ABSTRACT
The aim of this study is to analyze the microbiological profile of ventilator associated pneumonia (VAP) in relation to nasal swabs and prevalence of multi-drug resistant bacteria so as to implement effective treatment and prevention strategies in NICU (Neonatal Intensive Care Unit). One hundred neonates were ventilated for more than 48 h and met the inclusion criteria which were enrolled in the study. All cases were subjected to history taking, thorough clinical examination, Indications of mechanical ventilation, sputum and nasal swabs culture and sensitivity by conventional microbiological methods. As regard to positive sputum culture results, the rate of Klebsiella pneumoniae was 16.7% in cases with early onset VAP while it is 39.4% in cases with late onset VAP, Aceintobacter baumanni, Pseudomonas aeruginosa and combined P. aeruginosa and K. pneumoniae was 8.5% each in cases with late onset VAP. As regard, positive nasal swab culture results K. pneumoniae which was isolated from 16.7% of all cases, with early onset VAP while it was 23.4% in cases with late onset VAP, P. aeruginosa, combined P. aeruginosa and K. pneumonia was 8.5% each, and A. baumanni was 6.4% in cases with late onset VAP. Gram negative bacteria are the most common agents causing VAP in our study. There is a relationship between organisms in nasal swab and VAP. So, nasal screening for colonization may be a valuable tool for de-escalation of empiric therapy targeted to the organism.
Key words: Ventilator associated pneumonia (VAP), nasal swab, antimicrobial sensitivity.
Ventilator associated pneumonia (VAP) is a serious complication related to mechanical ventilation in the neonatal period. VAP is defined as nosocomial pneumonia in mechanically ventilated patients that develops more than 48 h after initiation of mechanical ventilation (MV) (Tripathi et al., 2010). VAP is the most common nosocomial infection in neonatal intensive care unit (NICU) (Awasthi et al., 2013).
Diagnosis of a VAP episode requires a combination of clinical, laboratory and radiological criteria. The most prevalent clinical signs associated with VAP refer to changes in the characteristics and volume of respiratory secretions and the appearance of purulent mucus in tracheal aspirate (TA). Other signs include hypo- or hyperthermia and worsening of the respiratory distress (Cernada et al., 2013).
Despite the advancements in antimicrobial regimes, VAP continues to be an important cause of morbidity and mortality. VAP requires a rapid diagnosis and initiation of appropriate antibiotic treatment, as there is adverse effect of inadequate antibiotic treatment on patient's prognosis and the emergence of multidrug-resistant (MDR) pathogen (Jakbrittu et al., 2012).
Specific biomarkers of VAP allowing differentiation of pneumonia from colonization have been extensively studied .The presence of bacterial pathogens will be sensed by specific cytosolic receptors such as Toll-like and Nod-like receptors triggering an inflammatory response (Cernada et al., 2014). Pro-inflammatory cytokines such as IL-1, IL-6, IL-8, IL-10, and TNF-α have been evaluated as markers of VAP, with discordant results (Conway et al., 2010).
The aim of this study is to analyze the microbiological profile of VAP in relation to nasal swabs, and prevalence of multi-drug resistant bacteria so as to implement effective treatment and prevention strategies in neonatal intensive care unit (NICUs).
This prospective observational study was carried out at Tanta University Hospitals, Egypt in Neonatal Intensive Care Unit, Pediatric and Medical Microbiology and Immunology Department on intubated neonates who were admitted to NICU over 1 year (from March 2016 to February 2017).
One hundred (100) cases were enrolled in the study which were divided into 2 groups according to onset of VAP; Early-onset VAP group: 6 patients [2 females (33.3%) and 4 males (66.7%)] and Late-onset VAP group: 94 patients [39 females (41.5%) and 55 males (58.5%)]. One hundred neonates were ventilated for more than 48 h and met the next inclusion criteria which were enrolled in the study.
Inclusion criteria
Inpatient without primary (1ry) underlying pneumonia; with chest x-rays showing newly progressive or persistent infiltrate, consolidation, pneumatocele, worsening of gas exchange as increase FIO2 requirement, increasing ventilation demand, and increasing oxygenation index, and three of the following: Temperature instability without recognized cause, Leucopenia, leucocytosis, left shift ≥10% or left shift >0.15, new onset of purulent tracheal aspirate or increasing respiratory secretion with increasing suctioning requirement, apnea, tachypnea and retraction, wheezing and rales, changes in heart rates, clinical decision to change antibiotics, elevation in CRP>10 mg/dl.
Exclusion criteria
Neonates requiring intubation less than 48 h and neonatal pneumonia as a cause of intubation. All cases were subjected to thorough history taking including prenatal, natal and postnatal history coupled with any maternal risk factor, mode of delivery, gestational age and postnatal age, thorough clinical examination on admission, indications of mechanical ventilation, sputum culture and sensitivity, nasal swab culture and sensitivity.
Microbiological procedure
Endotracheal aspirate and nasal swabs were collected under complete aseptic precaution for culture and sensitivity .The specimens collected were transported to laboratory within 1 h, All samples were subjected to conventional culture on blood agar, chocolate agar, and MacConkey,s agar medium for identification of the colonies by conventional microbiological and biochemical tests.
Antimicrobial susceptibility of all isolates were determined by the standard Kirby Bauer disc diffusion method according to Clinical and Laboratory Standard Institute Guidelines (CLSI, 2008). Disc diffusion test was performed using Muller Hinton agar (Oxoid, Basingstoke, United Kingdom).
The antimicrobial discs were obtained from Oxoid limited Basingstoke, Hamisphire and England. Antimicrobials were supplied and stored according to the manufacturer’s instructions. Escherichia coli ATCC 5922, Pseudomonas aeruginosa ATCC 27853 and Staphylococcus aureus ATCC 25923 were used as control strains. Disc zone diameter was interpreted according to the CLSI (2008) recommendations.
Antibiotics used
Gram negative panel: Imipenem 10 mg, meropenem 10 mg, cefotaxime 30 mg, ciprofloxacin 5 mg, sulfamethoxazole 300 mg, colistin 25 mg, polymyxin 300 mg, piperacillin-tazobactam 85 mg, amikacin 30 mg, levofloxacin 5 mg, ceftazidime 30 mg, gentamycin 10 mg, ampicillin-sulbactam 20 mg and tetracycline 30 mg.
Gram positive panel: Vancomycin 30 mg, erythromycin 15 mg, penicillin G 10 mg, cloramphenicol 30 mg, cefoxitine 30 mg, doxycycline 30 mg, clindamycin 2 µg, amikacin 30 mg, gentamycin 10 mg, ciproflxacin 5 mg, aztronam 30 mg, ceftriaxone 30 mg, cefoperazone 75 mg, cefotaxime 30 mg, cefuroxime sodium 30 mg and sulfamethoxazole 300 mg.
Statistical analysis
The data were statistically analyzed using SPSS software (Statistical Package for the Social Sciences, version 16, SPSS Inc. Chicago, IL, USA).
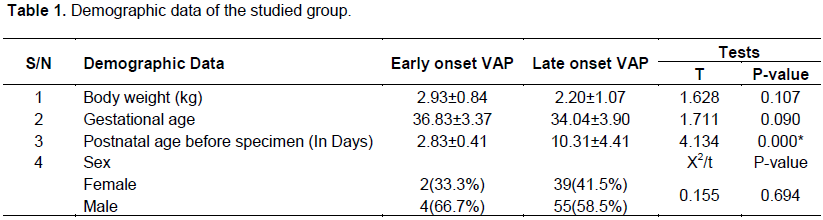
Demographic data of the studied patients showed that, there was no statistical significant difference between the two groups regarding body weight, gestational age, and sex. However, statistically significant increase in postnatal age in late onset VAP (10.31± 4.41 days) in comparison to early onset VAP (2.83 ± 0.41) was observed (Table 1).
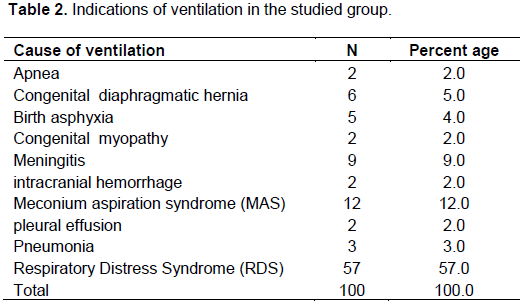
As regard the indications of ventilation in this study presented in Table 2 are respiratory distress syndrome (RDS) which accounts for 57% of cases, 12% of meconium aspiration syndrome, 9% of meningitis congenital diaphragmatic, 6% of hernia, 2% of congenital myopathy, 2% of intracranial hemorrhage and 2% of pleural effusion.
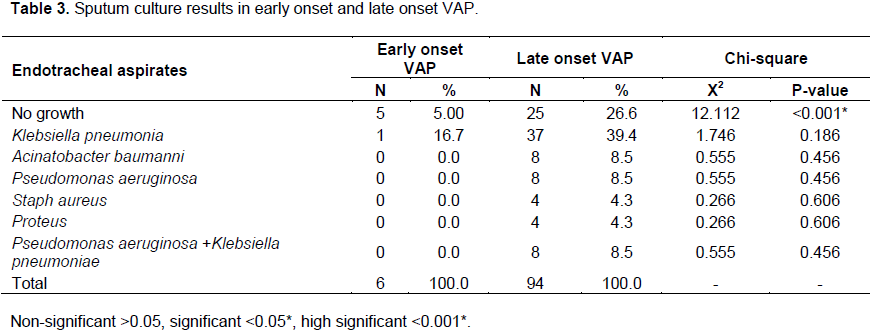
The results of culture of endotracheal aspirates in this study showed that, the rate of negative sputum culture results was significantly higher in cases with late onset VAP (26.3%) in comparison to early onset VAP where 5% of cases with early onset VAP showed no growth. As regard to positive sputum culture results, the rate of Klebsiella pneumoniae was 16.7% in cases with early onset VAP while it is 39.4% in cases with late onset VAP, Aceintobacter baumanni, P. aeruginosa and combined P. aeruginosa and K. pneumoniae was each 8.5% in cases with late onset VAP (Table 3).
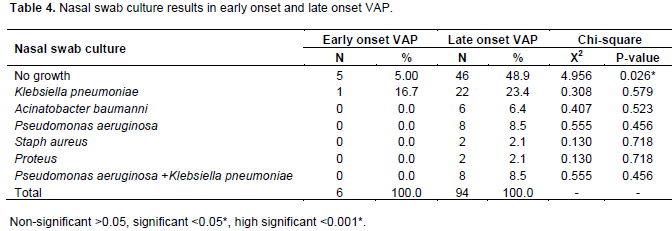
As regard to nasal swab culture, the results of the study reported that the rate of negative nasal swab culture results was significantly higher in cases with late onset VAP (48.9%) in comparison to early onset VAP where 5% of cases with early onset VAP showed no growth. As regard positive nasal swab culture results, K. pneumoniae was isolated from 16.7% of cases with early onset VAP while it was 23.4% in cases with late onset VAP, pseudomonas, combined P. aeruginosa and K. pneumoniae was each 8.5% and A. baumanni was 6.4% in cases with late onset VAP (Table 4).
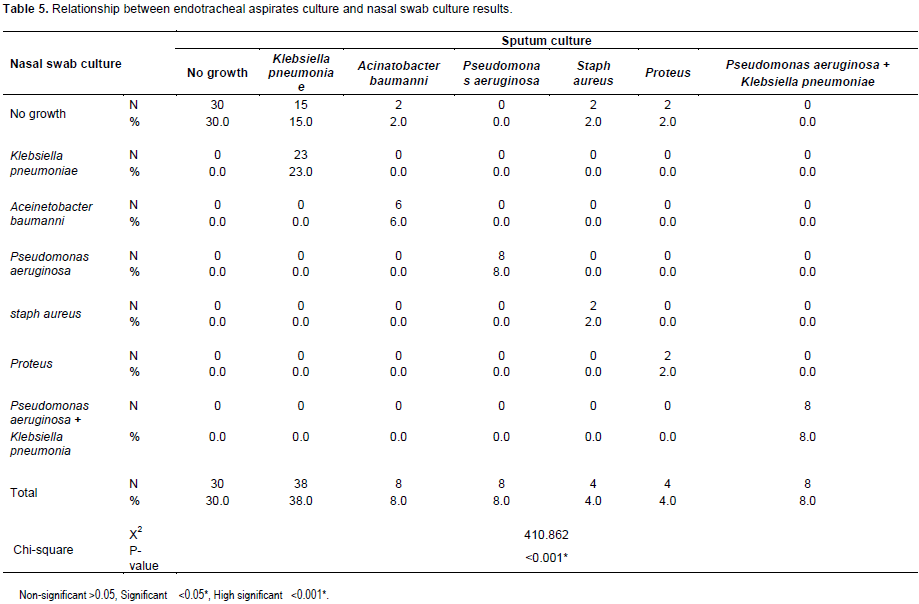
As regard the relationship between endotracheal aspirates and nasal swab cultures results showed that 30% of cases were negative in both cultures while the total number of cases which developed positive endotracheal aspirates culture for K. pneumoniae was 38. 23 of them was positive for Klebsiella in nasal swab culture, 8 cases developed A. baumanni in endotracheal aspirates culture, 6 of them was positive also in nasal swab culture, 8 deveolped P. aeruginosa and combined P. aeruginosa and K. pneumoniae in both sputum and nasal swab cultures (Table 5).
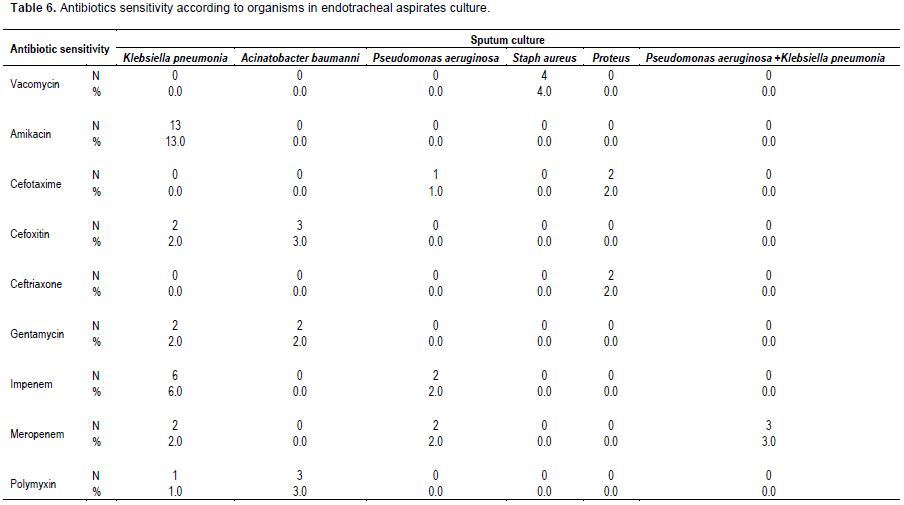
The pattern of antibiotics sensitivity of endotracheal aspirates culture shown in Table 6 where among K. pneumoniae strains the highest sensitivity was recorded to Amikacin (13%) followed by Imepenem (6%), among A. baumanni (3%) was sensitive to cefoxitim and Polymyxin, while all of the isolated staphylococci (4%) were sensitive to vancomycin.
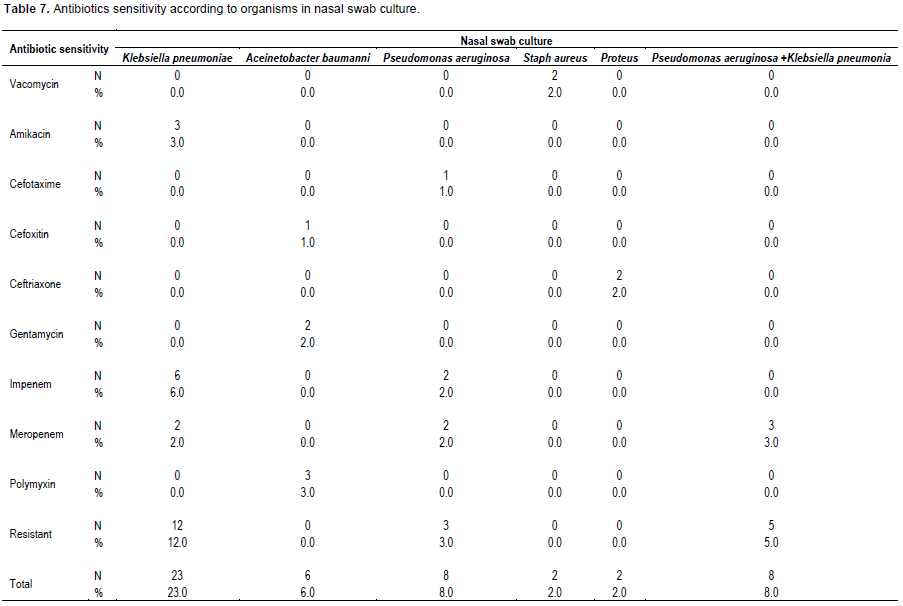
The pattern of antibiotics sensitivity of the nasal swab culture shown in Table 7 where among K. pneumoniae strains highest sensitivity was recorded to Imepenem (6%), among A. baumanni 3 strains were sensitive to polymyxin (3%), while 2 strains out of the isolated 4 S. aureus strains (2%) were sensitive to vancomycin.
VAP is a serious complication related to mechanical ventilation in the neonatal period. VAP is defined as nosocomial pneumonia in mechanically ventilated patients that develops more than 48 h after initiation of mechanical ventilation (MV) (Tripathi et al., 2010).
VAP continues to be the most common Health Care Acquired Infections (HCAIs) in the ICU, making up almost one third of the total HCAIs (Joseph et al., 2010). The pathogenesis of VAP is related to host and treatment related colonization factors. Aspiration of oropharyngeal pathogens and leakage of secretions containing bacteria around the endotracheal tube are principle factors for development of VAP. The progression from colonization to tracheobronchitis to VAP is a dynamic equilibrium (Al-Dorzi et al., 2012).
The aim of this study was to analyze the microbiological of VAP in relation to nasal swabs, risk factors and prevalence of multi-drug resistant bacteria so as to implement effective treatment and preventive strategies in NICU, Tanta University Hospitals, Egypt. The present study showed that regarding body weight, gestational age, and sex there was no significant difference between the 2 groups, while there was statistically significant increase in postnatal age in late onset VAP in comparison to early onset VAP.
As regard the indications of ventilation in this study presented in Table 3, RDS accounts for 57% of cases, 12% of Meconium Aspiration Syndrome, 9% of Meningitis, 6% of Congenital Diaphragmatic Hernia, 2% of Congenital myopathy, 2% of intracranial hemorrhage, and 2% of Pleural Effusion. The results shown were somewhat different from the results of Qazi et al. (2015) who reported RDS in 94%, asphyxia 68%, MAS 30%, Meningitis 22%, pneumonia 20% among cases of ventilated neonates.
The results of endotracheal aspirates culture in this study showed that K. pneumoniae was the most common organism in both groups as its rate was (16.7%) in cases with early onset VAP while (39.4%) in cases with late onset VAP, A. baumanni, P. aeruginosa and combined P. aeruginosa and K. pneumoniae was (8.5%) in cases with late onset VAP. The study performed by Hina et al., (2010) showed that the most common organism associated with VAP is P. aeruginosa (43.24%), followed by K. pneumoniae (18.91%). Also, the overall mortality rate was high in the Pseudomonas group (62.5%) (Hina et al., 2010).
In a study performed by Ravi et al. (2015) among the 52 samples collected and processed, 27 (51.92%) showed monomicrobial growth, 20 (38.46%) showed the polymicrobial growth and 5 (9.6%) showed no growth. A. baumanni (25.37%) was the most common isolate, followed by P. aeruginosa (17.91%), S. aureus (17.91%), K. pneumonia (10.44%), E. coli (8.9%) and Enterobacter (8.9%).
In other studies performed by Chastre and Fagon (2002) and Kollef et al. (1993) isolation of P. aeruginosa ranges from 15 to 25%. The study of Rocha et al. (2013) showed that S. aureus VAP represented 12.5% of the cases with statistical analysis which identified colonization as a risk factor for the development of this infection. This variability may be controlled by the guidelines of infection control followed and different antimicrobial policies in different institutions. Also, culture of endotracheal aspirates showed different bacterial colonization of the proximal airways in most patients in the ICU (Chastre and Fagon, 2002).
Similarly, another factor affecting the results (regardless of the bronchoscopic technique used) is the need for rapid processing of specimens for culture, in other to prevent loss of viability of pathogen (Chastre and Fagon, 2002). The results of nasal swab culture results in the rate of K. pneumoniae was (16.7%) in cases with early onset VAP while (23.4%) in cases with late onset VAP, P. aeruginosa combined P. aeruginosa and K. pneumoniae was (8.5%), and A. baumanni was (6.4%) in cases with late onset VAP.
This is in agreement with the study of Zhu and Paul (2007) in which the clinical data of 106 critical neonates who were treated with mechanical ventilator between 2003 and 2005 were studied, retrospectively and reported that among the patients in their study, the detection rate of gram negative Bacilli (76.9%) was the highest, followed by gram positive cocci (17.9%) in VAP patients. Also, in a study done by Afjeh et al. (2012), which determine the risk factors and outcomes of (VAP) in the neonatal intensive care unit (NICU), a retrospective cohort study was conducted on 259 patients who were ventilated 48 h and found that, the main pathogens were gram negative bacteria (82.1%) namely P. aeruginosa, K. pneumoniae and A. baumanni which were predominant.
As regard the Antibiotics sensitivity of sputum culture the results of this study showed that K. pneumonia strains recorded highest sensitivity to Amikacin (13%) followed by Imepenem (6%), among A. baumanni (3% ) which was sensitive to cefoxitim and Polymyxin. In the study of Abdulrahman (2005), K. pneumoniae strains showed sensitivity to aztereonam and imipinem, were 9 (100%), 6 (75%) to ciprofloxacin, 4 (44.4%) to sulbactam-ampicillin and amoxicillin-clavulinic acid, 2 (25%) to cefuroxime. 2 (22.2%) to amikacin and gentamicin, 1 (11.1%) to trimethoprim-sulphamethoxazole and cefotaxime, and 9 (100%) resistant to both ampicillin and cefoxitin.
In this study, all of the isolated staphylococci (4%) were sensitive to vancomycins which were resistant to all used antibiotics. This pattern of sensitivity to staphylococcal strains was to some extent different from the study of Abdulrahman (2005), who reported that S. aureus isolates sensitive to rifampin and vancomycin were 15 (100%), 11 (73.3%) to sulbactam-ampicillin, 10 (66.7%) to ciprofloxacin, 9 (60%) to imipinem and amoxicillin-clavulinic acid, 8 (53.3%) to tetracycline, 7 (46.7%) to cefuroxime and trimethoprim, 6 (40%) to ampicillin, while 2 (13.3%) were resistant to gentamicin; Methicillin-resistant Staphylococcus aureus (MRSA) represented 40%.
Gram negative bacteria are the most common agents causing VAP in our study. There is a relationship between organisms in nasal swab and VAP. So, Nasal screening for colonization may be a valuable tool for de-escalation of empiric therapy.
The authors have not declared any conflict of interests.
REFERENCES
|
Abdulrahman MA (2005). Antibiotic Susceptibility Patterns Of Different Bacteria Isolated From Patients With Ventilator Associated Pneumonia (VAP). J. Fam. Community Med. 12(3):139-144.
|
|
|
|
Afjeh SA, Sabzehei MK, Karimi A, Shiva F, Shamshiri AR (2012). Surveillance of ventilator-associatedpneumonia in a neonatal intensive care unit: characteristics, risk factors, and outcome. Arch. Iran Med. 15:567-571.
|
|
|
|
Al-Dorzi HM, El-Saed A, Rishu AH (2012). The results of a 6-year epidemiologic surveillance for ventilator-associated pneumonia at a tertiary care intensive care unit in Saudi Arabia. Am. J. Infect. Control 40(9):794-799.
Crossref
|
|
|
|
Awasthi S, Tahazzul M, Ambast A, Govil YC, Jain A (2013). Longer duration ofmechanical ventilation was found to be associated with ventilator-associatedpneumonia in children aged 1 month to 12 years in India. J. Clin. Epidemiol. 66:62-66.
Crossref
|
|
|
|
Cernada M, Aguar M, Brugada M, Gutiérrez A, López JL, Castell M, Vento M (2013).Ventilator-associated pneumonia in newborn infants diagnosed with an invasive bronchoalveolarlavage technique: a prospective observational study. Pediatr. Crit. Care Med.14:55-61.
Crossref
|
|
|
|
Cernada M, Brugada M, Golombek S, Vento M (2014).Ventilator-Associated Pneumonia in Neonatal Patients: An Update. Neonatology 105:98-107.
Crossref
|
|
|
|
Chastre J, Fagon JY (2002). Ventilator associated pneumonia. Am. J. Respir. Crit. Care Med. 165(7):867-903.
Crossref
|
|
|
|
CLSI-Clinical and Laboratory Standards Institute (2008). Performance Standards for Antimicrobial Susceptibility Testing. Sixteen Informational Supplement. Clinical and Laboratory Standards Institute, Chicago. Document M, 100:S16.
|
|
|
|
Conway Morris A, Kefala K, Wilkinson TS (2010). Diagnostic importance of pulmonary interleukin-1beta and interleukin-8 in ventilator-associated pneumonia. Thorax 65:201-207.
Crossref
|
|
|
|
Hina G, Arun V, Akhya KK (2010). A study of ventilator-associated pneumonia: Incidence, outcome, risk factors and measures to be taken for prevention. Indian J. Anaesth. 54(6):535-540.
Crossref
|
|
|
|
Joseph NM, Sistla S, Dutta TK (2010). Ventilator-associated pneumonia: role of colonizers and value of routine endotracheal aspirate cultures. Int. J. Infect. Dis. 14(8):723-729.
Crossref
|
|
|
|
Kollef MH (1993). Ventilator-associated pneumonia: A multivariate analysis. JAMA 270:1965-1970.
Crossref
|
|
|
|
Qazi I, Mir M, Asif A, Ikhlas A, Javed I, Bashir A (2015). Neonatal mechanical ventilation: Indications and outcome. Indian J. Crit. Care Med. 19(9):523-527.
Crossref
|
|
|
|
Ravi K, Ruchi D, Mirza AB, Reshmi C (2015). Incidence, microbiological profile and early outcomes of ventilator associated pneumonia in elderly in a Tertiary Care Hospital in India. Afr. J. Med. Health Sci. 14(1):66-69.
Crossref
|
|
|
|
Rocha LA, Marques R, da Costa AL, Gontijo PP (2013). Relationship between nasal colonization and ventilator-associated pneumonia and the role of the environment in transmission of Staphylococcus aureus in intensive care units. Am. J. Infect. Control 41(12):1236-1240.
Crossref
|
|
|
|
Tripathi S, Malik GK, Amita Jain A (2010). Study of Ventilator Associated Pneumonia in Neonatal Intensive Care Unit: characteristics, risk factors and outcome. Internet J. Med. Update 5(1):12-19.
Crossref
|
|
|
|
Zhu J, Paul WE (2008). CD4 T cells: fates, functions, and faults. Blood 112(5):1557-1569.
Crossref
|