ABSTRACT
In developing countries, urban surface waters are particularly affected by faecal pollution from domestic wastewaters due to the lack of sanitation and wastewater treatment plants. The presence of pathogenic microorganisms limits the uses of these waters for recreation and economic activities. In Ivory Coast, due to the important gap between water demand and water supply in urban areas, use of surface waters for the production of drinking waters becomes a serious alternative. Actually, there is no monitoring program to control pollution discharges into these surface waters. In this study, a monitoring study was planned from September 2015 to February 2017 in order to evaluate the level of faecal pollution of the Aghien lagoon, a potential drinking water supply located in Abidjan. Based on the enumeration of faecal indicator bacteria (Escherichia coli and Intestinal enterococci), microbiological water quality from Aghien Lagoon and its tributaries were evaluated. Abundance of faecal indicators ranged between 1.72 × 101 CFU.100 ml-1 and 1.48 × 102 CFU.100 mL-1 for E. coli and between 2.26 × 103 CFU.100 mL-1 and 7.72 × 103 CFU.100 mL-1 for IE in lagoon waters. The abundances of FIB observed in tributaries were higher than those observed in lagoon water. The tributaries comparison indicates that, the Djibi River is the most contaminated with an average value of 1.73 × 106 CFU.100 mL-1 for IE and 6.92 × 105 CFU.100 mL-1 E. coli mL. The contributions of tributaries in terms of faecal bacteria discharged into the Aghien lagoon are not negligible and these contributions are significantly different between the dry and rainy season. Therefore, lagoon water may be a potential drinking water supply if wastewater treatment plants are implemented in the Djibi and Bété basins.
Key words: Water quality, faecal bacteria, drinking water supply, tropical lagoon.
In urban area, population growth combined with urbanization poses a serious problem in relation to drinking water supply. This situation is particularly pronounced for urban area in developing countries.
Jacobsen et al. (2013), in a report of the World Bank Organization entitled “The Future of Water in African Cities: Why Waste Water?”, indicates that there is an important gap between water demand and water supply.
Water demand is increasing at a higher rate than the population growth. Whereas, water availability is shrinking due to the competing demands from agriculture, mining activities, industry deterioration of water quality and climate change. For a long time, ground waters were a major source of drinking water production in developing countries due to their relatively low cost of treatment and their high quantities. The increase of population in urban area is accompanied by a reduction of the quantity of these reservoirs due to pressure. Today, freshwaters are emerging as alternatives for the production of drinking water in developing countries.
To guarantee an access to drinking waters for the population of Abidjan (422 Km², 6 million inhabitants) in ten or twenty next years, the authorities are exploring the potential of lagoon water to serve as reservoir for drinking water production: the Aghien Lagoon. However, the most important problems which limit the use of this lagoon water are its quantity for long term uses and its quality due to industrial, domestic and agriculture pollutions (Traoré et al., 2012; Koffi et al., 2014). It is well known that surface waters from urban area are exposed to different types of pollutions including physicochemical and microbiological parameters (viruses, bacteria, protozoa and helminths) (Ouattara et al., 2011; Passerat et al., 2011; Anyona et al., 2014; Pandey 2014; Marcheggiani et al., 2015 ). These pollutions may result from industrialization and poor wastewater management strategies (Gigliola et al., 2012; Páll et al., 2013; García-Armisen et al., 2014). The main consequence is that, waterborne diseases that cause mortality of population are difficult to prevent or to control. An example of the absence of surface water management program is the presence of faecal pathogenic microorganisms (bacteria, viruses and protozoa) in these tropical waters (Lu et al., 2016; Nshimyimana et al., 2013; Vincy et al., 2017). The detection and enumeration of all these pathogenic microorganisms potentially present is impossible due to the large diversity of the pathogens, low abundance of each species and absence of standardized and low-cost methods for the detection of each of them. Thus, for routine monitoring, Faecal Indicator Bacteria (FIB) is usually enumerated to evaluate the level of microbial water contamination (Ouattara et al., 2011, Boehm et al., 2014). Escherichia coli and intestinal enteroccoci are considered as the best Faecal Indicator Bacteria to predict the sanitary risk associated with freshwaters (Edberg et al., 2000; Passerat et al., 2011).
A short review of literature showed that these indicators are used around the world to evaluate the microbiological water quality of surface waters (European Union Directive, 2006; Griffin et al., 2000). Even if the E. coli and intestinal enteroccoci are adopted in temperate area, there is a reasonable doubt concerning their use in tropical waters. In a short review focused on fecal indicators in tropical ecosystem, Rochelle-Newall et al. (2015) highlighted the fact that the fecal indicator bacteria chosen are sometimes applied to tropical systems without taking into account the potential specificities of the tropics such as higher temperature and humidity, differences in nutrient and organic matter availability and higher solar irradiation levels.
The objectives of our research are to evaluate the impact of wastewaters discharged in the Aghien Lagoon and its tributaries using E. coli and intestinal enterococci as faecal indicator bacteria and to determine the contribution of each tributary by mean of the quantification of microbial fluxes.
Study area
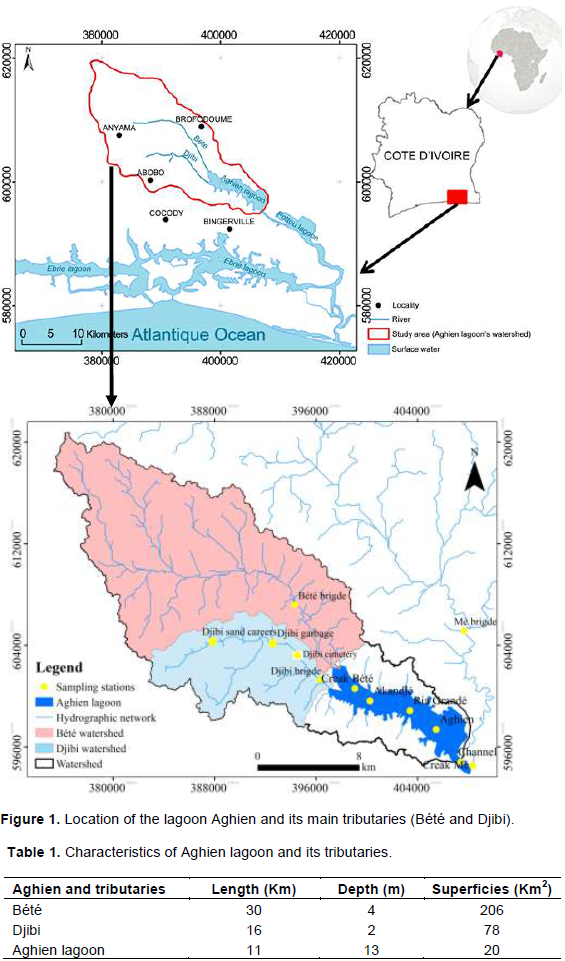
The study area is Aghien Basin located in South east of Ivory Coast (Figure 1). The Aghien watershed is composed of two basins: The Bété Basin (68%), Djibi Basin (26%) and an area covered by lagoon water (6%). The Djibi Basin and the Bété Basin are characterized by urbanization and agriculture (more than 60% of these basins) (Table 1). The water capacity of Aghien Lagoon is estimated to be 25 Km3 with a maximum depth of 13 m (Effebi et al. 2017; Koffi et al., 2014). The lagoon receives water from Bété and Djibi rivers before joining the Mé River in the downstream part of the lagoon. Some other small tributaries located in the Aghien watershed are diverted in the Bété and Djibi rivers so that their waters reach Aghien Lagoon. The Bété River and Djibi River receive domestic sewage from Anyama, Abobo, Brofodoume municipalities and villages located in the watershed without any treatment.
Environmental water sampling and processing
During the monitoring survey conducted in the scope of this study, water samples were collected in the Aghien Lagoon and its tributaries. Twelve sites were investigated from September 2014 to February 2017 (Figure 1); a total of ten sampling campaigns were thus performed. During these campaigns, water samples were collected in the lagoon (6 stations) and its tributaries (6 stations) with a sterile plastic bucket from bridges, halfway between the banks. Samples were stored in 1 L sterile bottles. All the bottles were labeled with the source name, date and time of collection of the samples. The bottles were transported to the laboratory, kept at 4°C and processed within a maximum of 2 h after collection for microbiological analysis.
Physical and chemical parameters
Nutrients, temperature, pH and dissolved oxygen are some of the important factors that play a vital role in the growth of microorganisms in the water body (Qureshimatva et al., 2015). Their importance on the evaluation of water quality affected by sewage waters is well described by Errich et al. (2016). The flow rate is used to calculate the fluxes of faecal bacteria discharged by different tributaries in order to estimate their contribution in terms of faecal pollution. In the scope of this study, three parameters were measured in situ during the whole study period. Temperature, dissolved oxygen and pH were measured using multi-parameter HACH HQ40D, according to standardized protocols of Rodier et al. (2009).
Water height and water flow of tributaries (Djibi, bété and Mé) were measured monthly by the Hydrology Department of Nangui Abrogoua University (Data collected from 2014 to 2017) in order to establish a standard curve (relationship water height-flow rate) for these tributaries. During the study period, water heights of tributaries were measured. The flow rate values of tributaries were calculated using this calibration curve. Results of physic and chemical parameters were expressed as maximum, average and minimum (Table 2).
E. coli and IE enumeration by plate count technique
E. coli and IE were enumerated in water samples by standard plate counts on TBX (E. coli) and Slanetz and Bartley agar (Bio-Rad Laboratories, Inc.). These two chromogenic growth media were shown to be highly specific to their corresponding indicator bacteria (ISO 7899-2 (08/2000) and ISO 16649-2:2001). These high levels of specificity were confirmed on samples from Aghien Lagoon and the three River samples at the beginning of the present study. Slanetz and Bartley supplemented with TTC (0. 2%) plates were incubated at 36°C for 24 h then at 44°C for 2 h before enumeration. TBX plates were incubated at 44°C for 24 h. Plate counts were expressed as colony forming units (CFU) per 100 mL of sample. The protocols used to detect E. coli (TBX agar) and IE (Slanetz and Bartley agar) are well described by Vergine et al. (2016) and Tiwari et al. (2018), respectively.
Contribution of tributaries in the microbial pollution of the lagoon
As presented in Figure 1, Aghien Lagoon received water from two main tributaries. In order to determine the contribution of the main tributary in the microbial pollution of the lagoon waters, the values of flow rate (l.h-1) was multiplied by the values of the abundance of faecal bacteria (CFU.l-1) observed in these tributaries during dry season. Then, a comparison was performed between the values of fluxes (CFU h-1) injected by each tributaries. Then, a statistical test (student’s t- test) was performed to determine the significance degree of these differences.
Statistical analysis
In this study, all data were subjected to descriptive statistical analysis (95% confidence limit). Statistical tool R was used to determine the variance, average, standard errors and ranges. Student test (t-test) was used to test differences among the sampling sites.
Physical and chemical characteristics of Aghien basin
A summary of the physical and chemical characteristics of the lagoon Aghien and its tributaries is presented in Table 1. Table 2 indicates that pH values vary between 6.6 and 7.2 in the tributaries and between 7.7 and 9.6 in the lagoon. Temperature value varies between 25 and 28°C for Aghien Lagoon, Bété and Mé rivers. High values of temperature were observed in the Djibi River (33°C). Dissolved oxygen values were around 65-90% for Aghien Lagoon, Bété and Mé Rivers. The values of dissolved oxygen observed in the Djibi River were particularly low (less than 10% along the river). The water flow rates expressed as m³.h-1 in the Table 2 were lower in the main tributaries (from 0.60 to 4.95 m³.h-1). The relatively low values were observed in the Djibi River. The most important values of water flow rates were observed in the Mé River located in the outlet of the Aghien Lagoon (Figure 1). For each of them, the high values were observed during the high rainy seasons and the low values were observed in the high dry season. A significant difference was observed between the high rainy seasons compared to the high dry seasons.
E. coli and IE in Aghien Lagoon and its tributaries
Abundance of E. coli and EI in water of Aghien Lagoon
All sampling sites (Channel, Akandje, Ria Grande and Aghien) were located in the proximity of small villages around the lagoon. Abundance of faecal bacteria in the lagoon varied between 1.72 × 101 CFU.100 mL-1 and 1.48 × 102 CFU.100 mL-1 for E. coli and between 2.26 × 103 CFU.100 mL-1 and 7.72 × 103 CFU.100 mL-1 for Intestinal enterococci (Figure 2). Higher abundances of faecal indicators were observed in the Channel site for E. coli and Akandjé site for Intestinal enterococci. Particularly, abundances of Intestinal enterococci were higher than those of E. coli at the entire sampling sites.
Level of E. coli and EI in the main tributaries of Aghien Lagoon
Three main tributaries received water from one sub- basin. Average abundance of faecal bacteria in the main tributaries varied between 3.18 × 102 and 8.22 × 103 for E. coli and between 8.30 × 103 and 1.41 × 104 for Intestinal enterococci (Figure 3). At the entire sampling sites, there were more abundances of Intestinal enterococci than E. coli. For both indicators, their abundances were significantly higher in Djibi River compared to that of Bété River and Mé River (p value <0.01).
Level of E. coli and EI in the Djibi River
The results showed that there were more abundances of faecal indicator observed in the Djibi River than in the other tributaries (Figure 4). To better appreciate why there was abundance of FIB determined in Djibi Basin than those in Bété and Mé basins, specific monitoring program was performed in the Djibi Basin. Results of abundances of E. coli and intestinal enterococci are presented in Figure 4. There were abundances of FIB at “Djibi cemetery” site than in the others sites of Djibi River. With an average value of 1.73 × 106 CFU.100 mL-1 for IE and 6.92 × 105 CFU.100 mL-1 E. coli, faecal bacteria was significantly abundant than that of the three other sites (p value <0.01).
The presence and abundance of faecal bacteria in the lagoon water clearly showed that sewage waters are drained into the Aghien Lagoon. The abundance of E. coli in the lagoon is lower than that recommended for recreational activities (US EPA, 1986; Havelaar et al., 2001; EU, 2006). The abundance of intestinal enterococci observed in lagoon is higher than that recommended by international guideline for microbiological water quality. International guideline recommended use of E. coli and Enterococci as indicators of faecal contamination of recreational waters even if the quality standards can vary from one country to another one terms of abundances. However, these guidelines do not indicate if it is enough to consider acceptable water quality when one of two criteria is not followed.
Levels of faecal contamination observed in the Aghien Basin are quite similar to those of surface waters encountered in several cities in Africa. For example, Musyoki et al. (2013) who carried out a study in Nairobi River, which crosses Kenyan capital city, Nairobi and its tributary (Athi River) showed that the abundances of faecal indicator bacteria in the waters of the rivers were 1.0 × 104 CFU.100 mL-1 for E. coli and 3.6 × 103 CFU.100 mL-1 for Enterococcus faecalis). Sibanda et al. (2013) also assessed the distribution of faecal-indicator bacteria in Tyume River in the Eastern Cape Province, South Africa. Faecal coliform (including E. coli) counts ranged from 1.0 × 102 to 1.6 × 104 CFU.100 mL-1 while enterococci counts were in the range of 3.3 × 101 CFU.100 mL-1 to 5.1 × 103 CFU.100 mL-1. High levels of faecal indicator bacteria were also observed in the Buffalo River (Chigor et al., 2013) and in the Apies River (Ekwanzala et al., 2017) where the abundance of Enterococci reached the concentration of 105 CFU.100 mL-1.
Based on the data analysis of E. coli and intestinal enterococci abundances observed in surface waters from many urban areas, it appears that their abundances are relatively low compared to the values observed in the outlet of wastewater treatment plants in Europe (Ouattara et al., 2014). This is very surprising when we consider the lack of sanitation systems in most cities in developing countries. We also observed that in most of the sampling sites, abundances of IE were higher than those of E. coli particularly in Aghien Lagoon and its tributaries. From literature, E. coli abundance measured in the waters (wastewaters and surface waters) is most of the time higher than that of intestinal enterococci. This result is reported by Ouattara et al. (2011, 2014) in wastewaters in Belgium. In surface water, several authors also showed that the abundance of E. coli is higher than that of IE. For example, Passerat et al. (2011) observed in Seine River that the abundance of E. coli (1.5E+06 (100 mL)-1) was three fold higher than that of IE (4.0E+05 (100 mL)-1). Indeed, good correlation between both indicators (E. coli and intestinal enterococci) has been demonstrated (Farnleitner et al., 2010; Ouattara et al., 2014). But in this study, due to the fact that sometimes IE abundance was higher than that of E. coli , the correlation found is not very good (R² = 0.6, n = 40). A possible response to the poor correlation observed in the Aghien Basin Lagoon and its tributaries is that they received wastewaters from septic tanks overfilling. After a long period of transition in these septic tanks, sewage water spilled from septic tank. And then, these waters are drained into lagoon and its tributaries. This fact combined to the high resistance of IE compare to E. coli in surface waters may explain this poor correlation between E. coli and IE.
In the main tributaries, abundances of faecal indicator bacteria are higher than those observed in the Aghien Lagoon. These tributaries are more impacted by faecal pollution than the Aghien Lagoon. Among these tributaries, Djibi River is much more affected by faecal pollution than the others tributaries. The lower levels of dissolved oxygen and higher abundance of faecal bacteria indicated that, Djibi River is strongly impacted by sewage water. Faecal pollution is particularly pronounced in Djibi River because of its low flow rate. In Bété River and Mé River, their relatively high flow rates contribute to reduce the impact of the faecal pollution (dilution effect). When comparing the contribution of tributaries which impacted the Aghien Lagoon, we observed that during the dry season, the fluxes of faecal bacteria injected by Djibi River in the lagoon are smaller than those of Bété River. During the rainy season, the fluxes of faecal bacteria discharged by Djibi River are in the same order of magnitude with those of the Bété River. At the same time, the student’s test performed to evaluate the contribution of the rivers indicated that there is no significant difference between Djibi River and Bété River (p value > 0.5).
Globally, the abundances of intestinal enterococci were higher than the acceptable level for bathing, recreational activities or drinking water, indicating that the water of Aghien Lagoon is impacted by domestic sewage waters. Among the tributaries, Djibi River presented the higher levels of faecal bacteria and low levels of dissolved oxygen, indicating that this tributary is much more affected by wastewater pollution compared to Mé River and the Bété River. The contributions of tributaries in terms of faecal bacteria discharged into the Aghien Lagoon are not negligible and these contributions are significantly different between the dry and the rainy season. In order to preserve the lagoon water quality and to promote its potential uses for bathing, irrigation or drinking water production, the implementation of wastewaters treatment plants in Djibi Basin and Bété Basin is recommended.
The authors have not declared any conflict of interests.
This research project is funded by the AMRUGE-C2D programme 2015-2017 of the Ivoirian Ministry of Higher Education and Scientific Research and by the French Institut de Recherche pour le Développement (IRD).
REFERENCES
|
Anyona DN, Dida GO, Abuom PO, Onyuka JO, Matano AS, Kanangire CK, Ofulla AVO (2014). Influence of anthropogenic activities on microbial and nutrient levels along the Mara River tributaries, Kenya. EurAsian Journal of BioSciences 8: 1-11.
Crossref
|
|
|
|
Boehm AB, Sassoubré LM (2014). Enterococci as Indicators of Environmental Fecal Contamination: In: Gilmore MS, Clewell DB, Ike Y, et al., editors. Enterococci: From Commensals to Leading Causes of Drug Resistant Infection. Boston: Massachusetts Eye and Ear Infirmary pp. 1-17.
|
|
|
|
|
Chigor VN, Sibanda T, Okoh AI (2013). Studies on the bacteriological qualities of the Buffalo River and three source water dams along its course in the Eastern Cape Province of South Africa. Environmental Science Pollution Research 20:4125-4136.
Crossref
|
|
|
|
|
Edberg SC, Rice EW, Karlin RJ, Allen MJ (2000). Escherichia coli: the best biological drinking water indicator for public health protection. Journal of Applied Microbiology 88:106S-116S.
Crossref
|
|
|
|
|
Effebi KR, Jeanne NY, Noufé DD, Diallo S, Armand TBZ, Fabrice N, Karoui H, Kamagaté B, Goné DL, Perrin JL, Séguis L (2017). Activities and uses of Aghien Lagoon (South-East of Côte d'Ivoire). Journal of Water Resource and Protection 9:11-19.
Crossref
|
|
|
|
|
Ekwanzala MD, Abia ALK, Ubomba‑Jaswa E, Keshri J, Momba NBM (2017). Genetic relatedness of faecal coliforms and enterococci bacteria isolated from water and sediments of the Apies River, Gauteng, South Africa. AMB Express 7(20):1-10.
Crossref
|
|
|
|
|
Errich A, El Hajjaji S, Mandi L, Fekhaoui M, Rezzouki B, Jodeh S, Azzaoui K, Lamhamdi A, Hamed O, Salghi R, Hasan AR, Hanbali G (2016). Impact of Waste Water on The Physico-Chemical Quality of Water Sources In Bed of Oued Essaquia Elhamra In South of Morocco. International Journal of Application or Innovation in Engineering and Management 5(9):249-261.
|
|
|
|
|
European Union Directive (2006). Directive 2006/7/EC of the European Parliament and of the Council of 15 February 2006 concerning the management of bathing water quality. Official Journal of the European Union 64:37-51.
|
|
|
|
|
Farnleitner AH, Ryzinska-Paier G, Reischer GH, Burtscher MM, Knetsch S, Kirschner AKT, Dirnböck T, Kuschnig G, Mach RL, Sommer R (2011). Escherichia coli and enterococci are sensitive and reliable indicators for human, livestock and wildlife faecal pollution in alpine mountainous water resources. Journal of Applied Microbiology 109(5):1599-1608.
|
|
|
|
|
García-Armisen T, InceoÄŸlu Ó¦, Ouattara NK, Anzil A, Verbanck MA, Brion N, Servais P, (2014). Seasonal Variations and Resilience of Bacterial Communities in a Sewage Polluted Urban River. PLoS ONE 9(3): e92579.
Crossref
|
|
|
|
|
Gigliola RB, Salloto AMC, Felipe HC, Leonardo HP, Ricardo PV, Catia C, Joyce LL, Rodolpho MA, Orlando BM, Maysa MC (2012). Pollution impacts on bacterioplankton diversity in a tropical urban coastal lagoon system. PLoS ONE 7(11):e51175.
Crossref
|
|
|
|
|
Griffin DW, Stokes R, Rose JB, Paul III JH (2000). Bacterial indicator occurrence and the use of an F+ specific RNA coliphage assay to identify fecal sources in Homosassa springs, Florida. Microbial Ecology 39:56-64.
Crossref
|
|
|
|
|
Havelaar A, Blumenthal UJ, Strauss M, Kay D, Bartram J (2001). Guidelines: the current position; World Health Organization (WHO). Water Quality: Guidelines, Standards and Health. Edited by Lorna Fewtrell and Jamie Bartram. Published by IWA Publishing, London.
|
|
|
|
|
Jacobsen M, Webster M, Vairavamoorthy K (2013). The Future of Water in African Cities - Why Waste Water? The World Bank Organiation 226 p.
|
|
|
|
|
Koffi KJP, N'Go YA, Yeo KM, Koné D, Savané I (2014). Détermination des périmètres de protection de la lagune Aghien par le calcul du temps de transfert de l'eau jusqu'à la lagune. Larhyss Journal 19:19-35.
|
|
|
|
|
Lu J, Struewing I, Vereen E, Kirby AE, Levy K, Moe C, Ashbolt N (2016). Molecular Detection of Legionella spp. and their associations with Mycobacterium spp., Pseudomonas aeruginosa and amoeba hosts in a drinking water distribution system. Journal of Applied Microbiology 120(2):509-21.
Crossref
|
|
|
|
|
Marcheggiani S, D'Ugo E, Puccinelli C, Giuseppetti R, D'Angelo AM, Gualerzi CO, Spurio R, Medlin LK, Guillebault D, Weigel W, Helmi K, Mancini L (2015). Detection of emerging and re-emerging pathogens in surface waters close to an urban area. International Journal of Environmental Research and Public Health 12:5505-5527.
Crossref
|
|
|
|
|
Musyoki AM, Suleiman MA, Mbithi JN, Maingi JM (2013). Water-borne bacterial pathogens in surface waters of Nairobi river and health implication to communities downstream athi river. International Journal of Life Science and Pharma Research 3(1):4-10.
|
|
|
|
|
Nshimyimana JP, Ekklesia E, Shanahan P, Chua LHC, Thompson JR (2013). Distribution and abundance of human-specific Bacteroides and relation to traditional indicators in an urban tropical catchment. Journal of Applied Microbiology 116:1369-1383.
Crossref
|
|
|
|
|
Ouattara KN, Passerat J, Servais P (2011). Faecal contamination of the water and sediment in the rivers of the Scheldt drainage network. Environmental. Monitoring. Assessment 183:243-257.
Crossref
|
|
|
|
|
Ouattara NK, García-Armisen T, Anzil A, Brion N, Servais P (2014). Impact of Wastewater Release on the Faecal Contamination of a Small Urban River: The Zenne River in Brussels (Belgium). Water Air and Soil Pollution 225:2043-2054.
Crossref
|
|
|
|
|
Páll E, Niculae M, Kiss T, Åžandru CD, Spînu M (2013). Human impact on the microbiological water quality of the rivers. Journal of Medical Microbiology 62:1635-1640.
Crossref
|
|
|
|
|
Pandey PK, Kass PH, Soupir ML, Sagor B, Singh VP (2014). Contamination of water resources by pathogenic Bacteria. AMB Express 4:51-66.
Crossref
|
|
|
|
|
Passerat J, Ouattara NK, Mouchel JM, Rocher V, Servais P (2011). Impact of an intense combined sewer overflow event on the microbiological water quality of the Seine River. Water Research 45: 893-903.
Crossref
|
|
|
|
|
Qureshimatva UM, Maurya RR, Gamit SB, Patel RD, Solanki HA (2015). Determination of physico-chemical parameters and water quality index (Wqi) of Chandlodia Lake, Ahmedabad, Gujarat, India. Journal of Environmental and Analytical Toxicology 5:288.
|
|
|
|
|
Rochelle-Newall E, Nguyen TMH, Le TPQ, Sengtaheuanghoung O, Ribolzi O, (2015). A short review of fecal indicator bacteria in tropical aquatic ecosystems: Knowledge gaps and future directions. Frontiers in Microbiology 6:308-322.
Crossref
|
|
|
|
|
Rodier J, Legube B, Merlet N (2009). The Analysis of the Water. 9th Edition, Dunod, Paris, 1203.
|
|
|
|
|
Sibanda T, Chigor VN, Okoh AI (2013). Seasonal and spatio-temporal distribution of faecal-indicator bacteria in Tyume River in the Eastern Cape Province, South Africa. Environmental Monitoring and Assessment 185:6579-6590.
Crossref
|
|
|
|
|
Tiwari A, Hokajärvi AM, Santo Domingo, JW, Kauppinen A, Elk M, Ryu H, Jayaprakash B, Pitkänen T (2018). Categorical performance characteristics of method ISO 7899-2 and indicator value of intestinal enterococci for bathing water quality monitoring. Journal of Water and Health 16(5):711-723.
Crossref
|
|
|
|
|
Traoré A, Soro G, Kouadio EK, Bamba BS, Oga MS, Soro N, Biémi J (2012). Evaluation des paramètres physiques, chimiques et bactériologiques des eaux d'une lagune tropicale en période d'étiage: la lagune Aghien (Côte d'Ivoire). International Journal of Biological and Chemical Sciences 6(6):7048-7058.
|
|
|
|
|
USEPA (U.S. Environmental Protection Agency) (1986). Ambient Water Quality Criteria for Bacteria – 1986. Bacteriological Ambient Water Quality Criteria for Marine and Fresh Recreational Waters; Office of Research and Development, Microbiology and Toxicology Div., Cincinnati Ohio and Office of Water Regulations and Standards, Criteria and Standards Division, Washington, D.C. (1986) EPA-440/5-84-002.
|
|
|
|
|
Vergine P, Salerno C, Barca E, Berardi G, Pollice A (2016). Identification of the faecal indicator Escherichia coli in wastewater through the β-D-glucuronidase activity: comparison between two enumeration methods, membrane filtration with TBX agar, and Colilert®-18. Journal of Water and Health 15 (2):209-217.
Crossref
|
|
|
|
|
Vincy MV, Brilliant R, Pradeepkumar AP (2017). Prevalence of indicator and pathogenic bacteria in a tropical river of Western Ghats, India. Applied Water Science 7:833-844.
Crossref
|
|