ABSTRACT
The current practice of using stanchion breeding cow houses results in a semi-dry manure that ideally should be used as the substrate for the anaerobic fermentation. The conventional liquid-slurry biogas fermentation is disadvantageous with high running costs due to requirement such as water addition and running efficiency, among others. This study aimed to establish a high-speed semi-dry methane fermentation system for cow manure, by comparing productivity in wet and semi-dry conditions (> 90 and 83-85% water content, respectively), and mesophilic and thermophilic conditions (38 and 55°C, respectively). The highest methane productivity was obtained for 30 days in semi-dry or wet, thermophilic conditions (0.4-16.2 L‧Kg-1VS‧day-1). The highest accumulated production was 60 days (192.1 L‧Kg-1VS) in semi-dry thermophilic conditions. The pronounced difference in methane production at different temperatures in semi-dry digesters was studied by characterizing the microbial community changes in mesophilic and thermophilic digesters. For bacteria, the class Clostridia (including family Clostridiaceae and the genus Clostridium) was dominant in the thermophilic digester. In the thermophilic digester, a succession of taxa from the genus Methanobrevibacter and family Methanobacteriaceae to genus Methanoculleus in archaea was observed. The convergence of archaeal microbiota in thermophilic conditions was more pronounced than in mesophilic conditions. These results indicate that the proposed procedure is capable of improving methane productivity per scale and lowering the running costs concomitant and downsizing the fermentation scale.
Key words: Semi-dry fermentation, methane yield, anaerobic digestion, cow manure, biogas.
Excessive utilization of fossil fuels induces environmental effects such as air pollution from carbon dioxide emission and decreases the energy resources on earth. Utilization of renewable energy carriers is currently receiving increasing attention to prevent future problems. Biogas production by methane- producing microorganisms using biomass or organic wastes is a promising strategy for renewable energy generation (Daniels, 1992; Angelidaki and Ellegaard 2003; Weiland 2003, 2010; Santosh et al., 2004; Yadvika et al., 2004). Biogas production occurs in natural ecological reactions in the rumen of cows (Ellis et al., 2007), rice paddy fields (Schütz et al., 1990), and wetlands (Segers, 1998). Thus, biogas production is an ecologically friendly procedure for energy production. The residues from biogas production can be utilized as fertilizers for agricultural crops (Horváth et al., 2016), which can reduce the use of fertilizers produced from petroleum.
Organic materials are degraded under anaerobic conditions resulting in biogas production (Kothari et al., 2014; Moset et al., 2015; Sun et al., 2015; Zhang et al., 2016). Blended raw materials with highly complex molecules are gradually degraded into the final substances, methane and CO2, by the action of microbial communities including varieties of eubacteria and archaea influenced by environmental factors such as temperature, water content, pH and substrate composition (Wirth et al., 2012). Therefore, it is important to understand the variation in microbial communities and their functions in response to changes in environmental factors to create a stable biological process.
Based on reported data, use of thermophilic conditions (≥ 45°C) in biogas production has several advantages over mesophilic conditions (25-40°C) with higher rates of organic matter degradation (Goberna et al., 2010) and annihilation of pathogenic mesophiles (Sahlström, 2003; Johansen et al. 2013). In addition to this, improved rates in thermophilic biogas production was compared with that of mesophilic fermentation (Zábranská et al., 2000; Ahn and Foster 2002; Yu et al., 2014; Moset et al., 2015) and the fluidity of solid fermentation sludge was found to be higher under thermophilic conditions than under mesophilic conditions. This difference results in the advantage that thermophilic-temperature fermentation is easily stirred (Brambilla et al., 2013; Grim et al., 2015). However, the rapid organization of the thermophilic microflora at start-up and the difficulty of maintaining an adequate thermophilic microflora at high fecal and urine loadings are major drawbacks in thermophilic operation compared to mesophilic operation (Fernandez et al., 2012; Labatut et al., 2014; Guo et al., 2014). Moreover, regardless of the reaction efficiency advantages in a thermophilic digester, mesophilic biogas production is still recommended with animal manure, because of the greater process robustness (Labatut et al., 2014).
Almost all biogas plants in Hokkaido, Japan operate using liquid slurry formed from the faeces and urine mixture which contains more than 90% water drained from free-stall (free-range) cows as the raw material. However, approximately 70% of all
dairy farmhouses in Hokkaido use stanchion-fixed breeding (stall barn) and the exhausted faeces and urine have less than 85% water content in the straw-rich semisolids. Introduction of liquid-slurry biogas plants in stanchion-fixed breeding farmhouses has not gained favor because of the increased volume of raw materials diluted by water and running costs concomitant with the addition of water and heating for methane fermentation when using semisolid faeces and urine. Therefore, procedures that use faeces and urine with less than 85% water content, derived from stanchion-fixed breeding, are desirable to facilitate biogas (methane) production, using the waste in its original form with a high organic loading rate (Pavan et al., 2000; Jha et al., 2013). This procedure will improve on the suitable management of waste, and the formation of a low-carbon and sustainable society, which has lower CO
2 emissions than procedures requiring high water content.
Previous authors have reported the functional analysis of microbiota (Ariesyady et al., 2007) and a trial of deification with core microorganisms (Rivière et al., 2009) in an anaerobic digester, in wet (> 90% water content) mesophilic conditions. In addition, intensive analyses of microbiota, over a year, took place in 20 different mesophilic digesters and showed the existence of different stable core microorganisms (Calusinska et al., 2018). Furthermore, a comparative study of transitional changes of microbiota in mesophilic versus thermophilic in wet anaerobic digesters was performed (Moset et al., 2015). In this study, methane productivity was compared between wet and semi-dry anaerobic biogas diesters under thermophilic and mesophilic conditions. In addition, to clarify the basis in the difference in methane productivity, the succession of microbiota changes in thermophilic digesters, in terms of different total solid (TS) contents, were analysed. Thus, this can contribute to establish a promising procedure for methane production, which will be suitable for treating semisolid faeces and urine drained from a stanchion breeding cow house.
Raw materials for the digester
Dairy manure was obtained from the cow house of a dairy farmer in Shihoro-cho, Hokkaido, Japan (43°10’N 143°15’E). The seed culture for the mesophilic temperature digester (40°C) was obtained from a large-scale biogas plant operated at a mesophilic temperature in Shihoro-cho, Hokkaido. The seed culture for the thermophilic-temperature digester was obtained from a large-scale biogas plant operated at a thermophilic temperature (55°C) in Ashoro-cho, Hokkaido, Japan (43°20’N, 143°32’E). Seed cultures for both mesophilic and thermophilic temperature digesters were derived from wet fermentation (>90% water content). The organisation of the biogas digester setup and their sample analyses are summarized in Table 1.
Small-scale biogas digester setup
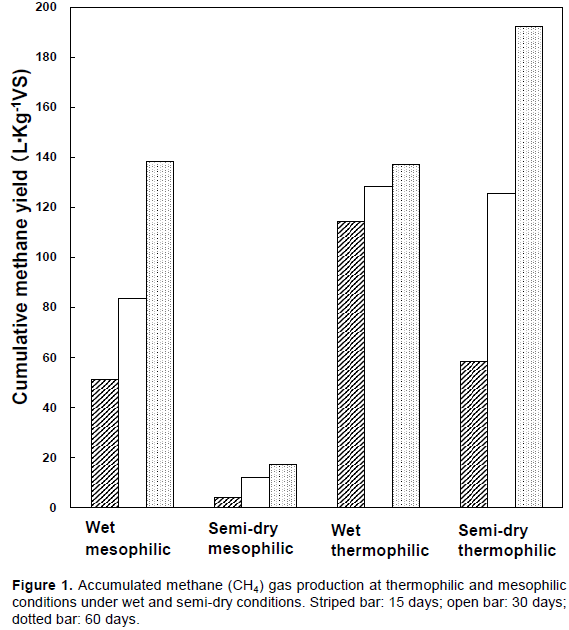
Anaerobic small-scale digester biogas production experiments were performed in 2 L Erlenmeyer flasks with a working volume of 1 L (dairy cattle manure: seed culture = 8:2). The initial pH in the four reactors was 7.3-7.5. The initial total solids (TS) values in the dry and wet fermentation digesters were 15.3-17.0% and 9.9-11.3%, respectively. The initial volatile solids (VS) values in dry and wet fermentation digesters were 14.1-15.2% and 8.7-10.2%, respectively (Figure 2). Anaerobic conditions were produced by substituting the air in the flask with N2 gas and sealing by a rubber stopper with a gas outlet connected to 5-L Tedlar bags (Omi Odor Air Service, Shiga, Japan) (Zhao et al., 2014). The experiments were performed with two different water contents, > 90% as the wet condition and 83-85% as the semi-dry condition (Abbassi-Guendouz et al., 2012); and at two temperatures, 38°C as the mesophilic condition and 55°C as the thermophilic condition (Shi et al., 2013). The temperature of the digesters was maintained using a water bath connected to a constant temperature controller; incubated in a walk-in incubator for 38°C as the mesophilic condition and 55°C as the thermophilic condition. Samples were withdrawn quickly from the flasks and were stirred by shaking the flask manually every 24 h. Sampling for DNA extraction used for microbiota analysis was performed from one fixed digester. All tests were run in triplicate digesters for 60 days.
Analytical methods
The yield of methane was determined using a gas chromatograph (GC-8A, Shimadzu, Kyoto, Japan) with a thermal conductivity detector equipped with a glass column molecular sieve 13X (3 m × 3.2 mm ID, GL Sciences, Tokyo, Japan) at 190°C; helium was the carrier gas. The amount of methane produced from each digester was estimated by multiplying the gas quantity in the gas pack, measured by a wet gas meter (WA-1A, Sinagawa Co., Tokyo, Japan), with the concentration of methane in the collected gas analysed by the gas chromatograph. All samples were collected in triplicate and averages are given; total solid (TS), volatile solids (VS) and pH were measured in accordance with the standard methods (APHA, 2005).
DNA extraction, polymerase chain reaction (PCR) and clone library construction
Sampling was performed four times in 30 days for the mesophilic temperature digester and sampling for the thermophilic temperature digester was performed five times in 60 days. In addition, samples of the thermophilic- and mesophilic- temperature reactions and the original manure were obtained for analysing the microbiota. For a culture-independent approach based on a 16S rRNA gene library, DNA was directly extracted from aliquots of samples frozen at -80°C, using ISOIL (Nippon Gene, Tokyo, Japan) according to the manufacturer’s instructions. The universal primers 27F (5′-AGAGTTTGATCMTGGCTCAG-3′) (Lane 1991; Sipos et al., 2007) and 926R (5′-CCGTCAATTCMTTTRAGTTT-3′) (Després et al., 2007; Weisburg et al., 1991) were used to amplify the bacterial 16S rRNA gene. The primers used for amplifying the archaeal 16S rRNA gene were Ar109F (5′-ACKGCTCAGTAACACGT-3′) (Großkopf et al. 1998) and Ar912R (5′-CTCCCCCGCCAATTCCTTTA-3′) (Lueders and Friedrich 2000). PCR was performed in 100 μl including 10 μl of 10 × PCR buffer, 8 μl of 2.5 mM dNTP mix, 100 ng of isolated DNA, 5 U of Ex Taq DNA polymerase (TaKaRa Bio Inc., Otsu, Japan) and 20 pmol of each primer. PCR was performed under the following conditions: 95°C for 3 min followed by 25 (for bacteria) or 28 cycles (for archaea) at 94°C for 30 s, 51°C (for bacteria) or 52°C (for archaea) for 30 s, and at 72°C for 90 s (for bacteria) or 52°C for 30 s (for archaea). The PCR products were purified using the QIAquick PCR Purification Kit (Qiagen, Manchester, UK) according to the manufacturer’s instructions. The PCR products for the 16S rRNA clone library were cloned in Escherichia coli DH5a using the pT7Blue-2 vector system (Novagen, Madison, WI, USA) according to the manufacturer’s instruction. Approximately 30 randomly selected clones were examined for the correct insert size using vector-targeted PCR followed by gel electrophoresis.
DNA sequencing and sequence assignment
DNA sequences were determined by the dideoxy chain termination method using a BigDye Terminator Cycle Sequence Kit (Applied Biosystem, Foster City, CA, USA) and an automated DNA sequencer (ABI Prism 3100 Genetic Analyzer, Applied Biosystems). The sequence assignments were determined by a BLAST search. Clone sequences that exhibited higher than 95 and 90% similarities with the closest sequence in the database were identified at the genus and family levels, respectively. The clone sequences that exhibited less than 90% similarity with the closest sequence were identified at the phylum level. However, one clone belonging to an unclassified family was identified at the order level. The sequences presented in this study were deposited in the DNA Data Bank of Japan (DDBJ) database under DDBJ/EMBL/GenBank accession numbers LC473176-LC473425 and LC473747-LC473425 for archaea and bacteria, respectively.
Statistical analysis of the clone libraries
The sequences derived from the 16S rRNA gene clone library were processed using the software “Quantitative Insight into Microbial Ecology” (QIIME) version 1.9.1 (Caporaso et al., 2010). Operational taxonomic units (OTUs) were selected based on 97% sequence similarity using the UCLAST algorithm (Edger, 2010). To evaluate coverage, rarefaction curves were generated based on the observed OTU metrics using QIIME. A three-dimensional principal coordinate analysis (PCoA) plot was generated using EMPeror software (Vázquez-Baeza et al., 2013) and was processed using QIIME.
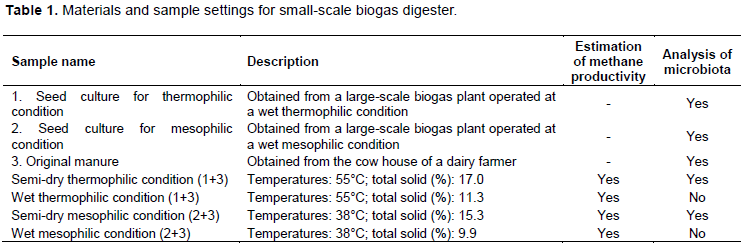
Methane gas productivity and changes in pH, TS and VS
Methane productivity was examined with raw manure material with different water contents, > 90% as wet conditions or 82–85% as semi-dry conditions and at different fermentation temperatures, 38°C as mesophilic temperature and 55°C as thermophilic temperature. The cumulative methane yields over 15, 30, and 60 days are presented in Figure 1. Poor methane production (0–0.68 L‧Kg-1VS‧day-1) was observed under semi-dry mesophilic conditions, but methane productivity was relatively high over 30 days in both semi-dry and wet thermophilic conditions (0.4-16.2 L‧Kg-1VS‧day-1). For wet digestion at 38°C, a high methane production (3.3-9.0 L‧Kg-1VS‧day-1) was observed for the first 5 days, production decreased from days 10 to 35 (1.7-2.8 L‧Kg-1VS‧day-1). However, from days 36 to 42, methane production increased to 3.5-4.6 L‧Kg-1 VS‧day-1 (data not shown). This may be for the appearance of microorganisms that could utilize the residual substrates. It should be noted that the methane production rate within the initial 15 days in the semi-dry conditions was lower than in the wet conditions. This difference relates to the delayed consumption of acid produced by bacteria in the mesophilic semi-dry digester (Figure 2A). The consortia of microorganisms in the seed culture took time to adapt to the semi-dry thermophilic conditions. However, there was a minor difference in the accumulated methane production mass between wet and semi-dry thermophilic conditions at 30 days. The wet thermophilic conditions resulted in the highest accumulated methane production (128.5 L‧Kg-1VS) until the 30th day concomitant with TS and VS decreasing to 4.7 and 3.7%, respectively (Figures 1, 2). However, the accumulated methane production was the highest in the semi-dry thermophilic conditions on the 60th day (192.1 L‧Kg-1VS) concomitant with TS and VS decreased to 6.4 and 6.8%, respectively (Figures 1, 2). The lowest accumulated methane production at 60 days was observed in the semi-dry mesophilic conditions (17.3 L‧ Kg-1VS). However, in contrast, equivalent amounts of methane were produced in the wet thermophilic conditions (137.2 L‧Kg-1VS) concomitant with TS and VS decreased to 6.5 and 5.5%, respectively and the wet mesophilic conditions (138.3 L‧Kg-1VS) concomitant with TS and VS decreased to 4.3 and 4.2%, respectively until the 60th day (Figures 1 and 2). The methane production in wet thermophilic digester was the highest within the first 20 days (data not shown). This indicates the fastest adaptation of microbiota in the trials was probably using seed culture, derived from the wet digester. On the 30th day, the semi-dry thermophilic digester was equivalent to wet thermophilic digester (Figure 1). The use of seed cultures derived from semi-dry thermophilic conditions may further improve methanogenesis during the first 20 days of semi-dry thermophilic fermentation. These results indicate that semi-dry thermophilic conditions are advantageous considering of the smaller bioprocess is favorable over large volume, and almost equivalent production rates in wet thermophilic digester are possible. The pH in the semi-dry mesophilic digester decreased within 3 days and continuously decreased until 5 days (pH 5.6); thereafter, the pH remained low (pH 5.3-5.6) until the 60th day (Figure 2A). In the wet mesophilic digester, acid consumption occurred after the pH decreased. The VS contents decreased under all the conditions which produced apparent production of methane (Figure 2C). The decrease was the greatest in the semi-dry thermophilic conditions, which may be related to the velocity of digestion of solid matters.
Analysis of microbial communities in the seed cultures and the manure used as the starting material
To understand the microbial community of the original raw manure material and seed cultures; the microbiota of the manure, and the mesophilic- and thermophilic-temperature seed cultures derived from large-scale biogas tanks, were analysed using constructed clone libraries (data not shown). The bacterial community of the raw original manure was predominated by the phylum Firmicutes (35.3%) and the family Ruminococcaceae (23.5%) followed by the phylum Bacteroidetes (11.8%). The archaeal community of the raw manure was predominated by the genera Methanobrevibacter (47.1%) and Methanocorpusculum (29.4%), followed by the family Methanocorpusculaceae (17.6%). The bacterial community of the seed culture from the thermophilic digester was predominated by the phylum Firmicutes (55.6%), followed by the family Halanaerobiaceae (11.2%) and the phylum Spirochaetes (11.2%), whereas the archaeal community was predominated by the genus Methanoculleus (91.3%), followed by the family Methanomicrobiaceae (8.7%). In seed the culture for mesophilic digesters, the bacterial community was predominated by the phyla Firmicutes (36.4%) and Bacteroidetes (27.3%), followed by family Clostridiaceae (9.1%), whereas the archaeal community was predominated by the genus Methanoculleus (71.4%) followed by the phylum Euryarchaeota (9.5%). The archaeal communities (involving 2-6 taxa in the classification in this study) were simpler than the bacterial communities (involving 7-9 taxa) across all these materials. In addition, the microbiotas in the seed culture for thermophilic digesters (involving two taxa) were the simplest of those among the three samples in both archaeal and bacterial communities. This could be because of the convergence of archaeal microbiota adapted to the high temperature digester in long-term where the seed culture was obtained.
Analysis of microbial community
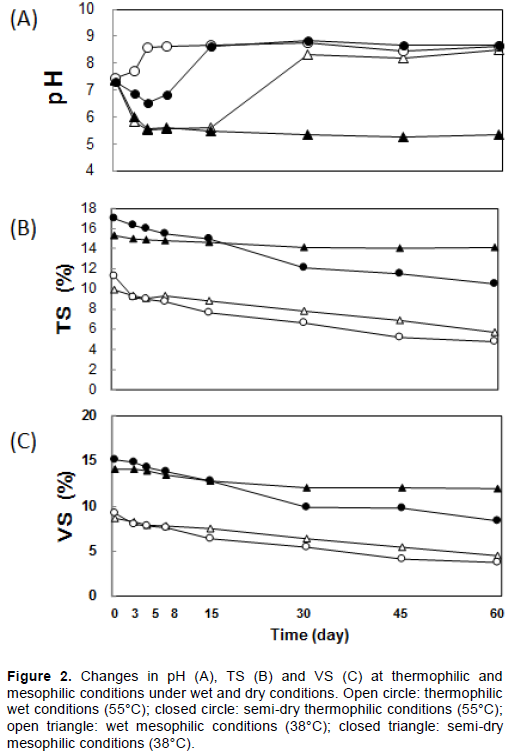
In the analyses of bacteria in the thermophilic condition, taxa involving the class Clostridia (34.8%) and phylum Bacteroidetes (21.7%) were dominant at initiation (0 day) (Figure 3A). The manure used in this trial was the main material in the fermentation, but the microbiota in the prepared digesters differed from each other. The predominant taxa on day 3 were like those on day 0, the relative abundance of phylum Bacteroidetes decreased by 5.9%, but the predominant taxon in the thermophilic digester was still class Clostridia (29.4%) (Figure 3A). By the 15th day, both class Clostridia and phylum Bacteroidetes showed decreased proportions in the microbiota and exhibited a slight increase on the 60th day. Although the genus Clostridium and class Clostridia predominated on the 7th day, their proportions were decreased on the 15th day. The methane production rate was not high on the 15th day, as the pH decrease in the mesophilic dry digester was faster than the thermophilic semi-dry wet digester and pH recovery, to neutral pH, did not occur in the mesophilic dry digester. In the mesophilic semi-dry conditions, the fluidity of the content may be lowest among the four conditions. Therefore, metabolites such as organic acids of the microorganisms tend localised. In addition, circulation of substances in the flask was stacked. Regarding mesophilic condition, the phyla Firmicutes (44.4%) and Bacteroidetes (27.8%) were predominantly observed at 0 days (Figure 4A). The phylum Bacteroidetes proportion (including class Bacteroidia and family Bacteroidaceae) was predominant throughout the observation period (Figure 3A and 4A). The phylum Bacteroidetes ratio in the mesophilic condition was higher than the thermophilic digester. Although the class Clostridia was observed on the 5th and 15th days, the class Clostridia (including genus Clostridium) ratio to the phylum Bacteroidetes was lower than the thermophilic temperature digester. It is considered that the microbiota in the mesophilic semi-dry conditions may reflect stacked substance circulation in the flask.
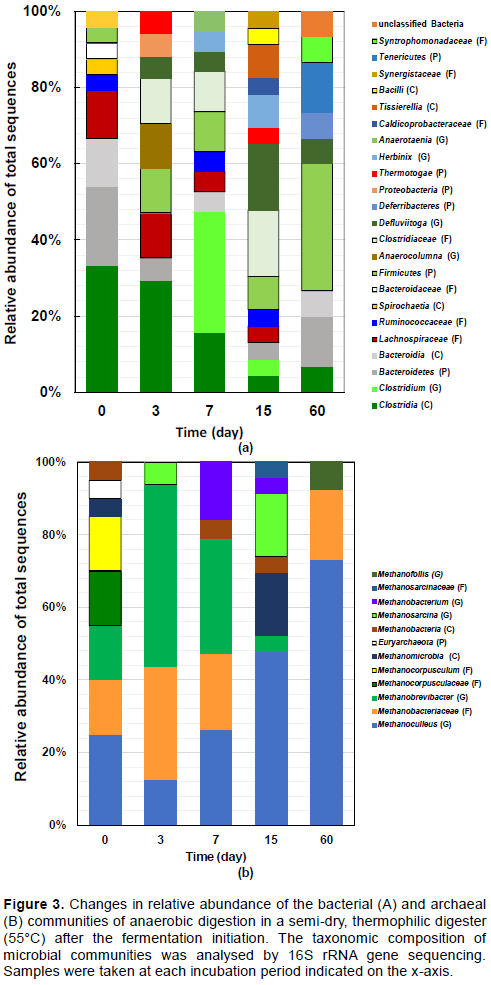
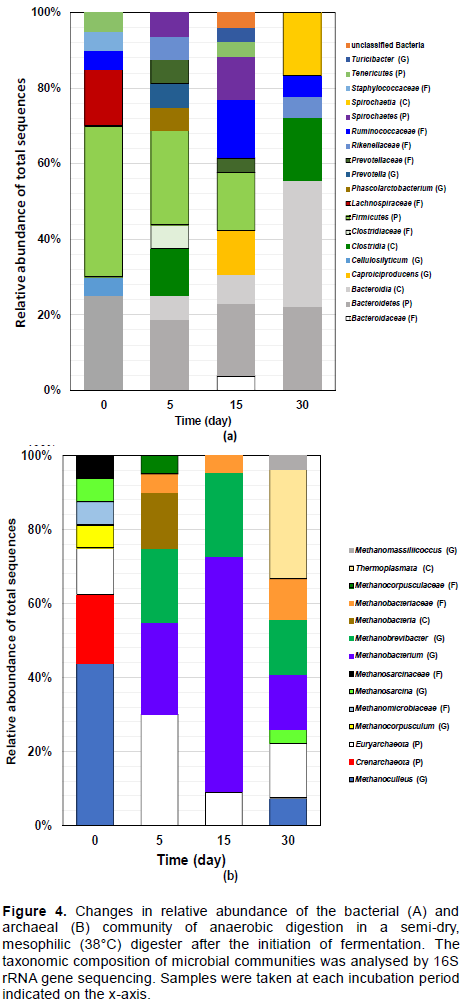
In the analyses of the archaea, genus Methanobrevibacter and family Methanobacteriaceae were predominantly observed until the 7th day, after which the proportion of these taxa decreased in the thermophilic digester (Figure 3B). From the 7th day, the relative abundance of genus Methanoculleus increased with the length of the incubation period; this continued until the 60th day. In the mesophilic digester, the genera Methanobrevibacter and Methanobacterium and the phylum Euryarcheota were the major taxa, and their proportion peaked on the 15th day (Figure 4B). However, the microbiota that was simplified on the 15th day became complicated on the 30th day. Still, the relative abundance of the genera Methanobrevibacter and Methanobacterium remained at 15% on the 30th day. This may exhibit that the archaeal community is more prone to convergence than the bacteria at elevated temperatures.
To observe the changes in the microbiota’s diversity, rarefaction curves were computed for the sequences and observed species based on the observed OTUs (Figure 5). There was little difference in diversity in both bacteria and archaea, regardless of the operational condition. Bacterial diversities (14.2±0.5 observed species/15 seqs; p=0.025) were always higher than archaeal diversities (10.0±1.2 observed species /15 seqs; p=0.025). In the thermophilic digester, although slightly higher diversity of bacteria was observed on the 7th day (14.6 observed species/15 seqs) than the other days (14.1 observed species/15 seqs), the overall diversity of bacteria was not significantly different. In contrast, the diversity of archaea in the thermophilic digester decreased with the length of the incubation period. In the mesophilic condition, although the bacterial diversity was slightly higher on the 5th day (15.0 observed species/15 seqs) than on the other days (14.3 observed species/15 seqs), it did not fluctuate significantly across the sampling periods. However, the archaeal diversity in the mesophilic digester increased with the length of the incubation period. Moreover, bacterial diversity in the mesophilic digester was almost the same as that in the thermophilic digester.
To observe the intensity of changes in the microbiota, an unweighted PCoA was performed for samples from thermophilic (Figure 6) and mesophilic conditions (Figure 7). It is considered that appropriate changing velocity in microbiota is significant for a proper initiation and endurance for methane production. In addition, there might be appropriate relative changing velocities between archaeal and bacterial communities. The rate of the archaeal community changed faster in the thermophilic digester than the mesophilic digester in the early phase (from day 0 to 3 and 5). The changing rate of microbiota in archaea was faster than the bacteria in both thermophilic and mesophilic conditions. Thus, low methane production rate in the mesophilic semi-dry digester is because of stagnation of an appropriate change in microbiota. However, appropriate circulation of substances in the system is important for a high methane production.
Due to small and effective bioprocess favourability, the capability of semi-dry anaerobic digestion was examined. Initiation of methane production depended on the recovery of a neutral pH at the early phase of the fermentation. The methane production rate and yield were the worst in the semi-dry mesophilic conditions among the four sets of anaerobic digesters. This poor performance was attributed to the long exposure period, to acidic conditions, of the archaeal microbiota (pH 5.3-5.6). Vigorous methane production occurred in the pH range of 8.2-8.9 in this experiment. The pH of the contents of anaerobic digestion for biogas production was 7.0-8.6 (Abouelenien et al., 2014; Stolze et al., 2015; Sun et al., 2015). Obvious acid consumption was finally observed in wet mesophilic conditions but not in semi-dry, mesophilic conditions. However, inhibition of methane production in semi-dry mesophilic conditions might be averted by adjusting the pH to neutral before excessive decrease of pH. In another study, methane production in semi-dry mesophilic conditions was comparable to wet mesophilic conditions (Jha et al., 2013). This study differs from the present study because of the possible differences in physicochemical conditions or physiological state of microorganisms in the digesters. In contrast, semi-dry mesophilic conditions for methane production have been reported to result in a long-term start-up phase (Chiumenti et al., 2018) or a poor start-up performance (Jha et al., 2011). A factor for promoting methane production in mesophilic semi-dry condition could be through the stagnation of the substances circulation within the system i.e., the consumption of the acid produced by the bacteria.
To determine the suitability of the semi-dry thermophilic conditions over the semi-dry mesophilic conditions, we analysed the microbiota in semi-dry thermophilic conditions (Figure 6A). The genus Methanoculleus (hydrogenotroph) increased lineally from day 3 (12.5%) to day 60, with a relative abundance of 73.1% at day 60. The genus Methanosarcina (Acetotroph) was observed at days 3 (6.3%) and 15 (17.4%). In a previous study, under wet (TS = 5%) thermophilic (50°C) conditions, although the most predominant taxon was the genus Methanosarcina (approximately 14% of the phylum Euryachaeota community), the genus Methanoculleus was present at approximately 4% after 20 days incubation (Moset et al., 2015). In contrast, succession from genus Methanosarucina to genus Methanothermobacter (hydrogenotroph) or from genus Methnaothermobacter to genus Methanocullues, within 30 days was observed in a solid-state thermophilic digester (Lin et al., 2017). Comparing the succession of thermophilic methanogenic microbiota in various conditions, they changed from acetogenic methanogens such as genus Methanosarcina or hydrogenotrophic methanogen such as genus Methanobrevibacter and genus Methnaothermobacter to hydrogenotrophic methanogens such as genus Methanocullues.
In this study, the family Methanosarcinaceae and genus Methanobrevibacte was predominantly observed in the semi-dry mesophilic conditions and the simple microbiota on the 15th day became complex by the 30th day. It is considered that this complication was because of an occurrence of taxa adapted to the decreased pH. A higher predominance of Methanoculleus marisnigri (4.5% in total microbial communities) in biogas fermentation in semi-dry mesophilic conditions (semi-dry matter content = 14% ± 2%; 40°C) than in wet mesophilic conditions has been reported previously (Wirth et al., 2012). In another study, under dry mesophilic conditions (≥20% TS; 37°C), almost all sequences were Methanosarcina thermophila-related methanogen (96.4-99.1%) as observed after 200 days of operation (Cho et al., 2013). In previous reports (Wirth et al., 2012; Cho et al. 2013), the succession of methanogenic microbiota in dry or semi-dry mesophilic conditions have not been reported. Therefore, the transitional change from initiation of fermentation to a stable state is not known.
In this study, according to bacterial analyses of the microbiota, the class Clostridia (including the family Clostridiaceae and the genus Clostridium) was the predominant observed bacteria in semi-dry thermophilic digester. Previous biogas production studies have reported the dominance of the class Clostridia in semi-dry mesophilic (37°C) operation (Wirth et al., 2012) and in the operation at 55°C (Rademacher et al., 2012). Moset et al. (2015) reported that, although the actual constituting taxa were different, the class Clostridia was dominant in both mesophilic and thermophilic wet conditions. In contrast, although the phylum Bacteroidetes (including the class Bacteroidia) peaked at 20% in semi-dry thermophilic conditions, a higher proportion of the taxon was observed (55.5% at maximum) in dry mesophilic conditions in this study. The phylum Bacteroidetes (including the class Bacteroidia) has often been observed in anaerobic biogas producing systems at both thermophilic and mesophilic conditions (Sun et al., 2015; Stolze et al., 2015). Thus, the high proportion of the phylum Bacteroidetes is not the reason for low biogas production in semi-dry mesophilic conditions. Bacteria belonging to class Clostridia and phylum Bacteroidetes could contribute to the hydrolysis of macromolecules and the production of volatile fatty acids and organic acids. These products will be used by methanogenic archaea for methane production via acetate, CO2 and H2 (Wirth et al., 2012). The decrease in pH, because of acid production, followed by concomitant acid consumption did not occur in the semi-dry mesophilic conditions. This may be because the high viscosity of the semi-dry mesophilic environment causes a local change, which causes stack of material cycling in the entire system.
Although the water content in manure is unknown, a previous study reported that the microbial community was relatively stable over 200 days, regardless of the operating temperature (37, 44 and 52°C) in semi-continuous stirred anaerobic digestor tanks (Sun et al., 2015). However, the microbial community fluctuated within 30 and 60 days in this study using a batch digester. This difference may be attributed to the operation procedure (semi-continuous or batch digester) and the operational period. Thus, the creation of a stable state over a long period will be possible by adjusting preparation procedure and appropriate management for the process, including the mass of the fermenter.
When the methane yield, in the semi-dry fermentation at mesophilic and thermophilic temperatures was compared, the PCoA plot for mesophilic condition showed the archaea in the microbiota changed faster than the bacteria. However, the acid produced by bacteria such as Clostridium spp. (Wirth et al., 2012) was faster than the appearance of the appropriate archaeal microbiota. However, under thermophilic condition, the appropriate archaeal microbiota for acid consumption appeared before the pH decreased to unfavourable levels for methane production. Deference in the velocity of microbiota changes in both bacterial and archaeal between the semi-dry thermophilic and semi-dry mesophilic conditions was not significant except at the fermentation initiation. The difference in pH recovery at the beginning of fermentation may be because of the differences in the physiological state induced by different microorganisms under different conditions. Thus, despite the increased cost for the energy required to maintain the high temperature (heating and cooling will be required in small and large scales, respectively), thermophilic anaerobic digestion is easier than operation under mesophilic condition for the semi-dry fermentation.
The semi-dry thermophilic conditions can be advantageous for methane productivity per scale, while lowering the running costs can assist with downsizing fermentation scale. In addition, less space is required, because of relatively condensed manure as the substrate for fermentation, and a decreased need to adjust the fermentation conditions.
The authors have not declared any conflict of interests.
The authors appreciate Ms. A Nishioka and Ms. S Nakagawa for providing technical assistance. They are also grateful to the New Energy and Industrial Technology Development Organization for funding the research.
REFERENCES
|
Abbassi-Guendouz A, Brockmann D, Trably E, Dumas C, Delgenès JP, Steyer JP, Escudié R (2012). Total solids content drives high solid anaerobic digestion via mass transfer limitation. Bioresource Technology 111:55-61.
Crossref
|
|
|
|
Abouelenien F, Namba Y, Kosseva MR, Nishi N, Nakashimada Y (2014). Enhancement of methane production from co-digestion of chicken manure with agricultural wastes. Bioresource Technology 159:80-87.
Crossref
|
|
|
|
|
Ahn J-H, Forster CF (2002). The effect of temperature variations on the performance of mesophilic and thermophilic anaerobic filters treating simulated papermill wastewater. Process Biochemistry 37(6):589-594.
Crossref
|
|
|
|
|
Angelidaki I, Ellegaard L (2003). Codigestion of manure and organic wastes in centralized biogas plants: Status and future trends. Applied Biochemistry and Biotechnology 109(1-3):95-105.
Crossref
|
|
|
|
|
APHA (2005) Standard Methods for Examination of Water and Wastewater, 21st edn. Greenberg AE, Clesceri LS, Eation AD (eds). Washington, DC, USA, American Public Health Association.
|
|
|
|
|
Ariesyady HD, Ito T, Okabe S (2007). Functional bacterial and archaeal community structures of major trophic groups in a full-scale anaerobic sludge digester. Water Research 41(7):1554-1568.
Crossref
|
|
|
|
|
Brambilla M, Romano E, Cutini M, Pari L, Bisaglia C (2013). Rheological properties of manure/biomass mixtures and pumping strategies to improve ingestate formulation: A review. American Society Agricultural Biologic Engineers 56(5):1905-1920.
Crossref
|
|
|
|
|
Calusinska M, Goux X, Fossépé M, Muller EEL, Wilmes P, Delfosse P (2018). A year of monitoring 20 mesophilic full-scale bioreactors reveals the existence of stable but different core microbiomes in bio-waste and wastewater anaerobic digestion system. Biotechnology for Biofuels 11:196.
Crossref
|
|
|
|
|
Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, Costello EK, Fierer N, Pena AG, Goodrich JK, Gordon JI, Huttley GA (2010). QIIME allows analysis of high-throughput community sequencing data. Nature Methods 7(5):335-336.
Crossref
|
|
|
|
|
Chiumenti A, de Borso F, Limina S (2018). Semi-dry anaerobic digestion of cow manure and agricultural products in a full-scale plant: Efficiency and comparison with wet fermentation. Waste Management 71:704-710.
Crossref
|
|
|
|
|
Cho S-K, Im D-H, Kim M-H, Shin H-S, Oh S-E (2013). Dry anaerobic digestion of food waste under mesophilic condition: performance and methanogenic community analysis. Bioresource Technology 131:210-217.
Crossref
|
|
|
|
|
Daniels L (1992). Biotechnological potential of methanogens. Biochemical Society Symposium 58:181-193.
|
|
|
|
|
Després VR, Nowoisky JF, Klose M, Conrad R, Andreae MO, Pöschl U (2007). Characterization of primary biogenic aerosol particles in fragment analysis of ribosomal RNA gene. Biogeosciences 4:1127-1141.
Crossref
|
|
|
|
|
Edger RC (2010). Search and clustering orders of magnitude faster than BLAST. Bioinformatics 26(19):2460-2461.
Crossref
|
|
|
|
|
Ellis JL, Kebreab E, Odongo N E, McBride BW, Okine EK, France J (2007). Prediction of methane production from dairy and beef cattle. Journal of Dairy Science 90(7):3456-3467.
Crossref
|
|
|
|
|
Fernandez Rodriguez J, Perez, M, Romero LI (2012). Mesophilic anaerobic digestion of organic fraction of municipal solid waste: Optimization of the semicontinuous process. Chemical Engineering Journal 193-194:10-15.
Crossref
|
|
|
|
|
Goberna M, Gadermaier M, Garcia C, Wett B, Insam H (2010). Adaptation of methanogenic communities to the cofermentation of cattle excreta and olive mill wastes at 37°C and 55°C. Applied Environmental Microbiology 76(19):6564-6571.
Crossref
|
|
|
|
|
Großkopf R, Janssen PH, Liesack W (1998). Diversity and structure of the methanogenic community in anoxic rice paddy soil microcosms as examined by cultivation and direst 16S rRNA gene sequence retrieval. Applied Environmental Microbiology 64(3):960-969.
Crossref
|
|
|
|
|
Grim J, Malmros P, Schnürer A, Nordberg Å (2015). Comparison of pasteurization and integrated thermophilic sanitation at a full-scale biogas plant - heat demand and biogas production. Energy 79:419-427.
Crossref
|
|
|
|
|
Guo X, Wang C, Sun F, Shu W, Wu W (2014). A comparison of microbial characteristics between the thermophilic and mesophilic anaerobic digesters exposed to elevated food waste loading. Bioresource Technology 152:420-428.
Crossref
|
|
|
|
|
Horváth IS, Tabatabaei M, Karimi K, Kumar R (2016). Recent updates on biogas production - A review. Biofuel Research Journal 10:394-402.
Crossref
|
|
|
|
|
Jha AK, Li J, Nies L, Zhang L (2011). Research advances in semi-dry anaerobic digestion process of solid organic wastes. African Journal Biotechnology 10(64):14242-14253.
Crossref
|
|
|
|
|
Jha AK, Li J, Zhang L, Ban Q, Jin Y (2013). Comparison between wet and semi-dry anaerobic digestions of cow dung under mesophilic and thermophilic conditions. Advances in Water Resource and Protection 1(2):28-38.
|
|
|
|
|
Johansen A, Nielsen HB, Hansen CM, Andreasen C, Carlsgart J, Hauggard-Nielsen H, Roepostorff A (2013). Survival of weed seeds and animal parasites as affected by anaerobic digestion at meso- and thermophilic conditions. Waste Management 33(4):807-812.
Crossref
|
|
|
|
|
Kothari R, Pandey AK, Kumar S, Tyagi VV, Tyagi SK (2014). Different aspects of semi-dry anaerobic digestion for bio-energy: An overview. Renewable and Sustainable Energy Review 39:174-195.
Crossref
|
|
|
|
|
Labatut RA, Angenet LT, Scott NR (2014). Conventional mesophilic vs. thermophilic anaerobic digestion: a trade-off between performance and stability? Water Research 53:249-258.
Crossref
|
|
|
|
|
Lane DJ (1991). 16S/23S rRNA sequencing. In: Stackebrandt E, Goodfellow M (eds) Nucleic acid techniques in bacterial systematics. Wiley, New York, pp. 115-147.
|
|
|
|
|
Lin L, Yu Z, Li Y (2017). Sequential batch thermophilic solid-state anaerobic digestion of lignocellulosic biomass via recirculation digestate as inoculum - Part II: Microbial diversity and succession. Bioresource Technology 241:1027-1035.
Crossref
|
|
|
|
|
Lueders T, Friedrich M (2000). Archaeal population dynamics during sequential reduction processes in rice field soil. Applied Environmental Microbiology 66(7):2732-2742.
Crossref
|
|
|
|
|
Moset V, Poulsen M, Wahid R, Højberg O, Møller HB (2015). Mesophilic versus thermophilic anaerobic digestion of cattle manure: Methane productivity and microbial ecology. Microbial Biotechnology 8(5):787-800.
Crossref
|
|
|
|
|
Pavan P, Battistoni P, Mata-Alvarez J, Cecchi F (2000). Performance of thermophilic semi-semi-dry anaerobic digestion process changing the feed biodegradability. Water Science and Technology 41(3):75-81.
Crossref
|
|
|
|
|
Rademacher A, Nolte C, Schönberg M, Klocke M (2012). Temperature increases from 55 to 75°C in a two-phase biogas digester result in fundamental alterations within the bacterial and archaeal community structure. Applied Microbiol Biotechnology 96(2):565-76.
Crossref
|
|
|
|
|
Rivière D, Desvignes V, Pelletier E, Chaussonnerie S, Guermazi, S, Weissenbach, J, Li T, Camacho P, Sghir A (2009). Towards the definition of a core of microorganisms involved in anaerobic digestion of sludge. The ISME Journal 3:700-714.
Crossref
|
|
|
|
|
Santosh Y, Sreekrishnan TR, Kohli S, Rana V (2004). Enhancement of biogas production from solid substrates using different techniques - A review. Bioresource Technology 95(1):1-10.
Crossref
|
|
|
|
|
Sahlström L (2003). A review of survival of pathogenic bacteria in organic waste using in biogas plants. Bioresource Technology 87(2):161-166.
Crossref
|
|
|
|
|
Schütz H, Seiler W, Conrad R (1990). Influence of soil temperature on methane emission from rice paddy fields. Biogeochemistry 11(2):77-95.
Crossref
|
|
|
|
|
Segers R (1998). Methane production and methane consumption: A review of processes underlying wetland methane fluxes. Biogeochemistry 41(1):23-51.
|
|
|
|
|
Sipos R, Szekely AJ, Palatinszky M, Revesz S, Marialigeti K, Nikolausz M (2007). Effect of primer mismatch, annealing temperature and PCR cycle number on 16S rRNA gene-targetting bacterial community analysis. FEMS Microbial Ecology 60(2):341-350.
Crossref
|
|
|
|
|
Shi J, Wang Z, Stiverson JA, Yu Z, Li Y (2013). Reactor performance and microbial community dynamics during solid-state anaerobic digestion of corn stover at mesophilic and thermophilic conditions. Bioresource Technology 136:574-581.
Crossref
|
|
|
|
|
Stolze Y, Zakrzewski M, Maus I, Eikmeyer F, Jaenicke S, Rottmann N, Siebner C, Pühler A, Schlüter A (2015). Comparative metagenomics of biogas-producing microbial communities from production-scale biogas plants operating under wet or semi-dry fermentation conditions. Biotechnolology for Biofuels 8(1):14.
Crossref
|
|
|
|
|
Sun L, Pope PB, Eijsink VGH, Schnürer A (2015). Characterization of microbial community structure during continuous anaerobic digestion of straw and cow manure. Microbial Biotechnology 8(5):815-827.
Crossref
|
|
|
|
|
Vázquez-Baeza Y, Pirrung M, Gonzalez A, Knight R (2013). EMPeror: A tool for visualizing high-throughput microbial community data. GigaScience 2(1):16.
Crossref
|
|
|
|
|
Weiland P (2003). Production and energetic use of biogas from energy crops and wastes in Germany. Applied Biochemistry Biotechnology 109(1-3):263-274.
Crossref
|
|
|
|
|
Weiland P (2010). Biogas production: Current state and perspectives. Applied Microbiology Biotechnology 85(4):849-860.
Crossref
|
|
|
|
|
Weisburg WG, Baens, SM, Pelletier DA, Lane DJ (1991). 16S ribosomal DNA amplification or phylogenetic study. Journal Bacteriology 173(2):697-703.
Crossref
|
|
|
|
|
Wirth R, Kovács E, Maróti G, Bagi Z, Rákhely G, Kovács KL (2012). Characterization of a biogas-producing microbial community by short-read next generation DNA sequencing. Biotechnology for Biofuels 5:41.
Crossref
|
|
|
|
|
Yadvika S, Sreekrishan TR, Kohli S, Rana V (2004). Enhancement of biogas production from solid substrates using different techniques - A review. Bioresource Technology 95(1):1-10.
Crossref
|
|
|
|
|
Yu D, Kruola, JM, Lähde K, Kymäläinen M, Shinkkonen A, Romantschuk M (2014). Biogas production and methanogenic archaeal community in mesophilic and thermophilic anaerobic co-digestion processes. Journal of Environmental Management 143:54-50.
Crossref
|
|
|
|
|
Zábranská J, ŠtÄ›pová J, Wachtl R, JeníÄek P, Dohányos M (2000). The activity of anaerobic biomass in thermophilic and mesophilic digesters at different loading rates. Water Science Technology 42(9):49-56.
Crossref
|
|
|
|
|
Zhang B, Zhao H. Yu H, Chen D, Li X, Wang W., Riao R, Cui Z (2016). Evaluation of biogas production performance and archaeal microbial dynamics of corn straw during anaerobic co-digestion with cattle manure liquid. Journal of Microbiology and Biotechnology 26(4):739-747.
Crossref
|
|
|
|
|
Zhao J, Ge X, Vasco-Correa J, Li Y (2014). Fungal pretreatment of unsterilized yard trimmings for enhanced methane production by solid-state anaerobic digestion. Bioresource Technology 158(4):248-252.
Crossref
|
|