases. Actually, about 85% of world population uses herbal medicines for prevention and treatment of diseases, and the demand is still on the rise both in developed and developing countries (Abramov, 1996; Abera, 2014). Reliance on traditional medicine could be even higher among the pastoral and agro-pastoral communities whose environment were reported to be rich in many of effective medicinal plants (Belayneh et al., 2012).
The survey data shown that knowledge on traditional practices has been acquired from parents to off springs, hence through generations, mainly from elderly religious men (80%), parents and close relatives (~20%) or very rarely through random trial and error method. In agreement with our observation, Tolasa (2007) reported that 91 and 9% of knowledge on the use of traditional medicine were acquired from parents/relatives and through trial and error, respectively, as observed in other part of Ethiopia. One of the major challenges confronting the sustainability of transfer of traditional knowledge and good practices of the use of traditional medicine is the tendency of keeping the traditional knowledge secrete from others, hence creating a gap in the flow of knowledge across generations. The confidentiality of traditional medical practice is a common phenomenon in other parts of the country, too (Giday et al., 2003), mainly for business interest. In agreement with our observation, the transfer of knowledge to people outside the family circle among the Zay people of Ethiopia takes place only on substantial payment (Giday et al., 2003). When such practice is accompanied by the new generation’s high dependence on modern medication (because of lack of ample information and practical skill), the future fates of traditional medication could be questionable when traditional medicines still have significant contribution to the health care system of larger part of the world population.
In the study area, more than ten traditional medicinal plants have been used for treatment of various ailments including toothache, infection of the GIT, and related diseases. However, the community’s preference to three of the traditional medicinal plants namely, R. multifidus, L. martinicensis, and K. begonifolia, were significantly high with preference rating of 66.6, 56.6 and 53.3%, respectively. The observed high reliance on the three traditional medicinal plants was scientifically sounding as all the three plants were observed to possess various phytochemical compounds of medicinal values including alkaloids, tannins, saponnins, flavonoids, steroids, terpenoids and cardiac glycosides. Flavonoids are found in almost all parts of plants (Ahmed et al., 2015; Cushnine and Lamb, 2005) protect the plant from insect pests and ultraviolet radiations besides imparting color to flowers and fruits. In addition to their antioxidant, anti-depressant and anti-inflammatory role in human body, flavonoids act as bactericidal and bacteriostatic by damaging cytoplasmic membrane, inhibiting energy metabolism and synthesis of nucleic acids in different microorganisms (Ahmed et al., 2015). Earlier report (Kaya et al., 2010) also showed that flavonoids are among the major constituents isolated from the Ranunculus species and have been considered as the main components of the same plants antioxidant activity besides its antibacterial activity against S. aureus, Bacillus subtilis, B. cereus, E. coli, Helicobacter pylori, P. aeruginosa, P. fluorescens, Enterobacter aerogenes (Ahmed et al., 2015) and infections of Methicillin-resistant S. aureus strains (Alcaraz et al., 2000). Tannins, the other common phytochemical isolated from traditional medicinal plants, inhibit plasma coagulation by S. aureus (Akiyama et al., 2001) and also form chelate with metal ions. The possible antimicrobial mechanisms of tannins could be: induction of complexation with enzymes or substrates, act on the membranes of microorganisms; and toxicity due to complexation with metal ions (Akiyama et al., 2001).
The emergence of multiple drug resistant pathogens, including Multi-Drug Resistant TB (MDR TB), Methicillin-Resistant Staphylococcus aureus (MRSA), and Vancomycin-Resistant Entero-cocci (VRE), is making the treatment and control of infectious diseases more difficult (Bacha et al., 2016), with over 480 000 new cases of MDR-TB only in 2013 [31]. According to recent review made in Ethiopia (Mogese et al., 2014), some of the antibiotics have become virtually useless, alarming for the urgent need to apply antibiotic restriction policies as well as measures to prevent further spread of resistant clones. Thus, the pressure due to drug resistance necessitates search for novel antimicrobial substances from plants as the development of resistance to bioactive substance from plants is low, if any (Bacha et al., 2016). Although not evaluated against resistance bacterial strains, our extract could have potent activity against resistance strains as it did against the sensitive test strains.
The antibacterial activity of chloroform extract of leaves of R. multifidus (LRMCF) (conc. 200 mg/mL) against S. aureus (IZ, 26.67 ± 0.83) was comparable to the activity of commercial antibiotic ciprofloxacin (IZ, 35 mm). The same extract displayed almost similar activity (IZ, 25.33 ± 0.83) even at lower concentration (100 mg/mL) against the same strain, with minimal activity against E. coli (IZ, 18.00 ± 1.00) at the lowest concentration (50 mg/mL) used in this study. Likewise, report from different parts of the world (including Turkey and Lebanon) indicated strong antibacterial activities of extracts of Ranunculus bulbosus against S. aureus (Didry et al., 2006), Ranunculus myosuroudes against E. coli and S. typhi with 88.8% susceptibility of the test strains (Barbour et al., 2004), and Ranunculus sceleratus against S. aureus and E. coli (Bissa and Bohra, 2012).
The activities of methanol extract of leaves of L. martinicensis (LLMME) against E. coli at relatively higher concentration (200 mg/mL) (IZ, 26.67 ± 3.33 mm) and even lower concentration (100 mg/mL) (25.33 ± 3.33 mm) were very promising as it had comparable activity to that of commercial antibiotic ciprofloxacin (IZ, 34 mm) against the same tested strain. However, chloroform extract of leaves of L. martinicensis against S. aureus at relatively lowest concentration (50 mg/mL) had intermediate activity with mean inhibition zone diameter of 16.33 ± 1.17 mm. Besides its antimicrobial activities, L. martinicensis has been used as repellant of mosquito due to minty odor (Imam and Tajuddeen, 2013; Muhammad et al., 2012). The presence of different phytochemical compounds could be responsible for its antibacterial activities as phyto-chemical screening of the leaves extract already revealed the presence of flavonoids, alkaloids and volatile oils (Muhammad et al., 2012).
Chloroform extract of leaves of K. begonifolia also revealed activity (conc. 100 mg/mL) against P. aeruginosa (IZ, 22.67 ± 4.17 mm) followed by petroleum extract (IZ, 22.33 ± 3.33 mm) against S. typhimurium with activity closer to standard commercial antibiotic ciprofloxacin (IZ, 32 mm) . Leaves of plants are among the commonly used plant parts although there are contradictory reports in this regards (Mesfin et al., 2009; Teklay et al., 2013). Of 114 medicinal plant species identified being used to treat 47 human and 19 livestock diseases (Teklay et al., 2013), leaves parts were the most commonly used section accounting for almost 50% of the total sample analyzed. To the contrary, roots were reported the most commonly used plant parts (35.8%) among seventy-two plant species documented for having medicinal value as reported from Wonago district of South Nations and Nationalities Peoples Region (SNNPR), South Ethiopia (Mesfin et al., 2009).
Similar to leaves crude extracts, the stems extract of the three medicinal plants (R. multifidus, L. martinicensis, K. begonifolia) were evaluated for their antibacterial activities and the results revealed presence of moderate to high activities. Accordingly, chloroform extracts of stems of R. multifidus displayed comparable activity against S. aureus both at 200 mg/mL (IZ, 26.67 ± 0.83 mm) and 50 mg/mL (IZ, 25.00 ± 0.50 mm) although it had lower activity against E. coli (IZ, 18.00 ± 1.00 mm) at the same lower concentration (50 mg/mL). Our findings are in corroboration with related activity observed in roots of Ranunculus repens (Noor et al., 2006) assessed in Pakistan and whole plants of Ranunculus marginatus var. trachycarpus and Ranunculus sprunerianus from Turkey (Noor et al., 2006). The later authors reported that the extracts had both antibacterial as well as antioxidant activities, with the antioxidant activity having positive correlation with the total phenolic and flavonoid contents of the extracts (Kaya et al., 2010).
Likewise, petroleum extracts of stems of L. martinicensis ( SLMPE) revealed strong activity even at lowest concentration (50 mg/mL) against S. aureus, S. typhimurium, E. coli and P. aeruginosa with mean inhibition zone diameter of 29.33 ± 0.67, 29.33 ± 1.67, 28.00 ± 0.00 and 27.00 ± 0.50, respectively. The same extract also showed amazingly maximum activity against S. aureus which (IZ, 28.67 ± 0.83 and 28.00 ± 1.00 mm) at 200 and 100 mg/mL, respectively. As observed from the above activity, the extract had broad spectrum with inhibitory activity against both Gram positive and Gram negative bacteria as supported by earlier observation made by Pandey et al. (2011). Exceptionally, high activities were observed in petroleum ether extracts of the stems of K. begonifolia (SKBPE) against both Gram positive and Gram negative bacteria as recorded for S. aureus (28.33 ± 0.83 - 30.00 ± 1.50 mm), P. aeruginosa (27.00 ± 1.50 - 28.67 ± 1.67 mm), E. coli (28.33 ± 0.83 - 31.00 ± 0.50 mm) and S. typhimurium (28.00 ± 1.00 - 30.33 ± 0.33) for the three concentrations of the extract (50-200 mg/mL). Both Gram positive and Gram negative bacteria were sensitive to the extracts. In contrast to this finding, some of the earlier study (Pandey et al., 2011) reported that many of the traditional medicinal plants had lesser activities against Gram-negative bacteria as compared to their activities in Gram-positive.
The minimum and maximum MIC values observed in all the three stems extracts using the different solvents were 5.6 mg/mL (in S. aureus, P. aeruginosa, and S. thyphimurium) and 16.6 mg/mL (E. coli). Likewise, most of the isolates had MBC values of 16.6 mg/mL with the exception of 50 mg/mL of all the extracts against E. coli. Usually, plant extracts are bacteriostatic at relatively lower concentrations and bactericidal at higher concentrations (Jaya et al., 2008) although antibacterial activities could depend on the actual concentration of active compounds in the crude extract (Mazumder et al., 2006). In contrary to the current observation, the MIC values of extracts of two other species Raninculus (R. marginatus var. trachycarpus and R. sprunerianus) were reported to be between 128 and 256 μg/mL (Kaya et al., 2010). In the same report, the maximum inhibition zones ranged between 7 and 12 mm, values much lesser than what we observed (19.33-23.67mm) for extracts of Ranunculus multifidus (inclusive of data on stems and leaves extracts). The presence of many bioactive phytochemical compounds mainly alkaloids, tannins, flavonoids, saponins, steroids and cardiac glycosides could be accounted to the antimicrobial activities observed in many of the extracts assessed in this study either individually or possibly in combinations. Likewise, many of the earlier reports made on antibacterial activities of various medicinal plants linked the observed activities to many of these phytochemicals (CSA, 2005; Bissa and Bohra, 2012). The optimal effectiveness of medicinal plants may not be due to one main active constituent, but to the combined action of different compounds originally present in the plants (Farombi. 2003; Gonzalez et al., 1994).
The activities of extracts of the same plant could vary depending on the polarity of solvents used for extraction, the concentration of active compounds. In the present study, petroleum ether extract of stems of K. begonifolia revealed remarkably high inhibition on both Gram positive and Gram negative with significantly high mean inhibition zone diameter (31.00 ± 0.50). Generally, the antibacterial activities of medicinal plants against the test strains showed that the crude preparations of traditional medicinal plants are among the candidate resources for novel antimicrobial agents and calls for further strengthening of the search for alternative potent antimicrobial agents from the available pool of resources.
The traditional medicinal plants evaluated in the current study have shown promising activities against both Gram negative and Gram positive bacteria. The highest antimicrobial activities were recorded for petroleum ether extract of Kosteletzkya begonifolia stems against S. aureus [Inhibition Zone diameter (IZ), 28.3-30 mm], P. aeruginosa (IZ: 27 to 28.67 mm), E. coli (IZ: 28.3 to 31 mm) and S. typhimurium (IZ, 28 to 30.3)]. The extract displayed activity significantly closer to that of the control antibiotics, ciprofloxacin (IZ, 30 to 35 mm). The finding strengthens the fact that traditional plants could represent new sources of antibacterial substances with stable, biologically active components. As the bioactive substances present in the extracts of the study plants justify the rationale for the use of the same plants in traditional medicine, isolation, purification and structural elucidation of the bioactive constituents are recommended.
LRMPE, Leaf of Ranunculus multifidus petroleum ether extract; LRMCF, leaf of R. multifidus chloroform extract; LRMME, leaf of R. multifidus methanol extract; LLMPE, leaf of Leucas martinicensis (Jacq.) R. Br petroleum ether extract; LLMCF, leaf of L. martinicensis(Jacq.) R. Br chloroform extract; LLMME, leaf of L. martinicensis (Jacq.) R. Br methanol extract; LKBPE, leaf of Kosteletzkya begonifolia (Ulbr.) Ulbr petroleum ether extract; LKBCF, leaf of K. begonifolia (Ulbr.) Ulbr chloroform extract; LKBME, leaf of K. begonifolia (Ulbr.) Ulbr methanol extract; SRMPE, stem of Ranunculus multifidus petroleum ether extract; SRMCF, stem of R. multifidus chloroform extract; SRMME, stem of R. multifidus methanol extract; SLMPE, stem of Leucas martinicensis (Jacq.) R. Br petroleum ether extract; SLMCF, stem of L. martinicensis (Jacq.)R. Br chloroform extract; SLMME, stem of L. martinicensis (Jacq.)R. Br methanol extract; SKBPE, stem of Kosteletzkya begonifolia (Ulbr.) Ulbr petroleum ether extract; SKBCF, stem of K. begonifolia (Ulbr.) Ulbr chloroform extract; SKBME, stem of K. begonifolia (Ulbr.) Ulbr methanol extract.
The authors have not declared any conflict of interests.
TB was fully involved in all phases of the study including designing of the study, data collection, data analysis and write up. YT supervised data collection and involved in laboratory work, and KB designed the study, supervision the study both in field and laboratory, data analysis and interpretation, and preparation of the manuscript for publication.
The authors would like to thank Jimma University for financial support.
REFERENCES
|
Abebe D (2011). The Role of Medicinal Plants in Healthcare Coverage of Ethiopia, the possible integration. In: Conservation and Sustainable Use of Medicinal Plants in Ethiopia. Proceeding of The National Workshop on Biodiversity Conservation and Sustainable Use of Medicinal Plants in Ethiopia, held from 28 April-01 May 1998, pp. 6- 21.
|
|
|
|
Abera B (2014). Medicinal plants used in traditional medicine by Oromo people, Ghimbi District, Southwest Ethiopia. J. Ethnobiol. Ethnomed. 10:40
Crossref
|
|
|
|
|
Abramov V (1996). Traditional medicine. World Health Org. 134:1-3.
|
|
|
|
|
Ahmed A, Kaleem M, Ahmed Z, Shafiq H (2015). Therapeutic potential of flavonoids and their mechanism of action against microbial and viral infections - A review. Food Res. Int. 77: 221-235
Crossref
|
|
|
|
|
Akiyama H, Fujii K, Yamasaki O, Oono T, and Iwatsuki K (2001). Antibacterial action of several tannins against Staphylococcus aureus, J. Antimicrobial. Chemother. 48(4):487-491
Crossref
|
|
|
|
|
Alcaraz L, Blanco S, Puig O, Tomas F, Ferretti F (2000): Antibacterial activity of flavonoids against methicillin-resistant Staphylococcus aureus strains, J. Theor. Biol. 205(2):231-240
Crossref
|
|
|
|
|
Bacha K, Tariku Y, Gebreyesus F, Zerihun S, Mohammed A, Weiland-Bräuer N Schmitz R A, Mulat M (2016). Antimicrobial and anti-Quorum Sensing activities of selected medicinal plants of Ethiopia: Implication for development of potent antimicrobial drugs. BMC Microbiol. 16:139
Crossref
|
|
|
|
|
Barbour EK, Al Sharif M, Sagherian VK, Habre AN,Talhouk RS and Talhouk SN (2004). Screening of selected indigenous plants of Lebanon for their antimicrobial activity. J. Ethnopharmacol. 93:1-7.
Crossref
|
|
|
|
|
Belayneh A, Asfaw Z, Demissew S, Bussa NF (2012). Medicinal plants potential and use by pastoral and agro-pastoral communities in Erer Valley of Babile Wereda, Eastern Ethiopia. J. Ethnobiol. Ethnomed. 8:42.
Crossref
|
|
|
|
|
Bissa SH, Bohra A (2012). Evaluation of Antibacterial Potential of Ranunculus sceleratus. Bot. Res. Intern. 5:10-13.
|
|
|
|
|
Branham LA, Carr MA, Scott CB, Callaway TR (2005). E. coli O157 and Salmonella spp. in white-tailed deer and livestock. Curr. Issues Intest. Microbiol. 6:25-29.
|
|
|
|
|
Canter PH, Thomas WH, and Ernst E (2005). Bringing medicinal plants into cultivation: opportunities and challenges for biotechnology. Trends Biotechnol. 23:180-185.
Crossref
|
|
|
|
|
Central Statistics Agency of Ethiopia (CSA) (2005). National Statistics, Tables B.3 and B.4. 2005
|
|
|
|
|
Cunningham AB (1993). African Medicinal Plants: Setting priorities at the interface healthcare between conservation and primary health care. (Sample, A. Ed.). People and Plants Working Paper. Paris, UNESCO. pp. 1-50.
|
|
|
|
|
Cushnine T, Lamb AJ (2005). Antimicrobial activity of flavonoids. Int. J. Antimicrob. Agents 26(5):343-356.
Crossref
|
|
|
|
|
Dabassa A, Bacha K (2012). The prevalence and antibiogram of Salmonella and Shigella Isolated from abattoir, Jimma town, South West Ethiopia. Int. J. Pharm. Biol. Res. 3(4):143
|
|
|
|
|
Didry N, Dubrenil L, and Pinkas M (2006). Microbiological properties of protoanemonin. isolated from Ranunculus bulbosus. Phytother. Res. 7:21-24.
Crossref
|
|
|
|
|
Farombi EO (2003). African Indigenous Plant with Chemotherape utic Potentials and biotechnological Approach to the production of bioactive prophylacudiestic agents. Afr. J. Biotechnol. 2(12):662-671
Crossref
|
|
|
|
|
Giday M, Asfaw Z, Elmqvist T, Woldu Z. (2003) An ethnobotanical study of medicinal plants used by the Zay people in Ethiopia. J. Ethnobiol. Pharm. 85:43-52.
Crossref
|
|
|
|
|
Gonzalez A, Moujir G, Bazzocchi I, Correa IL and Guptha MD (1994). Screening o antimicrobial and cytotoxic activities of Panamanian plants. Phytomedicine 1:149-153.
Crossref
|
|
|
|
|
Imam TS, Tajuddeen UM (2013). Qualitative phytochemical screening and larvicidal potencies of ethanolic extracts of five selected macrophyte species against Anopheles mosquitoes (diptera: culicidae), J. Res. Environ. Sci. Toxicol. 2(6):121-125.
|
|
|
|
|
Jaya Prakash Goud M, Komraiah A. Narasimha Rao K, Ragan A, Raju VS and Singara Charya MA (2008). Antibacterial activity of some folklore medicinal plants from south India. Afr. J. Tradit. Complement. Altern. Med. 4:421-426.
Crossref
|
|
|
|
|
Kamatenesi M, Oryem-Origa H (2007). Medicinal plants used to induce labor during childbirth in Western Uganda. J. Ethnopharmacol. 109:1-9
Crossref
|
|
|
|
|
Kaya GI, Somer NU, Konyalioglu S, Yalcin HT, Yavasoglu NU, Sarikaya B and. Onur MA (2010). Antioxidant and antibacterial activities of Ranunculus marginatus var. trachycarpus and Ranunculus sprunerianus. Turk. J. Biol. 34:139-14
|
|
|
|
|
Khulbe K, Sati SC (2009). Antibacterial activity of Boenninghausenia albiflora Reichb. (Rutaceae). Afr. J. Biotechnol. 8:6346-6348
Crossref
|
|
|
|
|
Komuraiah A, Bolla K, Rao KN, Ragan A, Raju VS and Singara Charya, MA (2009). Antibacterial studies and phytochemical constituents of South Indian Phyllanthus species. Afr. J. Biotechnol. 8:4991-4995.
|
|
|
|
|
Lewington A (1993). Medicinal plants and plant extracts: A Review of their Importation into Europe. Cambridge, UK. P 92.
|
|
|
|
|
Mackeen MM, Ali AM, El-Sharkawy SH, Manap MY, Salleh KM, Lajis N H, and Kawazu K (1997). Antimicrobial and Cytotoxic Properties of Some Malaysian Traditional Vegetables (Ulam). Int. J. Pharmacogn., 35(3):174-178
Crossref
|
|
|
|
|
Mazumder A, Mahato A, and Mazumder R (2006). Antimicrobial potentiality of Phyllanthus amarus against drug resistant pathogens. Nat. Prod. Res. 20:323-326
Crossref
|
|
|
|
|
Mesfin F, Demissew S, Teklehaymanot T (2009). An ethnobotanical study of medicinal plants in Wonago Woreda, SNNPR, Ethiopia, J Ethnobiol. Ethnomed. 5:28
Crossref
|
|
|
|
|
Mogese F, Endris M, Mulu A, Tessema B, Belyhun Y, Shiferaw Y, Huruy K, Unakal C, Kassu A (2014). The growing challenges of antibacterial drug resistance in Ethiopia, J. Global Antimicrob. Resist. 2(3):148-154.
Crossref
|
|
|
|
|
Muhammad S, Fatima A, Yahaya MM (2012). The Phytochemical Components of Leucas Martini-censis that Cause Repellence of Adult Mosquito. Int. J. Mod. Bot. 2(1):1-5.
Crossref
|
|
|
|
|
Mulat M, Chali K Tariku Y, Bacha K (2015). Evaluation for in vitro antibacterial activities of selected medicinal plants against food-borne pathogens. Int. J. Pharma. Sci. Rev. Res. 32 (2):45-50.
|
|
|
|
|
Noor W, Gul I, Ali, Chaudhary MI (2006). Isolation and antibacterial activity of compounds from Ranunculus repens L. J. Chem. Soc. Pak. Microbiol. 42:361-363.Owolabi J, Omogbai EK and Obasuyi O. (2007). Antifungal and antibacterial activities of the ethanolic and aqueous extract of Kigella africana (Bignoniaceae) stem bark. Afr. J. Biotechnol. 6:1677-1680
|
|
|
|
|
Pandey P, Mehta A, and Hajra S (2011). Evaluation of Antimicrobial Activity of Ruta graveolens Stem Extracts by Disc Diffusion Method. J. Philos., 3:92-95.
|
|
|
|
|
Sofowora A (1993). Medicinal Plants and Traditional Medicines in Africa. Chic Hester John, Willey & Sons, New York. P 256.
|
|
|
|
|
Teklay A, Abera B, Giday M (2013). An ethnobotanical study of medicinal plants used in Kilte Awulaelo District, Tigray Region of Ethiopia. J. Ethnobiol. Ethnomed. 9(1):65.
Crossref
|
|
|
|
|
Tesema T, Mirutse G, Nigusu A (2002). National Biodiversity Strategy and Action Plan Project. Resource base of medicinal plants of Ethiopia, first phase report, Addis Ababa, Ethiopia
|
|
|
|
|
Tolasa E (2007). Use, Treat and Conservation of Traditional Medicinal Plants by Indigenous People in Gimbi Woreda, Western Wollega, West Ethiopia. M.Sc. Thesis, Addis Ababa University, Ethiopia.
|
|
|
|
|
Trease GE, Evans WC (1998). Pharmacognosy. Brailliar Tiridel and Macmillian Publishers, London.
|
|
|
|
|
WHO (2002). Traditional medicine: Growing Needs and Potentials, Geneva.
|
|