ABSTRACT
Production of extended-spectrum beta-lactamases (ESBLs) can lead to treatment failures when the antibiotics are used. This study detected ESBLs genes on Multidrug Resistant Escherichia coli from HIV-infected individuals attending some hospitals in Jos. Eighty (80) isolates of multidrug resistant (MDR) E. coli were screened for plasmids. ESBLs genes including bla-CTX, bla-TEM and bla-SHV were detected on the plasmids using polymerase chain reaction (PCR) with 100 bp DNA ladder as DNA molecular weight marker. Out of the total 38 plasmids tested, ESBLs genes occurred in 13 (34.21%), with the bla-TEM dominating [7 (53.85%)] over the bla-CTX [4 (30.77%)]. Two (15.38%) of the isolates carried both genes. None of the isolates carried the bla-SHV gene in our study. All the strains showed resistance to SXT, AMC and CTX. Resistance was most frequently observed against SXT (13), AMC (13), CTX (13), CFM (12), F (8), NA (6), GN (5), CRO (4), OFX (2) and CIP (1). None of the ESBLs-bearing strains showed resistance to IPM. Result showed 34.21% prevalence of ESBLs and suggested the need to be more cautious with the clinical use of third generation Cephalosporins especially for the treatment of acute infections caused by E. coli due to the high resistance recorded.
Key words: Extended spectrum beta-lactamase, resistance genes, antimicrobial, plasmids, Escherichia coli, HIV-infected, Jos.
The infections caused by antibiotic resistant microorganisms, may be very difficult to treat due to limited choices of antibiotics. Extended-Spectrum-Beta-Lactamase antibiotics such as third generation cephalosporin (3GC) form the major component of the empiric antibacterial chemotherapy in most clinical setups
and especially in tertiary care center (Chaudary and Aggarwal, 2004).
Beta-lactamases are enzymes that are major cause of bacterial resistance to the beta-lactam family of antibiotics such as penicillins, cephalosporins, cephamycins, and carbapenems. They catalyze the hydrolysis of the amide bond of four-membered beta-lactam ring and render the antibiotic inactive against its original cellular target, the cell wall transpeptidase. Extended-spectrum beta-lactamases (ESBLs) are derived from the narrow-spectrum beta-lactamases (TEM-1, TEM-2, or SHV-1) by mutations that alter the amino acid configuration around the enzyme active site (Bajpai et al., 2017). They mediate resistance to all penicillins, third generation cephalosporins (e.g. ceftazidime, cefotaxime, and ceftriaxone) and aztreonam, but not to cephamycins (cefoxitin and cefotetan) and carbapenems (Bonnet, 2004).
ESBLs are plasmid-mediated and organisms producing beta-lactamase enzymes exhibit co-resistance to many other classes of antibiotics (Kruse and Sørum, 1994). These enzymes are most commonly produced by the members of the Enterobacteriaceae, especially Escherichia coli and Klebsiella (Chika et al., 2017). Gram negative Enterobacteriaceae expressing ESBLs are among the most multidrug-resistant pathogens in hospitals and are spreading worldwide. The infections caused by ESBLs-producing organisms have resulted in poor prognosis, prolonged hospital stay and greater hospital expenses (Paterson et al., 2004).
Available literature has demonstrated a risen prevalence of multidrug resistant ESBL-producing E. coli globally (Hassuna et al., 2020; Abdulaziz et al., 2018; Falgenhauer et al., 2019). Our study is predicated on the paucity of data from the West African sub-region on ESBLs resistant genes in HIV-infected individuals. Most available reports (Aibinu et al., 2003, 2004; Iroha et al., 2010; Yusuf et al., 2011; Oli et al., 2017) focused on phenotypic detection of ESBLs in HIV-non-infected populations. Such reports have been shown to be inconsistent in revealing the actual prevalence of ESBLs genes in the region, as also earlier advocated by Founou et al. (2018) and Bajpai et al. (2017). Accurate epidemiologic data can enable effective empirical therapy plan and infection control program.
Study area, design and period
The antibiotic resistance and plasmids profile of eighty E. coli isolates obtained from HIV-infected individuals attending Bingham University Teaching Hospital and Faith Alive Foundation Hospital Jos, were determined. Identity of the isolates was confirmed using MicrobactTM Gram-Negative Identification System (24E) kits, while ESBLs genes were detected using PCR. This is part of a cross-sectional study conducted between February 2018 and December, 2019. The consenting participants were enrolled and their stool samples screened for E. coli. Both prevalence and susceptibility studies of the isolates have been earlier reported. Only isolates showing multidrug resistance were used in this study.
Ethics approval and consent to participate
This research was ethically cleared and approved by the Jos University Teaching Hospital Institutional Review Board. Written and informed consent was obtained from study participants after explaining the purpose and aim of the study.
Consent to publish
Informed consent was obtained from all individual participants included in the study.
Antibiotic susceptibility test
Antibiotic susceptibility was assessed using the disc diffusion method of Bauer et al. (1996) and further described by CLSI (2013). Antibiotics discs used include Imipinem (IPM) 10 µg, Trimethoprim-Sulfamethoxazole (SXT) 25 µg, Gentamycin (GN) 10 µg, AmoxicilinClavulanic acid (AMC) 30 µg, Nitrofurantoin (F) 200 µg, Cefotaxime (CTX) 30 µg, Nalidixic acid (NA) 30 µg, Ofloxacin (OFX) 5 µg, Ceftriazone(CRO) 30 µg, Cefixime (CFM) 5 µg, and Ciprofloxacin (CIP) 5 µg from Oxoid (UK). A cell suspension of organisms equivalent to a 0.5 McFarland standard was used for the susceptibility testing. Tests were standardized using E. coli 25922 from the American Type Culture Collection (ATCC) as reference strain. Clear zones of inhibition were measured in mm using a transparent metre rule.
Plasmids DNA extraction and profiling
Plasmids DNA were extracted using the Plasmid extraction protocol with Zippy plasmid Miniprep kit (Inqaba biotech West Africa Ltd) as follows.
Six hundred microliters of bacterial culture grown in LB medium was added to a 1.5 ml microcentrifuge tube. This was centrifuged for 30 s at 12000 rpm. The supernatant was discarded and the procedure repeated to get a clear pellet. The cell pellet was then resuspended after adding 600 µl of TE to it. This was followed by the addition of 100 µl of 7X lysing buffer. The mixture was inverted for about 4 to 6 times in the tube and then incubated for 1 to 2 min. It was then mixed with 350 µl of cold neutralization buffer thoroughly before centrifuging for 2 to 4 min at 12 rpm. The supernatant was transferred into a Zymo-Spin IIN column placed in a collection tube. This was centrifuged for 15 s for 12000 rpm. The flow-through was discarded and the Zymo-Spin IIN column returned to the collection same tube. 200 µl of the Endo-Wash Buffer was added to the column and centrifuged for 30 s at 12000 rpm. The Zippy Wash Buffer (400 µl) was then added to the column and centrifuged for 1 min at 12000 rpm. The column was then transferred into a sterile RNAse/DNAse-free 1.5 ml microcentrifuge tube. The column was then transferred into a clean 1.5 ml microcentrifuge tube where 30 µl of zippy elution buffer was directly added to the column matrix and incubated for 1 min at room temperature. The whole mixture was finally centrifuged for 30 s at 12 rpm to elute the plasmid DNA.
Plasmids were characterized using agarose gel electrophoresis (Sambrook and Fritsch, 1989) and DNA-Hind 111 Digest as DNA ladder to identify the plasmid copies present in different isolates. For this purpose, an agarose gel of 0.8% was used, while ethidium bromide was used for staining of DNA fragments, which were visualized by UV-Trans illumination. Samples with visible bands were cut from the gel and purified using the Zymoclean Large Fragment DNA Recovery Kit. Molecular weights of plasmids were calculated using molecular weight calculator by Bikandi et al. (2004) at Insilico.ehu.es.
PCR amplification
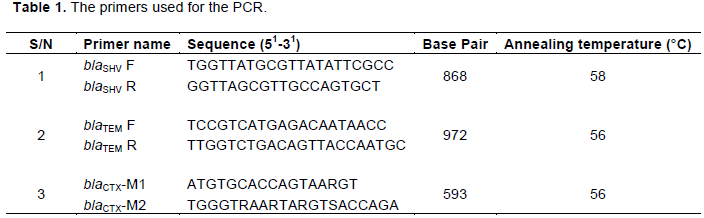
ESBLs belonging to the CTX, TEM, and SHV families primers (Bla-CTX, TEM and SHV) were used for amplification of resistance genes using PCR. The PCR reaction was carried out using the Solis Biodyne 5X HOT FIREPol Blend Master mix. PCR was performed in 20 µl of a reaction mixture, and the reaction concentration was brought down from 5x concentration to 1X concentration containing 1X Blend Master mix buffer (Solis Biodyne), 1.5 mM MgCl2, 200 µM of each deoxynucleoside triphosphates (dNTP) (Solis Biodyne), 20 pMol of each primer (Jena Bioscience, Germany), 2 unit of Hot FIREPol DNA polymerase (Solis Biodyne), Proofreading Enzyme, 5 µl of the extracted DNA, and sterile distilled water was used to make up the reaction mixture. Thermal cycling was conducted in an Pielter thermal cycler (MJ Research Series) for an initial denaturation of 95°C for 5 min followed by 30 amplification cycles of 30 s at 95°C; 1 min at 56°C and 1 min 30 s at 72°C. This was followed by a final extension step of 10 min at 72°C. The amplification product was separated on a 1.5% agarose gel and electrophoresis was carried out at 80V for 1 h 30 min. After electrophoresis, DNA bands were visualized by ethidium bromide staining using 100 bp DNA ladder (Solis Biodyne) as DNA molecular weight marker. Table 1 shows primers used in the present study.
Data processing and analysis
Data were entered into excel sheets and analyzed using SPSS version 2010 software. The correlation analysis was used to determine possible relationship between plasmid copies and number of antibiotics resisted. The 95% confidence limit and probability (P value) of 0.05 were used to determine level of significance of associations.
Antibiotic resistance of isolates as shown in Figure 1, revealed that SXT had the highest resistance level of 98.75% (79 isolates), while IPM was the least (3.75%). In all, CTX (86.3%) and CFX (83.00%) were the least effective among the cephalosphorins, while there was minimal resistance to the aminoglyosides (OFX=23% and CIP=22.5%). Also, isolates showed 67.5 and 66.3% resistance to NA and F, respectively.
Thirty-eight/eighty (47.50%) of the isolates had plasmids of various sizes ranging from 1.029 to 23.485 kbp. The most frequent plasmid having a molecular weight of 12.371 kbp occurred in 11 of the isolates, three of which also bear the ESBLs genes. Plasmid copies varied from 1 to 7, with those bearing 3 plasmids having the highest frequency (31.58%), while those harboring 7 occurred the least (5.26%) (Figure 2). No significant correlation exist between plasmid copies and number of antibiotics resisted (r= 0.295, P= 0.072).
The PCR results to detect ESBLs belonging to the TEM, SHV and CTX-M families genes are as shown in Figure 3. It revealed that ESBLs genes occurred in 13/38 (34.21%) of the plasmids, with the bla-TEM dominating [7/13 (53.85%)] over the bla-CTX [4/13 (30.77%)]. Two (15.38%) of the isolates however, carried both bla-TEM and bla-CTX genes. None of the isolates in our study carried the bla-SHV gene. Both bla-TEM and bla-CTX were detected on the most frequently encountered plasmid in our studies.
Table 2 shows the resistance pattern of ESBLs genes-bearing E. coli in this study. They were generally resistant to 5 antibiotics and above. Isolates harboring both bla-CTX and bla-TEM genes exhibited resistance to more than 8 antibiotics compared to other isolates harboring either of the 2 genes alone. All the strains showed absolute (100%) resistance to SXT, AMC and CTX. Resistance was most frequently observed against SXT 13, AMC 13, CTX 13, CFM 12, F 8, NA 6, GN 5, CRO 4, OFX 2, and CIP 1. Furthermore, resistance to GN and CRO is associated with the bla-TEM gene, as none of the bla-CTX genes-bearing strains was resistant to the drugs as shown in the table. None of the strains bearing ESBLs genes showed resistance to IPM in this study.
High resistance to SXT and cephalosphorins exhibited by E. coli in our study is quite significant since these drugs are among the most widely used in the treatment of infectious diseases. Persistent exposure of bacterial strains to a multitude of β-lactams may have induced a selective pressure in favour of the resistant strains having eliminated the sensitive strains in the process. This could expand the activity of the resistant strains, even against newly developed β-lactam antibiotics (Shaikh et al., 2015). Earlier reports (Coudron et al., 1997; Piroth et al., 1998) have suggested the use of β-lactam antibiotics (including CTX, CFM, CRO, etc.) in combination with AMC to be used in the treatment of resistant bacterial infections. This must be done with caution in the light of our findings. Our result revealed the increasing resistance of E. coli to antimicrobials in the region and portends great danger to the treatment of infectious diseases since E. coli could transfer resistant genes to enterobacterial pathogens and other normal flora in the body. Our study agrees with that of Igwe et al. (2016), but differ from that of Adenipekun et al. (2016) and Aworh et al. (2019), who reported lower resistance to 3rd and 4th generation cephalosporins in the South-Western and North-Central part of the country, respectively.
The present study showed that most of the plasmids isolated are of smaller to medium sizes (1.029-23.485 kbp), suggesting that majority may be of the mobilizable category (Usually ≤10 kbp). This is further supported by the fact that the most frequent plasmid having a molecular weight of 12.371 kbp occurred in 11 (28.95%) of the isolates, three of which also bear the ESBLs genes.
The present study did not show any significant correlation between plasmid copies and number of antibiotics resisted (r= 0.295, P= 0.072). This is expected as there are isolates resisting fewer (1-3) antibiotics but bearing up to 7 plasmids, just as there were also those resisting 9 antibiotics and bearing up to 7 plasmids, some of which also contain resistant genes. The former could indicate that most of those plasmids are either non-resistant or mobilizable in nature. Such resistance could as well be on the chromosomes as previously suggested (Aibinu et al., 2003).
Strains bearing ESBLs genes in this study were generally resistant to 5 antibiotics and above (Table 1). However, isolates habouring both bla-CTX and bla-TEM genes exhibited resistance to more (Aibinu et al., 2004; Iroha et al., 2010) antibiotics compared to other isolates habouring either of the 2 genes alone. This could be due to the multiplied effect of the genes on such isolates.
The prevalence of ESBLs genes in our study is high (34.21%). This portends serious risk of resistance to treatment of infections with antibiotics, as earlier reports have shown that organisms producing beta-lactamase enzymes exhibit co-resistance to many other classes of antibiotics (Kruse and Sørum, 1994), leading to highly limited available drugs for the treatment of infectious diseases. The prevalence in our study is however lower than the 56.7, 70.0, 72, 83.0 and 95.5% reported by Irith et al. (2007), Igwe et al. (2016), Horsefall et al. (2017) Husam et al. (2009) and Wani et al. (2009), respectively. It is however higher than the 18.6% earlier reported by Onyedibe et al. (2018) in Jos. This difference may be attributed to the use of molecular technique in our study as against phenotypic detection in theirs, since the latter is known to be less sensitive compared to the former. Our result is very similar to the 33 and 33.5% earlier reported from Saudi Arabia and Egypt by Abdulaziz et al. (2018) and Hassuna et al. (2020), respectively.
Previous workers (Saravanan et al., 2018; Abdulaziz et al., 2018; Hassuna et al., 2020), have also reported higher prevalence of bla-CTX gene in the middle-East and Africa, but our study showed the bla-TEM gene dominating over the bla-CTX gene with the bla-SHV being completely absent. This may be due to the fact that the bla-TEM gene was originally known to be associated with E. coli where it was originally isolated. Our result however agrees with that of Olugbenga et al. (2015) and Aibinu et al. (2003), who reported higher prevalence of the bla-TEM from Osun and Lagos states respectively, in Nigeria. This suggests an increasing regional spread of the resistant genes. This suggests an increasing spread of the bla-TEM gene in Nigeria possibly due to human and animal migration.
High resistance of plasmid-borne ESBL positive isolates to 3rd generation Cephalosporins (CTX 100% and CFM 92.31%, AMC 100%) recorded in our study is expected. These are beta lactams, and Beta-lactamases are known to hydrolyze the amide bond of the β-lactam ring resulting in an inactive compound (Bajpai et al., 2017).
The ESBLs resistant isolates showed the least resistance to the quinolones (CIP 1 or 7.70% and OFX 15.39%) in our study, even as all were sensitive to IPM. Our result differs from that of Igwe et al. (2016) from Zaria, in North-Western Nigeria, where higher levels of resistance were reported against the quinolones (CIP 76.55% and OFX 74.5%). Similar to our findings however, their study and that of Aibinu et al. (2003) also reported no resistance to IPM. This indicates that IPM is still a choice drug for the treatment of MDR bacteria in the region. Nevertheless, other similar studies (Onyedibe et al., 2018, Adesola et al., 2020) reported varying levels of resistance (18.6 and 20.6%, respectively) to the drug. This suggests the need for close monitoring, so as to track and control the spread of resistance.
The results suggest that we could experience high levels of clinical failure when using third generation cephalosporins for the treatment of acute infections caused by E. coli. These findings are of concern as E. coli are among the most frequent causes of intra-abdominal, soft tissues and community-acquired urinary tract infections word-wide.
Most of the plasmids isolated are of small to medium sizes (1.029-23.485 kbp) and therefore mostly mobilizable. Beta lactamase resistance genes (bla-CTX and bla-TEM) were prevalent (34.21%) in the region. All ESBLs-borne isolates showed absolute resistance to SXT and 100% susceptibility to IPM. The result suggested the need to be more cautious with the clinical use of third generation cephalosporins especially for the treatment of acute infections caused by E. coli as resistance to the drugs by plasmid-borne ESBLs positive isolates was high in the study.
THE LIMITATION AND STRENGTH OF THE STUDY
The study was done in hospital setting so that the result may not be representative samples of other patients attending other health sectors in the same area. The number of isolates was small and this may affect the estimation of the prevalence of ESβL producing strains in the study area.
The research was supported by the Fogarty International Center (FIC); Office of the Director (OD/NIH); National Institute of Neurological Disorders and Stroke (NINDS/NIH); and the National Institute of Nursing Research (NINR/NIH) of the National Institutes of Health under Award Number D43 TW010130.
The authors have declared any conflict of interests.
REFERENCES
|
Abdulaziz A, Ahmad AJ, Abdullah AA (2018). Prevalence of Multidrug Resistance and Extended-Spectrum β-Lactamase Carriage of Clinical Uropathogenic Escherichia coli Isolates in Riyadh, Saudi Arabia. International Journal of Microbiology pp. 1-9.
Crossref
|
|
|
|
Adenipekun EO, Jackson CR, Ramadan H, Iwalokun BA, Oyedeji KS, Frye JG, Barrett JB, Hiott LM, Woodley TA, Oluwadun A (2016). Prevalence and multidrug resistance of Escherichia coli from community-acquired infections in Lagos, Nigeria. Journal of Infection in Developing Countries 10(9):20-931.
Crossref
|
|
|
|
|
Adesola O, Onwugamba F, Iwalokun B, Mellmann A, Becker K, Schaumburg F (2020). High proportion of carbapenemase-producing Escherichia coli and Klebsiellapneumoniae among extended-spectrum β-lactamase-producers in Nigerian hospitals. Journal of Global Antimicrobial Resistance 21:8-12.
Crossref
|
|
|
|
|
Aibinu I, Aednipekun E, Odugbemi T (2004). Emergence of quinolone resistance amongst Escherichia coli strains isolated from clinical infections in some Lagos state hospitals, in Nigeria. Nigerian Journal of Health and Biomedical Sciences 3(2):73-78.
Crossref
|
|
|
|
|
Aibinu IE, Ohaegbulam VC, Ademipekun EO, Ogunsola FT, Odugbemi TO, Mee BJ, (2003). Extended-spectrum beta-lactamase enzymes in clinical isolates of Enterobacter species from Lagos, Nigeria. Nigerian Journal of Health and Biomedical Sciences 2:53-60.
|
|
|
|
|
Aworh MK, Kwaga J, Okolocha E, Mba N, Thakur S (2019). Prevalence and risk factors for multi-drug resistant Escherichia coli among poultry workers in the Federal Capital Territory, Abuja, Nigeria. PLoS ONE 14(11):e0225379.
Crossref
|
|
|
|
|
Bajpai T, Pandey M, Varma M, Bhatambare GS (2017). Prevalence of TEM, SHV, and CTX-M Beta-Lactamase genes in the urinary isolates of a tertiary care hospital. Avicenna Journal of Medicine 7(1):12-16.
Crossref
|
|
|
|
|
Bauer AW, Kirby WMM, Sherris JKC, Turk MN (1996). Antibiotic susceptibility testing by a standardized single disc method. American Journal of Clinical Pathology 45(4):413-496.
Crossref
|
|
|
|
|
Bikandi J, San Millán R, Rementeria A, Garaizar J (2004). In silico analysis of complete bacterial genomes: PCR, AFLP-PCR and endonuclease restriction.Bioinformatics Application Note 20(5):798-799.
Crossref
|
|
|
|
|
Bonnet RG (2004). Group of extended-spectrum beta-lactamases: the CTX-M enzymes. Antimicrobial Agents and Chemotherapy 48(1):1â€14.
Crossref
|
|
|
|
|
Chaudary U, Aggarwal R (2004). Extended spectrum beta lactamases; AN emerging threat to clinical therapeutics. Indian Journal of Medical Microbiology 22(2):75-80.
|
|
|
|
|
Chika E, Ifeanyichukwu I, Clement OA, Malachy U, Peter E, Iroha CS, Ogene L, Chinedu O (2017). Multiple Antibiotic Resistance, Antibiogram and Phenotypic Detection of Metallo-Beta- Lactamase (MBL) from Escherichia coli of Poultry Origin. Journal of Applied Microbiology and Biochemistry 1(4):15-20.
Crossref
|
|
|
|
|
Clinical Laboratory Standard Institute (CLSI) (2013). Performance Standard for Antimicrobial Disk and Dilution Susceptibility Test for Bacteria Isolated from Animals; Approved Standard - Third edition. CLSI document M31-3A, Wayne, PA: C L SI 24 p.
|
|
|
|
|
Coudron PE, Moland ES, Sanders CC (1997). Occurrence and Detection of Extended-Spectrum b-Lactamases in Members of the Family Enterobacteriaceae at a Veterans Medical Center. Journal of Clinical Microbiology 35(10):2593-2597.
Crossref
|
|
|
|
|
Falgenhauer L, Imirzalioglu C, Oppong K, Akenten CW, Hogan B, Krumkamp R, Poppert S, Levermann V, Schwengers O, Sarpong N, Owusu-Dabo E, May J, Eibach D (2019). Detection and Characterization of ESBL-Producing Escherichia coli From Humans and Poultry in Ghana . Frontiers in Microbiology 9:3358.
Crossref
|
|
|
|
|
Founou LL, Founou RC, Allam M, Ismail A, Djoko CF, Essack SY (2018). Genome Sequencing of Extended-Spectrum β-Lactamase (ESBL)-Producing Klebsiellapneumoniae Isolated from Pigs and Abattoir Workers in Cameroon. Frontiers in Microbiology 9:188-199.
Crossref
|
|
|
|
|
Hassuna NA, Khairalla AS, Farahat EM, Hammad AM, Abdel-Fattah M (2020). Molecular characterization of Extended-spectrum β lactamase- producing E. coli recovered from community-acquired urinary tract infections in Upper Egypt. Scientific Report 10:2772.
Crossref
|
|
|
|
|
Horsefall SJ, Abbey SD, Nwokah E, Okonko IO (2017). Prevalence of Extended-Spectrum Beta-lactamases (ESBLs) and Plasmid status of Escherichia coli and Klebsiellapneumoniae isolates from clinical sources in UPTH, Port-Harcourt, Nigeria. New York Scientific Journal 10(3):29-39.
|
|
|
|
|
Husam SK, Khalid MB, Abiola CS, Giuseppe AB (2009). Extended spectrum beta-lactamases (ESBL) in Escherichia coli and Klebsiellapneumoniae: trends in the hospital and community settings. Journal of Infections in Developing Countries 3:295-299.
Crossref
|
|
|
|
|
Igwe JC, Olayinka BO, Ehnimidu JO, Onaolapo JA (2016) Virulent Characteristics of Multidrug Resistant E. coli from Zaria, Nigeria. Clinical Microbiology 5(6):268.
|
|
|
|
|
Irith W, Heinrich KG, Dietrich M, Enno S, Harald S (2007). Detection of Extended-Spectrum Beta-Lactamases among Enterobacteriaceae by Use of Semi-automated Microbiology Systems and Manual Detection Procedures. Journal of Clinical Microbiology 45:1167-1174.
Crossref
|
|
|
|
|
Iroha IR, Amadi ES, Oji AE, Nwuzo AC, Ejike-Ugwu PC (2010). Detection of Plasmid Borne Extended Spectrum Beta-Lactamse Enzymes from Blood and Urine isolates of Gram-negative Bacteria from a University Teaching Hospital in Nigeria. Current Research in Bacteriology 3:77-83.
Crossref
|
|
|
|
|
Kruse H, Sørum H (1994). Transfer of multiple drug resistance plasmids between bacteria of diverse origins in natural microenvironments. Applied and Environmental Microbiology 60(11):4015â€4021.
Crossref
|
|
|
|
|
Oli AN, Eze DE, Gugu TH, Ezeobi I, Maduagwu, UN, Ihekwereme CP (2017). Multi-antibiotic resistant extended-spectrum beta-lactamase producing bacteria pose a challenge to the effective treatment of wound and skin infections. Pan African Medical Journal 27:66-77.
Crossref
|
|
|
|
|
Olugbenga OA, Adewumi O, Odewale G, Ojurongbe O, Adefioye OJ (2015). Phenotypic and Molecular Characterisation of Extended-Spectrum Beta-Lactamase Producing Escherichia coli Obtained from Animal Fecal Samples in Ado Ekiti, Nigeria. Journal of Environmental and Public Health 7 p.
Crossref
|
|
|
|
|
Onyedibe KI, Shobowale EO, Okolo MO, Iroezindu MO, Afolaranmi TO, Nwaokorie FO, Opajobi SO, Isa SE, Egah DZ (2018). Low Prevalence of Carbapenem Resistance in Clinical Isolates of Extended Spectrum Beta Lactamase (ESBL) Producing Escherichia coli in North Central, Nigeria. Advances in Infectious Diseases 8:109-120.
|
|
|
|
|
Paterson DL, Ko WC, Von Gottberg A, Mohapatra S, Casellas M, Goossens H, Mulazimoglu L, Trenholme G, Klugman KP, Bonomo RA, Rice LB, Wagener MM, McCormack JG (2004). Antibiotic therapy for Klebsiellapneumoniae bacteremia: implications of production of extended-spectrum beta-lactamases. Clinical and Infectious Disease 39(1):31-37.
Crossref
|
|
|
|
|
Piroth L, Aube H, Doise JM, Martin MV (1998). Spread of ESBL-producing K. pneumoniae: are β-lactamase inhibitors of therapeutic value?. Clinical and Infectious Disease 27:76-80.
Crossref
|
|
|
|
|
Sambrook J, Fritsch EF (1989). Maniatis T.Isolation of high molecular weight DNA from mammalian cells. Molecular Cloning, a Laboratory Manual 1:9-14.
|
|
|
|
|
Saravanan M, Ramachandran B, Barabadi H (2018). The prevalence and drug resistance pattern of extended spectrum β-lactamases (ESBLs) producing Enterobacteriaceae in Africa. Microbial Pathogens 114:180-192.
Crossref
|
|
|
|
|
Shaikh S, Fatima J, Shakil S, Rizvi SM, Kamal MA (2015). Antibiotic resistance and extended spectrum beta-lactamases: Types, epidemiology and treatment. Saudi Journal of Biological Sciences 22(1):90-101.
Crossref
|
|
|
|
|
Wani KA, Thakur MA, Siraj FA, Fomdia B, Gulnaz B, Maroof P (2009). Extended Spectrum B-Lactamase Mediated Resistance in Escherichia coli in a Tertiary Care Hospital. International Journal of Health Sciences 3:155-163.
|
|
|
|
|
Yusuf I, Arzai AH, Umar A, Magaji N, Salisu N, Tukur A, Hamid KM, Haruna M (2011). Prevalence of Extended Spectrum β-Lactamases (ESBL) producing Escherichia coli. Bayero Journal of Pure and Applied Sciences 4(2):182-185.
Crossref
|
|