The current study assessed the drinkability of water in 25 schools in Cruz das Almas BA Brazil. Total coliforms, Escherichia coli, Enterococcus, mesophylls and psichrotrophic bacteria were evaluated, coupled with color, turbidity, free residual chlorine, dissolved oxygen and biochemical oxygen demands. Water samples were collected at four sites: the first tap within the supply network or within the alternative supply (first site), main reservoir (second site), kitchen water (third site) and drinking water troughs (fourth site). When the two assessment periods (during dry and wet seasons) were taken into account, contamination by total coliforms in approximately 65.3% of samples was detected, whilst E. coli and Enterococcus micro-organisms respectively occurred in 18.4 and 36.7% of samples. Counts of mesophyll microorganisms were greater during the dry period than during the rainy season. More than 34.7% of counts were above the legal limit. Although no apparent variation in color occurred, irregularity in turbidity and pH was detected in two schools. Inadequate rates for dissolved oxygen occurred in only two schools, during the dry period, whereas biochemical oxygen demand complied with legislation. Although, no coliform was extant in several samples, others had bacterial contamination requiring more efficacious practices for quality improvement. Results show that health risks in schools in Cruz das Almas may be solved by adequate water treatment, periodical cleaning of reservoirs and proper maintenance of taps and filters.
Water is important for all living organisms. Life, the functioning of ecosystems, communities and economies depend totally on water. However, water scarcity occurs in almost all developing countries, with immense expenses spent on its availability, quality, use and mortality by water-caused diseases (Brasil, 2014; Khan, 2012). Life quality is strictly linked to the quality of drinkable water since it is a relevant element employed in several daily tasks such as cooking, personal hygiene and cleaning. Personal well-bring requires access to drinkable water free from pathogen agents and/or toxic chemical products which transmit diseases to consumers (Brasil 2011; Xavier et al. 2011; WHO, 2011).
Several factors such as domestic drainage, industrial and agricultural effluents, deforestation, mining, solid wastes, effluents from swine breeding, diffuse pollution in urban areas, salinization, environmental accidents, dam building and aquiculture contribute towards water contamination (Brasil, 2006; Xavier et al., 2011; WHO, 2011). However, the best way for water preservation is avoiding contamination by animal and human wastes which contain a great number of bacteria, viruses, protozoa and helminths. In fact, failure in the effective treatment of water causes great risks to consumer´s health (Brasil, 2006; Azizullah et al., 2011; WHO, 2011; Rodrigues and Barros, 2012).
Several studies have shown that approximately two million tons of industrial drainage and agricultural wastes are deposited in water courses worldwide, resulting in illnesses due to contaminated water (UNEP, 2010). In fact, 2.2 million people, including 1.8 million children less than five years old, die every year from diarrhea and related diseases. United Nations Organization estimates show that almost 900 million people have some difficulty in accessing drinkable water and approximately 2.6 million do not have basic sanitation facilities. In Brazil, water availability reaches 91% of the population, with 98% in urban areas. Approximately 70% of rural populations have access to drinkable water, or rather, a rise in 85% within the last 25 years (UNEP, 2010; Brasil, 2012; Instituto Trata Brasil, 2015).
Brazilian legislation (Decree 2.914 of 2011, issued by the Ministry of Health, Brasil, 2011) forwards several parameters to characterize water as drinkable. Its parameters are based on universal rules derived from the Guidelines for Drinking-Water Quality of the World Health Organization (WHO, 2011). Current analysis evaluates drinking water available for consumption in several schools of Cruz das Almas BA Brazil.
The current study was performed in 25 public school institutions (labeled A to Y) in the municipality of Cruz das Almas, Brazil. Analyses were undertaken during two seasonal periods. During the dry season (September to March), 25 water samples were collected between November and December, whereas samples were retrieved between June and August during the rainy season (April to August) (Bahia, 2013). Water samples were collected and transported to the Laboratory of Animal Parasitology and Microbiology of the Universidade Federal do Recôncavo da Bahia (UFRB) for analysis.
Water was collected at four sampling points in each school by retrieving 500 ml from each sampling point, amounting to 2 L of water from each school: water collected from the first tap of the water supply network or from the alternative supply (1st sampling point), main reservoir (2nd sampling point), kitchen water (3rd sampling point) and the main drinking water troughs (4th sampling point). Information on the water supply source was also retrieved. Collection points (tap or trough) were kept open with water flowing during two or three minutes; hygiene with alcohol 70% was undertaken for sample collection.
Total coliforms and Escherichia coli were analyzed by chromogenic substrates (Colilert®) based on two active substrates, σ-nitrophenyl-β-D-galactopyranoside (ONPG) and 4-methylumbelliferil-β-D-glucuronide (MUG), to detect total coliforms and E. coli, respectively. Coliforms produce the enzyme β-galactosidase which hydrolyzes ONPG and releases σ-nitrophenol, providing a yellowish color to the medium. E. coli produces enzyme β-glucuronidase which hydrolyzes MUG, forming the fluorescent compound 4-methylumbelliferone under ultraviolet light at 365 nm.
Microorganisms were analyzed quantitatively, or rather for their presence or absence, by adding 100 mL of water samples in transparent plastic flasks (previously sterilized in autoclave). A powder substrate was added to each flask, homogenized and distributed on IDEXX™ Quanti Tray cards. The material was incubated in a buffer at 36°C for 24 h. After incubation, the yellowish color indicated total coliforms, whereas fluorescence blue under UV light (365 nm) in the dark indicated E. coli. Estimates of MPN.100 ml-1 at 95% confidence for each MPN rate quantified total coliforms and E. coli.
MPN of microorganisms Enterococcus was calculated by multiple tube technique, in a series of 5 or 10 tubes, in which 5/10 ml of water were inoculated in tubes with Glucose Azide Broth in double concentration and then incubated at 36±1°C for 48 h. Positivity would be demonstrated by the medium´s turbidity. Further, plates with medium Pfizer selective enterococcus (PSE) agar were striated in all tubes with turbidity to confirm the presence of typical colonies, featuring a dark brownish color with a brown halo (Apha, 1998).
Mesophyll microorganisms were counted by the Pour Plate technique in which 1 mL of each sample was deposited on the bottom of petri plates, adding (Plate Count Agar (PCA) culture medium previously fused and cooled at 40°C. After homogenized and solidified, the content was incubated at 35°C for 48 h. Counting was performed by colony counters on plates with 10 - 300 colony-forming units (CFU) (Apha, 1998).
Psichrotrophic microorganisms were calculated by the above-mentioned method described for mesophylls, with the exception of temperature and incubation period, respectively at 7°C for 10 days, in CFU.mL-1 (Apha, 1998).
Colorimeter and turbidity-meter were employed to determine the physical parameters color (UHazen) and turbidity (UNT), respectively. Further, pH rates of samples were obtained by pH-meter, while free residual chlorine was determined by NN diethyl paraphenylene diamine (DPD) with colorimeter HACH (Hanna, 1998).
Dissolved oxygen and biochemical oxygen demand of samples were measured by dissolved oxygen meters Hanna DO-5519 and Lutron DO-5519 (Apha, 1998).
Microbiological assessment
Coliform microorganisms occurred only in samples collected during the rainy period, with approximately 66.6% contamination of the water analyzed at one out of the four collection sites. There was a 64% contamination of analyzed sites in samples during the dry period and thus inadequate for human consumption. A higher contamination rate was detected during the dry period when compared with that during the rainy one as averages (Table 1). In fact, results ranged between 0.0 (absence) and 2419.6 MPN.100 mL-1 of water. The above demonstrates that, regardless of the seasonal period or the supply form, and due to total coliforms, intervention of the sites under analysis should be made so that water could be safe for consumption (Table 1). Samples from the alternative supply had even higher contamination rates (Table 1), probably due to lack of chlorine treatment, mandatory by current legislation for drinkable water.
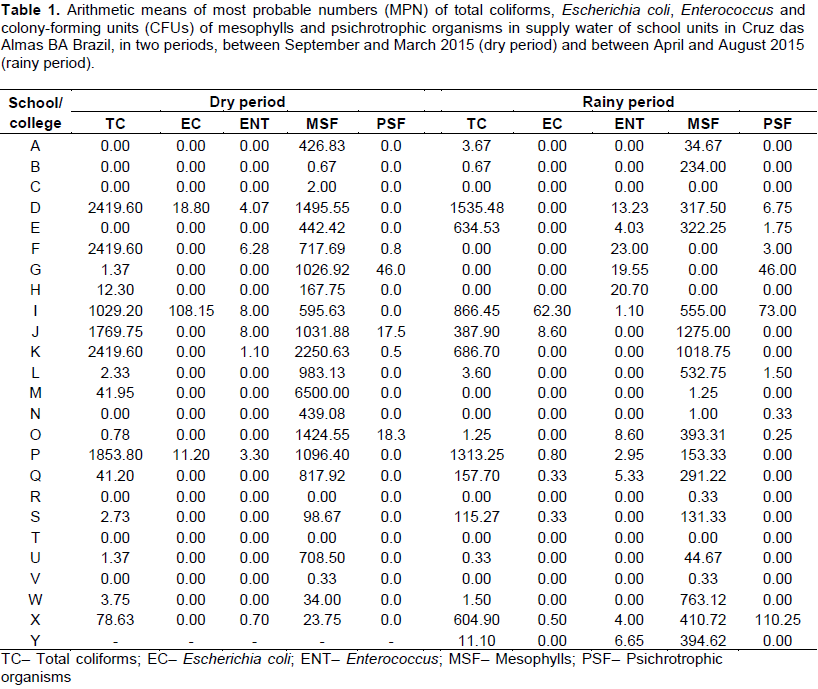
Rates contradict several studies that underscore the importance of data collection at different season periods. Review by Kostyla et al. (2015) on 22 studies on water quality and seasons in developing countries revealed that contamination is highest during the rainy period when bacteria, indicating fecal contamination, measurement methods, population definition, source type and equatorial climate zone are taken into account. The above is due to the fact that research related to water quality gives priority to the dry period because of accessibility, time, roads and other factors. However, there has been a recent trend to underscore the fact that water microbiological contamination is greater during the rainy season (WHO, 2010; UNICEF, 2010), which did not occur in the current study. Since collection periods were coupled with hygiene at the collection site in each school analyzed, the final result must have been affected.
Collection sites of the samples may also affect the research´s conclusion data since each specific site may produce a different result (WHO, 2010; UNICEF, 2010). In fact, the collection of samples at different sites in schools is very relevant so that greater representativeness would be obtained and the precise focus would be detected. Samples collected from WSS + ACS (B and U) show mild contamination by coliforms,or rather, 0.67 and 0.33 MPN.100 mL-1 for averages of the four collection sites for the rainy season, and 0.0 and 1.37 MPN.100mL-1 for the dry period. In spite of low contamination rate, the samples are not the best for consumption and cross contamination of treated water may have occurred (Conama, 2005; Brasil, 2011, WHO, 2011).
The microorganism, Escherichia coli indicates recent fecal contamination and the possible presence of pathogens, since these microorganisms are strictly fecal. The detection of their presence is relevant since they cause several diseases, such as E. coli O157: H7 which may be lethal due to hemolytic-uremic syndrome (HUS), a very serious disease in children, elderly and immune-compromised people. Similar to legislation on total coliforms, the microorganism E. coli should be absent in water for human consumption in WSS and ACS (Conama, 2005; Brasil, 2011; WHO, 2011; Eden, 2014)
Table 1 shows averages in the collection sites. Variation in mean rates between 0.0 (absence) and 108.15 MPN.100mL-1 of the sample could be detected in the two season periods when the research was conducted. In fact, the microorganism was present in six schools during the rainy season and in three schools during the dry period. All the schools where E. coli was detected urgently require intervention due to harm caused to people by drinking contaminated water.
In the case of the two evaluation periods, the highest contamination levels occurred at collection sites 1 and 2. Since all water for consumption should be free from E. coli, all drinkable water should be treated and exhibit free residual chlorine rate so that micro-biological contaminations would be avoided (Brasil, 2011). Greater control is required to verify possible flaws in the water distribution system or in the building structure, hygiene of reservoir and water troughs.
Several studies by Jasper et al. (2012) have shown that school children exposed to inadequate water conditions and deficient sanitary installations may develop infectious, gastrointestinal, neural-cognitive and psychological diseases. The hygiene-sanitary segment of the premises should receive greater investments. Education is a right and the availability of water and sanitation in schools is highly relevant and requires criteria, norms and control so that the educational process would not be jeopardized.
Water may be contaminated in diverse manners as Rocha et al. (2010) exemplify in a similar study on schools. Contamination may initially occur in the public supply system, even though it commonly occurs by faulty distribution or by lack of hygiene of the water reservoir which supplies the schools. Faults in the maintenance of pipes and principally of the reservoir develop favorable conditions for microbial growth. Hygiene and the maintenance of water integrity for consumption avoid contamination.
Moosa et al. (2015) evaluated water quality in drinking troughs in schools and at the university in Ajman, United Arab Emirates, and detected Pseudomonas aureginosas, total coliforms and E. coli: 32.65% of the 49 samples assessed were contaminated by total coliforms and unfeasible for consumption according to the legislation of the country mentioned above. The authors reported that the drinking water should not contain the bacterium. As underscored by Rocha et al. (2010), cleansing of the premises and its surroundings has a key role in the maintenance of water quality for drinking. Another important factor in the study are stricter attitudes by health authorities to guarantee drinking water quality. The control and monitoring of water quality by school administrators and educators should involve the student community and the general society to supply water within the mandatory parameters. These activities must be backed by governmental policies to inform the population on quality parameters and how to maintain the best water quality (Souza et al., 2015). In a similar study, the above authors assessed input supply water, water exit from the reservoir and water in the drinking troughs and verified that 60.6% of water in the 33 school institutions in Mossoró RN Brazil were inadequate for human consumption, featuring total coliforms, and/or thermotolerant coliforms, with a high contamination index by total coliforms and E. coli in the schools´ drinking troughs.
The presence of significant amounts of coliform bacteria (total coliforms and Escherichia coli) in underground water indicates irregularities deep in the earth or a break down in the sanitary integrity of water wells. Inadequate protection associated with sanitary flaws highlights the need to establish plans for the protection of water supply for rural communities where water treatment is still unavailable. Hedging the premises adequately, enhancing regular maintenance and disposing correctly human and animal wastes (which infiltrate the soil and contaminate the place) are mandatory. They are strategies that ensure the safety of the water sources (Tsega et al., 2013; Bain et al., 2014).
Although, no specific legislation exists to determine the most probable number (MPN) for enterococcus micro-organisms in water for human consumption, high counts indicate potential sources of contamination caused by deficiencies in water treatment or in the distribution system (Conama, 2005; Brasil, 2011; WHO, 2011). Table 1 registers MPN of Enterococcus microorganisms during the two periods analyzed in the current study.
Positive results occurred during the dry and rainy periods, respectively in 29.16 and 44% of samples. Although, they may be found in the feces of animals or even in the environment as free organisms, enterococci are closely associated with human wastes, featuring fecal pollution in the water and lack of intervention when high rates were detected (Brasil, 2006). Further, these microorganisms may be of great help to detect fecal contamination, albeit with certain restrictions, since they are integrated to food microflora. However, they are more resistant to chlorination when compared with the coliform group (WHO, 2011, 2003).
There were greater contamination levels by enterococci during the rainy period, confirming position by WHO and UNICEF (2010) and Kostyla et al. (2015) and by Table 1. In fact, the microbiological contamination of water is greater during the rainy period, confirming seasonal influences that enhance false-positive results in research that evaluates consumption water quality.
Assessment of water samples in plastic tanks and in wells (89 and 177) in an area within the metropolitan region of São Paulo, Brazil, revealed that enterococcus microorganisms were extant in 21 samples (23.5%) for plastic tanks and in 142 samples (80.2%) for wells, enhancing possible interventions due to fecal contamination (Razzolini et al., 2011).
Several studies have focused on enterococci especially with regard to their resistance capacity. A study assessed enterococci in water in bottles, hospitals and wells in Kerala, India, and detected contamination in 74% of the 270 samples. Results revealed that water may contain bacteria resistant to anti-microbial agents with severe health risks to consumers. Resistance is acquired by the microorganisms’ capacity to form protecting films or biofilms (Peter et al., 2012). Concern on these microorganisms is highly important since they may damage human health regardless of their requirement by Brazilian legislation. Mesophyll and psychrotrophic microorganisms were selected for heterotrophic bacteria. In the case of strict and facultative aerobic mesophyll microorganisms, contamination level ranged between 0.0 (absence) and 6.500 CFU.mL-1, respectively, reached high rates (Table 1) considered irregular by Decree 2,914/2011 of the Ministry of Health, with a limit of 500 CFU.mL-1 in water samples. During the dry period, greater population counts of mesophyll microorganisms were reported as compared to those during the rainy period, with 45.83 and 20.00% of samples above the rates allowed by law.
No psychrotrophic microorganism rates above those permitted by Brazilian legislation were detected in the two periods analyzed and only small counts were registered. Since these microorganisms have an wide temperature range (between -10 and 30°C) for their development, great care should be taken to quantify them (Tortora et al., 2012) since they may be found in places without any refrigeration, such as sites 1 (reservoir) and 4 (water troughs), at room temperature or even regardless of temperature.
When they are used to indicate water quality, heterotrophic bacteria counts (HBC) have a wide range of results, or rather, they reveal bacteria of fecal origin or bacteria naturally occurring in water, since rates above 500 CFU.mL-1 may indicate the occurrence of coliforms. Although they may not be prejudicial to health, high counts of these microorganisms are a warning on flaws in the water treatment, either due to disinfection and to the formation of biofilms, or in storage or faults in the distribution systems or the presence of organic matter in water. They may also indicate the existence of pathogenic agents such as Acinetobacter, Aeromonas, Flavobacterium, Klebsiella, Moraxella, Serratia, Pseudomonas and Xanthomonas (Ana, 2005; Brasil, 2011; WHO, 2011; Chowdhury, 2012). Similar to requirements for enterococcus microorganisms, the above is also required to evaluate bottled mineral water (Falcone-Dias and Farache Filho, 2013).
The parameter also indicates the efficiency of the disinfection process, improvement in the installations for water distribution and evaluation of water storage in tanks or in reservoirs (Diduch et al., 2016).
Physical and chemical assessment
Physical assessment showed no variation when the parameter color of water was evaluated. Only two of all the sites analyzed failed to comply with legislation on turbidity (Table 2), with rates up to 5.0 UNT (BRASIL, 2011). Means from each environment assessed revealed a variation between 0.54 and 7.26, with higher rates during the rainy period than during the dry period. Soil erosion, mining activities, drainage and industrial effluents were the main causes. Turbidity above the best legal rates directly affects consumer´s water acceptability. The less the turbidity rates, the more acceptable will the water be. Further, suspended particles may protect pathogenic microorganisms (BRASIL, 2006; WHO, 2011). High turbidity levels may indicate high levels of microbial contamination and other physical and chemical parameters. Therefore, turbidity rates may be used to select water sources. In fact, turbidity analysis is a low-cost key parameter and indicates the efficient removal of pathogens resistant to chlorination (Mann et al., 2007, WHO, 2011; Castaño and Higuita, 2016).In the case of chemical assessment, pH of samples B, D and O (Table 2) during the two collection periods featured rates below the ideal range (between 6.0 and 9.5, according to Brasil, 2011). Although, pH does not have a direct impact on the water used by the final consumer, it is a very important parameter. When it exceeds the ideal range, it may influence disinfection and chlorine clarification in the water. Flaws in the maintenance of the range may cause several liabilities, such as corrosion and incrustations in the pipes, which may change the water’s taste and aspect due to the aggregation of the material that the water tubes are made of (Brasil, 2006; WHO, 2011). Free Residual Chlorine count was the parameter with the greatest variation. Decree 2,914 of 2011 of the Brazilian Ministry of Health specifies an acceptable standard for the parameter, with a minimum of 0.2mg.L-1 of Free Residual Chlorine in the water for consumption up to 2 mg.L-1 throughout the supply system. When means in Table 2 are analyzed, samples I, J, K and L show that during the two assessment periods, rates were below the best level. Within the set of samples evaluated, only 34% of the schools during the dry period had rates which complied with the range proposed by the Ministry of Health. On the other hand, FRC rates comply with legislation in 48% of the schools during the rainy period.

Since chlorine is the principal active compound for water disinfection, several analyses have focused on the manner the product works without causing economic liabilities or any harm to people´s health. There are several strategies for its maintenance at adequate levels to avoid re-contamination after treatment and harm to the consumer´s health. Decrease in FRC levels frequently occurs when the pipes of the distribution system are made of copper as compared to PVC and galvanized pipes.
However, several motives, such as frequent stagnations, temperature increase, reduced discharge, stocking for large periods and others, may alter FRC levels (Zheng et al., 2015).
Contrastingly, high levels may also cause serious health risks to people. Although, chlorine´s residual load combats the risk of contamination by the pathogenic microorganisms in the water, contrary effects may occur when rates are high. Associated with high levels of FRC, one should mention the formation of trihalomethanes, compounds formed by the reaction between chlorine and organic compounds, originating cancerigenous chloroforms. Besides allergic symptoms, such as skin eruptions and intestinal symptoms, when ingested, the bacteria of lactic acid that line the colon are destroyed and the intestine is exposed to foreign pathogenic agents (Brasil, 2006; Siddique et al., 2011; Zheng et al., 2015). When they quantified the minimum legal rate of 0.2 mg.L-1 for FRC, Sanches et al. (2014) verified that 40.62% of samples from kitchen taps and 31.25% of samples from drinking troughs from eight schools in Uberaba MG Brazil failed to comply with the accepted minimum. Moreover, Cardoso et al. (2007) reported that FRC rate in 33% of samples from 83 schools in Salvador BA Brazil was below the legal parameter. The above proves a common denominator in many Brazilian municipalities.
Dissolved oxygen is one of the most relevant parameters to evaluate the quality of water environments. Although, DO averages of samples analyzed for the two periods were very similar, three school institution registered rates lower than 5 mg.L-1 during the dry period. Consequently, they failed to comply with legislation which states that rates should not be lower than 5 mg/L (Conama, 2005). Alterations in DO levels are due to temperature variations, pressure and water salinity. In other words, physical, chemical and biological processes act directly on water bodies (Brasil, 2014).
Several studies report that the association between temperature, pH and DO in irregular rates may affect water quality, especially in supply systems with copper pipes. When the re-contamination of water is evaluated, one may observe a close relationship between temperature, DO and heterotrophic bacteria counts. Best temperature rates and available oxygen enhance re-contamination of the environment since they lack FRC to eliminate re-contamination (Vargas et al., 2010; WHO, 2011; Lu et al., 2014).
All BOD rates complied with current legislation up to 5 mg.L-1 (Table 2) (Brasil, 2014). There was a variation in averages between 0.50 and 0.79 mg.L-1, considered the best rates. Although, BOD is a parameter to assess domestic and industrial effluents in natural non-polluted environments, the Brazilian Health Foundation registers that the ideal concentration in such environments varies between 1 and 10 mg.L-1. This is a low rate, with alterations only with possible contaminations affecting color, turbidity and DO consumption by decomposing organisms (Brasil, 2014; Lu et al., 2014). Wanda et al. (2015) studied the quality of water in certain African regions and reported that high BOD, availability of organic matter in the medium, greatly interferes with water classification. When associated with a low DO rate plus microorganisms, great care must be taken with the assessed water treatment system
Although, assessments during two periods have beenundertaken to obtain a greater representation of the hygiene-sanitary quality of available water in schools in Cruz das Almas, a constant follow-up of the water quality is needed since students, school officers and teachers spend a large part of their time on the premise and use the water available.
Immediate intervention is required in sites where FRC rates exceed the acceptable range, since otherwise microbial growth and re-contamination will occur during the dry and rainy periods. Immediate provisions should be taken when fecal contamination occurs in the water. Conditions should be considered irregular even though high rates occur during the dry period. Current data are a help to school and municipal authorities so that water quality problems would be solved. Adequate and regular hygiene of water reservoirs that provide water to the kitchen and water troughs, change of water filters and the establishment of standard procedures are alternatives for the elimination of microorganisms to comply with Brazilian legislation.
In the case of schools that use alternative water supply solutions, the issue on the implantation of the public supply system and other options should be raised for the solution of the problem. In fact, the Ministry of Health has already recommended alternative disinfection such as diffused chlorination, lozenge chlorination, liquid chlorination and the establishment of home-treatment units.