Full Length Research Paper
ABSTRACT
Streptococcus pneumoniae is the main bacterial cause of community-acquired pneumonia. In children, 22% of the deaths are due to pneumonia as the single leading cause of death. The local people in Uganda use herbs like Curcuma longa Linnaeus and Garcinia buchananii Baker to manage upper respiratory tract infections (URTIs). The ethanolic extracts of the C. longa rhizome and G. buchananii stem bark have individually demonstrated antimicrobial activity against bacteria, protozoa, and viruses. Crude extracts of C. longa rhizome powder and G. buchananii fresh back were obtained through maceration using ethanol. In vitro disc diffusion method and serial dilution method were used to determine antibacterial susceptibility and minimum inhibitory concentration (MIC), respectively of the plant extracts against S. pneumoniae. Both ethanolic extracts of C. longa rhizome and G. buchananii stem bark individually showed activity against S. pneumoniae and this antibacterial effect was largely dose-dependent. However, ceftriaxone had superior antibacterial activity (p< 0.0001) than all the individual extracts and combinations. The MICs of C. longa and G. buchananii ethanolic extracts were 3.125 and 1.5625 mg/mL, respectively. The 50:50 C. longa - G. buchananii combination showed superior activity compared to other combinations, though it was not statistically significant (p > 0.05). The Fractional Inhibitory Concentration Index (FICI) was 11.68. This study concluded that the ethanolic extracts of both the rhizome of C. longa and the stem bark of G. buchananii, when used singly and in combination, demonstrated antibacterial activity against S. pneumoniae. However, the combination of the ethanolic extracts of these two plants demonstrates antagonistic activity.
Key words: Curcuma longa, Garcinia buchananii, Streptococcus pneumoniae, combined antibacterial activity.
INTRODUCTION
Streptococcus pneumoniae is the leading cause of community-acquired pneumonia. It is one of the most common pathogens that cause invasive diseases such as sepsis, meningitis, and pneumonia, bacteremia, and sinusitis (Soto-Noguerón et al., 2016; Wessels et al., 2012). Pneumonia due to S. pneumoniae accounts for numerous hospitalizations and deaths among all age groups (Baldo et al., 2015). In children, acute respiratory tract infections account for 22% of deaths; with pneumonia as the single leading cause of death. The World Health Organization (WHO) estimates that about 14.5 million episodes of serious pneumococcal disease occur, resulting in about 826,000 deaths in children under five years. The highest number of deaths due to S. pneumoniae occurs in developing countries than in industrialized country settings (WHO, 2012). Seventy percent of the deaths occur in African and Asian countries (Krumkamp et al., 2012).
The first-line management of URTIs caused by S. pneumoniae is penicillins like amoxicillin. However, due to the emergence of penicillin-resistant bacterial strains, many other drugs are often used to manage these infections including fluoroquinolones, cephalosporins like ceftriaxone, cefotaxime. Other drugs used include clindamycin, doxycycline, vancomycin, and linezolid (UCG, 2016). However, resistance to penicillins, macrolides, fluoroquinolones, tetracycline, clindamycin, and trimethoprim-sulfamethoxazole combination is on the rise and occurs through various mechanisms (Cherazard et al., 2017).
Medicinal plants in Africa constitute a large but still largely untapped pool of natural product remedies. World Health Organization (WHO) estimates that 80% of the population in developing countries still relies on plant-based medicines for some part of primary health care (Ekor, 2014). In parts of East Africa like the Lake Victoria basin, numerous medicinal plants including Garcinia buchananii B. and Curcuma longa L. are used individually or in combination to manage upper respiratory tract infections (URTIs). In Africa, G. buchananii (family Clusiaceae) bark extract is widely used traditionally for the management of gastrointestinal diseases like diarrhea, and dysentery (Balemba et al., 2010), and other conditions such as respiratory tract infections, eye diseases, hypertension, and diabetes (Okullo et al., 2014). G. buchananii contains an isoprenylated benzophenone derivative garcinol as one of its phytochemicals (Schobert and Biersack, 2019; Stark et al., 2015). Garcinol has been reported to have antibacterial activity against Staphylococcus aureus, Escherichia coli, Bacillus subtilis, and Enterobacter aerogenes (Varalakshmi et al., 2010). C. longa L. (Tumeric) belongs to the Zingiberaceae family and is used largely as a coloring agent, as a spice, and also as a medicine (Gurning, 2020). As a medicine, it is used widely as an antimicrobial, anti-inflammatory, anticancer, antidiabetic, and antioxidant agent (El-Kenawy et al., 2019; Teow et al., 2016). Rhizome extracts of C. longa have been found to possess broad-spectrum antibacterial activity (Kumar et al., 2020), with this activity attributed to its major constituent curcumin (Teow et al., 2016).
Studies on C. longa rhizome extracts have shown activity against S. aureus, Klebsiella pneumonia, E. coli, and Staphylococcus epidermidis (Feghali et al., 2018; Singh et al., 2017).
Mixtures of different plants extracts are widely used to manage various diseases. The rationale of the use of combinations is to benefit from the possible synergistic or potentiating effects (Ozioma and Okaka, 2019). There is however a great need to evaluate whether the different combinations of the crude plant actually achieve the desired benefits. In various studies, the antibacterial activity of individual extracts of C. longa rhizome and G. buchananii stem back extracts have been established for various micro-organisms but not S. pneumoniae. This study aimed at establishing the antibacterial activity of individual and combination of ethanolic extracts of C. longa rhizome and G. buchananii stem bark against S. pneumoniae.
MATERIALS AND METHODS
Reagents and chemicals
Ethanol (70%), distilled water, concentrated sulfuric acid, ferric chloride, sodium hydroxide, hydrochloric acid, iodine, potassium iodide, chloroform, dimethylsulfoxide (DMSO), and ceftriaxone standard.
Collection and identification of plant
Fresh forms of turmeric rhizome were obtained from a garden in Rakai-Uganda, while fresh forms of the stem bark of G. buchananii were obtained from a garden in Kisaasi, Uganda in February 2018. The plant materials were transported to the pharmacognosy laboratory at the Department of Pharmacy, Makerere University. Herbarium specimens were submitted to the Makerere University herbarium for authentication by a botanist.
Preparation of plant extracts
The fresh rhizomes of C. longa were washed using distilled water. The rhizomes were then dried in the open air under shade for 10 days after which, they were ground using a motor and pestle. The obtained powder was macerated in ethanol (70%) in a closed glass container; in a ratio of 1:6 at room temperature, for 5 days with occasional agitation. The obtained mixture was then filtered using Whatman filter paper number 1. The filtrate was concentrated by evaporation in a rotary evaporator at 90°C. This process was also repeated for the fresh bark of G. buchananii. The percentage yield for the plant materials was then calculated using the formula:
Percentage yield of extract = (Mass of dried crude extract / Mass of powdered plant material macerated) ×100%
Phytochemical analysis
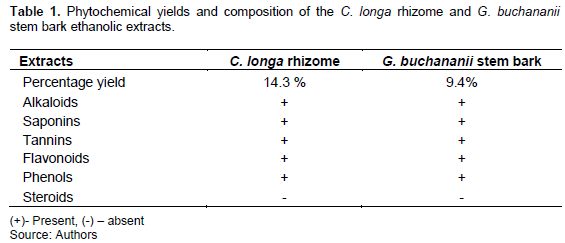
Phytochemicals screening for tannins, alkaloids, saponins, flavonoids, steroids, and phenols was done following standard methods (Evans, 2009) (Table 1).
Test for tannins (Ferric chloride test)
Ferric chloride (1 mL) was added to the extract. The formation of a blue-green precipitate confirmed the presence of tannins.
Test for alkaloids
Extracts (10 g) were dissolved in dilute hydrochloric acid and then filtered. To 2 mL of the filtrate was added Wagner’s reagent (Iodine in Potassium iodide). The formation of a reddish-brown precipitate indicated the presence of alkaloids.
Test for saponins
The extract was added to distilled water (20 mL) in a measuring cylinder and agitation was done for 15 min. The formation of a 1 cm layer of foam confirmed the presence of saponins.
Test for flavonoids
Sodium hydroxide (2 mL) was added to the extract. The formation of a yellow color, that became colorless once diluted acid was added confirmed the presence of flavonoids.
Tests for steroids
To extract (1 mg) in a test tube was added chloroform (10 mL). Concentrated sulfuric acid (10 mL) was added slowly on the sides of the test tube. The formation of a brown ring at the intersection of the two layers and with the upper layer turning green confirmed the presence of steroids.
Test for phenols
The extract was dissolved in distilled water (2 mL) and then a few drops of ferric chloride were added. The formation of a deep blue or greenish colour confirmed the presence of phenols.
Antimicrobial susceptibility testing
The disc diffusion method was used to screen for antibacterial activity. S. pneumoniae ATCC 49619 (Provided by Microbiology department-Makerere University) was inoculated on blood agar plates by streaking. Sterile Whatman filter paper discs measuring 6 mm in diameter were sterilized by autoclaving at 121°C and a pressure of 15 psi for 15 min. These filter disks were then impregnated with crude plant extracts of different concentrations 3.125, 6.25, 12.5, 25, 50, 100 mg/mL, and 25:75, 50:50 and 75:25 fractional combinations of the extracts under aseptic conditions. The positive control used was ceftriaxone (100 mg/mL) and the negative control was DMSO (1.5%). This was done in triplicate for all the extracts and the controls. DMSO was the solvent used to make all the different concentrations of the extracts and positive control. Impregnated filter paper discs for both controls and those with plant extracts were then placed on the surfaces of the blood agar media, onto which S. pneumoniae had been inoculated, and incubated at 37°C for 24 h. After incubation, the plates were removed from the incubator and the diameters of the zones of inhibition were measured using a Vernier caliper.
Determination of the MIC and FICI
The minimum inhibitory concentration (MIC) of the ethanolic extracts was determined by the serial dilution method. Ethanolic extracts of both C. longa and G. buchananii were prepared using Brain Heart Infusion (BHI) agar to make different concentrations ranging from 0.78125 to 200 mg/mL in their respectively labeled test tubes. S. pneumoniae (strain ATCC 49619) was inoculated into the BHI with different concentrations of both the individual and with the most potent combination of C. longa and G. buchananii (50:50). Controls containing only the nutrient broth without the extracts were included. The test tubes were then incubated at 37°C for 24 h. The presence of turbidity after the incubation period denoted the presence of S. pneumoniae, while the absence of any turbidity indicated inhibition of microbial growth. The concentration of the extract in the test tube with the lowest dilution with no detectable growth by visual inspection was considered the MIC. Due to the colored nature of the plant extracts, which made the observation for turbidity difficult, the samples were subsequently sub-cultured at 37°C in an incubator for 24 h to observe for any growth. The Fractional Inhibitory Concentration Index (FICI) was calculated using the following standard formula. The effects of the combinations were then classified as: synergistic, additive, indifference and antagonistic, if the FICI is <1, =1, >1 ≤2, and >2, respectively.
FIC (G. buchananii extract) = MIC (G. buchananii extract in combination) / MIC (G. buchananii extract alone)
FIC (C. longa extract) = MIC (C. longa extraction in combination) / MIC (C. longa extract alone)
FICI = FIC (G. buchananii extract) + FIC (C. longa extract).
Data management and analysis
Graphpad Prism Ver.7.0 was used to compute descriptive statistics of the mean and standard deviation (SD) inhibition zone diameter. The data was then subjected to one-way analysis of variance (ANOVA) followed by Dunnett’s multiple comparison tests to compare the antibacterial activities of the different extract concentrations and the controls. A value of p<0.05 was considered significant.
RESULTS
Extraction yield and phytochemicals
The percentage yield of ethanolic extracts of C. longa rhizome and G. buchananii stem bark was 14.3 and 9.4%, respectively. Both ethanolic plant extracts contained alkaloids, saponins, tannins, flavonoids, and phenols and lacked steroids (Table 1).
Antimicrobial susceptibility of S. pneumoniae to C. longa and G. buchananii ethanolic extracts
Ceftriaxone 100 mg/mL (positive control) had the largest zone of inhibition of 43.33 (± 3.05) on the S. pneumoniae strain ATCC 49619 used when compared with all other test substances. Among the 3.125 to 100 mg/mL ethanolic plant extracts of G. buchananii and C. longa, the highest dose concentrations of 100 mg/mL had the largest zones of inhibition of 26.67 (± 1.15) and 25.33 (±2.31), respectively. Of the C. longa and G. buchananii extracts combinations, the 50% C. longa + 50% G. buchananii, had the largest zone of inhibition (Table 2). There was no significant difference (p> 0.05) in activity between the different G. buchananii and C. longa extracts combinations, and between similar concentrations of G. buchananii and C. longa when used singly.
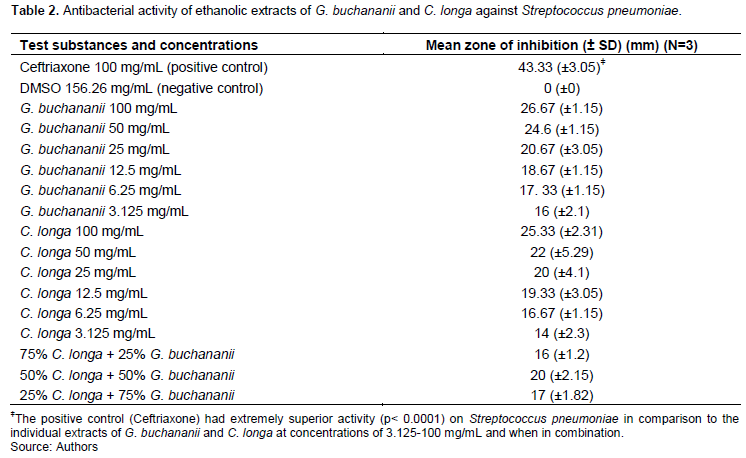
The MICs for ethanolic extracts C. longa rhizome and G. buchananii stem bark against S. pneumoniae were 3.125 and 1.5625 mg/mL, respectively. The most potent combination of extracts (50% C. longa and 50% G. buchananii) had a MIC of 12.162 mg/mL and a Fractional Inhibitory Concentration Index (FICI) of 11.68.
DISCUSSION
The percentage yield of ethanolic extract of C. longa from this study was lower than that observed in a previous study that reported 17.39% (Tanvir et al., 2017). Variations in yields and phytochemical composition of plant extracts are reported to be caused by variations in climatic and agronomic factors, and as well as differences in the extraction procedures used (Borges et al., 2018; Dhanani et al., 2017). Both C. longa and G. buchananii contained significant amounts of alkaloids, saponins, tannins, flavonoids, and phenols. These findings are similar to those in previous studies (Gurning, 2020; Tanvir et al., 2017) and the anti-bacterial inhibitory effects identified could be attributed to some or all of these phytochemicals in the plant extracts (Oghenejobo et al., 2017; Stark et al., 2015). For instance, Garcinol which is a flavonoid in G. buchananii has antibacterial activity against S. aureus, E. coli, Bacillus subtilis, and Enterobacter aerogenes (Varalakshmi et al., 2010). Curcumin, a phenolic compound in C. longa has been reported to be responsible for its broad antibacterial effects (Oghenejobo et al., 2017; Teow et al., 2016), acting through reducing bacterial membrane integrity resulting in membrane leakages in both Gram-positive and Gram-negative bacteria (Tyagi et al., 2015).
Both C. longa rhizome and G. buchananii stem bark extracts have been reported to have activity against various microbes (Afrose et al., 2015; Moghadamtousi et al., 2014; Wise et al., 1998). In this study, ethanolic extracts of C. longa at a concentration of 100 mg/mL showed sensitivity against S. pneumoniae while its concentrations of 50 and 25 mg/mL showed intermediate antibacterial activity. For G. buchananii, ethanolic extracts of concentration 50 and 100 mg/mL showed sensitivity against S. pneumoniae, while the 25 mg/mL had intermediate activity. S. pneumoniae was sensitive to ceftriaxone (positive control) with a mean diameter of the zone of inhibition of ≥ 23 mm (CLSI, 2013). There was no significant difference (p> 0.05) in the antibacterial activity of ethanolic extracts of C. longa rhizome and G. buchananii stem bark of the same concentrations on S. pneumoniae. Furthermore, the antibacterial effect of the two ethanolic plant extracts on S. pneumoniae was largely dose-dependent. The dose-dependent antibacterial property of C. longa rhizome extracts has been reported in other studies (Izui et al., 2016). There were however no significant differences in activity between G. buchananii concentrations of 50 and 100 mg/mL or C. longa concentrations of 12.5, 25, 50, and 100 mg/mL used in our study. Ceftriaxone 100 mg/mL was more active (p< 0.0001) on S. pneumoniae when compared with all the different ethanolic extracts of C. longa rhizome and G. buchananii stem bark at the different combinations used.
In this study, the MICs for ethanolic extracts C. longa rhizome and G. buchananii stem bark against S. pneumoniae were 3.125 and 1.5625 mg/mL, respectively. In previous studies, the MICs of C. longa ethanolic and methanolic extracts against other micro-organisms such as S. aureus, S. epidermidis, E. coli, and K. pneumoniae, have been reported to be in the range of 0.2 to 16 mg/mL (Niamsa and Sittiwet, 2009; Raji et al., 2018; Wise et al., 1998). Cephalosporins like ceftriaxone and cefotaxime, as well as β-lactams such as penicillin G, have MICs ≤ 2 mg/L on S. pneumoniae strains, and are effective treatments for pneumococcal bacteraemia and pneumonia caused by S. pneumoniae (Kaplan and Mason, 1998). Basing on the MIC ranges obtained in this study and in related studies, the plant extracts of C. longa rhizome and G. buchananii stem bark are potential treatments for infections caused by S. pneumoniae strains such as URTIs, pneumonia, otitis media, and sinusitis.
Synergistic antibacterial activity has been reported for extracts of C. longa when used in combination with various conventional antibiotics such as ampicillin, ciprofloxacin, gentamycin, amikacin, and cefepime among others (Moghadamtousi et al., 2014; Teow et al., 2016). Furthermore, mixtures of various plant extracts are widely used to benefit from potential synergistic or additive effects; however, combining plant extracts can also result in antagonism. Antagonistic effects from combination of plant extracts are reported to be a result of the masking of the active principles by other components in the complex mixture (Caesar and Cech, 2019).
CONCLUSION
In this study, the Fractional Inhibitory Concentration Index (FICI) of the most potent combination of extracts (50% C. longa and 50% G. buchananii) was 11.68. A calculated FICI greater than 2 indicates antagonism (Ofokansi et al., 2012). This indicates that combining C. longa and G. buchananii ethanolic extracts using earlier combination ratio provides no advantage to the antibacterial activity of each of these plant extracts on S. pneumoniae. The use of a broader range of C. longa and G. buchananii ethanolic extracts concentrations ratios is required to confirm this reported antagonism. Variations in concentration ratios of different plant extracts can result in either synergism or antagonism (van Vuuren and Viljoen, 2011).
CONFLICT OF INTERESTS
The authors have not declared any conflict of interests.
ACKNOWLEDGEMENTS
The authors thank the laboratory technical staff at the Department of Pharmacy and Department of Medical Microbiology, Makerere University who provided the technical guidance in carrying out the different procedures and for providing some of the materials including S. pneumoniae ATCC 49619. Special appreciation goes to the Institutional Review Board (IRB) of the School of Health Sciences, Makerere University (MakSHS-IRB) that approved this study.
REFERENCES
|
Afrose R, Saha SK, Banu LA, Ahmed AU, Shahidullah AS, Gani A, Sultana S, Kabir MR, Ali MY (2015). Antibacterial effect of Curcuma longa (Turmeric) against Staphylococcus aureus and Escherichia coli. Mymensingh Medical Journal 24(3):506-515. |
|
|
Baldo V, Cocchio S, Lazzari R, Furlan P, Bertoncello C, Russo F, Baldovin T (2015). Estimated hospitalization rate for diseases attributable to Streptococcus pneumoniae in the Veneto region of north-east Italy. Preventive Medicine Reports 2:27-31. |
|
|
Balemba OB, Bhattarai Y, Stenkamp-Strahm C, Lesakit MSB, Mawe GM (2010). The traditional antidiarrheal remedy, Garcinia buchananii stem bark extract, inhibits propulsive motility and fast synaptic potentials in the guinea pig distal colon. Journal of Neurogastroenterology and Motility 22(12):1332-1339. |
|
|
Borges C, Seabra S, Ponce F, Lima G (2018). Agronomic factors influencing Brassica productivity and phytochemical quality. Brassica germplasm characterization, breeding and utilization. Mohamed AE (Ed). IntechOpen. |
|
|
Caesar LK, Cech NB (2019). Synergy and antagonism in natural product extracts: when 1 + 1 does not equal 2. Natural Product Reports 6(6):869-888. |
|
|
Cherazard R, Epstein M, Doan TL, Salim T, Bharti S, Smith MA (2017). Antimicrobial resistant Streptococcus pneumoniae: Prevalence, mechanisms, and clinical implications. American Journal of Therapeutics 24(3):361-369. |
|
|
CLSI (2013). Performance standards for antimicrobial susceptibility testing; Twenty-Third informational supplement. Pennsylvania, USA: Clinical and Laboratory Standards Institute. |
|
|
Dhanani T, Shah S, Gajbhiye NA, Kumar S (2017). Effect of extraction methods on yield, phytochemical constituents and antioxidant activity of Withania somnifera. Arabian Journal of Chemistry 10:1193-1199. |
|
|
Ekor M (2014). The growing use of herbal medicines: issues relating to adverse reactions and challenges in monitoring safety. Frontiers in pharmacology 4:177-177. |
|
|
El-Kenawy AELM, Hassan SMA, Mohamed AMM, Mohammed HMA (2019). Tumeric or Curcuma longa Linn. Nabavi SM and Silva AS (Eds.). Non-vitamin and Non-mineral Nutritional Supplements Academic Chapter 3; 43:447-453. |
|
|
Evans WC (2009). Trease and Evans Pharmacognosy. Daphine E (Ed.). Saunders Limited, Elsevier, Edinburgh, UK. 16th Edition. Chapter 17:135-147. |
|
|
Feghali P, Ibrahim R, Na'was T (2018). Antibacterial activity of Curcuma longa, Opuntia ficusindica and Linum usitatissimum. MedCrave Online Journal of Toxicology 4(3):214-220. |
|
|
Gurning K (2020). Antimicrobial activity of ethanol extract of rhizome turmeric (Curcuma Longa L.) for growth of Escherichia coli, Staphylococcus aureus and Candida albicans. Asian Journal of Pharmaceutical Research and Development 8(3):5-8. |
|
|
Izui S, Sekine S, Maeda K, Kuboniwa M, Takada A, Amano A, Nagata H (2016). Antibacterial activity of Curcumin against periodontopathic bacteria. Journal of Periodontology 87(1):83-90. |
|
|
Kaplan SL, Mason Jr EO (1998). Management of infections due to antibiotic-resistant Streptococcus pneumoniae. Clinical Microbiology Review 11(4):628-644. |
|
|
Krumkamp R, Schwarz NG, Sarpong N, Loag W, Zeeb H, Adu-Sarkodie Y, May J (2012). Extrapolating respiratory tract infection incidences to a rural area of Ghana using a probability model for hospital attendance. International Journal of Infectious Diseases 16(6):429-435. |
|
|
Kumar M, Kaur P, Garg R, Patil RK, Patil HC (2020). A study on antibacterial property of curcuma longa -herbal and traditional medicine. Adesh University Journal of Medical Sciences and Research 2(2):103-108. |
|
|
Moghadamtousi SZ, Kadir HA, Hassandarvish P, Hassan TH, Abubakar S, Zandi K (2014). A review on antibacterial, antiviral, and antifungal activity of Curcumin. BioMed Research International 2014:186864. |
|
|
Niamsa N, Sittiwet C (2009). Antimicrobial activity of Curcuma longa aqueous extract. Journal of Pharmacology and Toxicology 4(4):173-177. |
|
|
Ofokansi K, Attama A, Uzor P, Mo O (2012). Antibacterial activities of the combined leaf extract of Phyllanthus muellerianus and ciprofloxacin against urogenital isolates of Staphylococcus aureus. Clinical Pharmacology and Biopharmaceutics 1(4):1-5. |
|
|
Oghenejobo M, Opajobi OA, Bethel OUS, Uzoegbu U (2017). Antibacterial evaluation, phytochemical screening and ascorbic acid assay of turmeric (Curcuma longa). MedCrave Online Journal of Bioequivalence and Bioavailability 4(2):232-239. |
|
|
Okullo JBL, Omujal F, Bigirimana C, Isubikalu P, Malinga M, Elias B, Namutebi A, Obaa B, Agea J (2014). Ethno-medicinal uses of selected indigenous fruit trees from the Lake Victoria basin districts in Uganda. Journal of Medicinal Plants Studies 2(1):78-88. |
|
|
Ozioma EO, Okaka A (2019). Herbal medicines in African Traditional Medicine. Herbal Medicine. Intechopen 10:191-214. |
|
|
Raji EFPA, Ibrahim R, Tarek N (2018). Antibacterial activity of Curcuma longa, Opuntia ficus-indica and Linum usitatissimum. MedCrave Online Journal of Toxicology 4(3):214-220. |
|
|
Schobert R, Biersack B (2019). Chemical and biological aspects of Garcinol and Isogarcinol: Recent developments. Chemistry and Biodiversity 16(9):e1900366. |
|
|
Singh N, Gupta S, Rathore V (2017). Comparative antimicrobial study of ethanolic extract of leaf and rhizome of Curcuma longa Linn. Pharmacognosy Journal 9(2):208-212. |
|
|
Soto-Noguerón A, Carnalla-Barajas MN, Solórzano-Santos F, Arrendondo-García JL, Arzate-Barbosa P, Tinoco-Favila JC, Echániz-Aviles G (2016). Streptococcus pneumoniae as cause of infection in infants less than 60 days of age: serotypes and antimicrobial susceptibility. International Journal of Infectious Diseases 42:69-73. |
|
|
Stark TD, Salger M, Frank O, Balemba OB, Wakamatsu J, Hofmann T (2015). Antioxidative compounds from Garcinia buchananii stem bark. Journal of Natural Products 78(2):234-240. |
|
|
Tanvir EM, Hossen S, Hossain F, Afroz R, Gan HS, Khalil I, Karim N (2017). Antioxidant properties of popular Turmeric (Curcuma longa) varieties from Bangladesh. Journal of Food Quality 2017:847-1785. |
|
|
Teow S, Liew K, Ali SA, Khoo AS, Peh S (2016). Antibacterial action of Curcumin against Staphylococcus aureus: A brief review. Journal of Tropical Medicine Article ID 2853045, 10 pages, 2016. |
|
|
Tyagi P, Singh M, Kumari H, Kumari A, Mukhopadhyay K (2015). Bactericidal activity of curcumin I is associated with damaging of bacterial membrane. PloS One 10(3):e0121313. |
|
|
Uganda Clinical Guidelines (UCG) (2016). National guidelines for management of common conditions. Ministry of Health, The Republic of Uganda. |
|
|
Van Vuuren S, Viljoen A (2011). Plant-based antimicrobial studies, methods and approaches to study the interaction between natural products. Planta Medica 77(11):168-1182. |
|
|
Varalakshmi KN, Sangeetha CG, Shabeena AN, Sunitha SR, Vapika J (2010). Antimicrobial and cytotoxic effects of Garcinia indica fruit rind extract. American-Eurasian Journal of Agricultural and Environmental Sciences 7(6):652-656. |
|
|
Wessels E, Schelfaut JJ, Bernards AT, Claas EC (2012). Evaluation of several biochemical and molecular techniques for identification of Streptococcus pneumoniae and Streptococcus pseudopneumoniae and their detection in respiratory samples. Journal of Clinical Microbiology 50(4):1171-1177. |
|
|
WHO (2012). Measuring impact of Streptococcus pneumoniae and Haemophilus influenzae type b conjugate vaccination: Immunisation, vaccination and biologicals. World Health Organisation. Geneva-Switzerland WHO/IVB/12.08. |
|
|
Wise R, Hart T, Cars O, Streulens M, Helmuth R, Huovinen P, Sprenger M (1998). Antimicrobial resistance. Is a major threat to public health. British Medical Journal (Clinical Research Edition) 317(7159):609-610. |
|
Copyright © 2024 Author(s) retain the copyright of this article.
This article is published under the terms of the Creative Commons Attribution License 4.0