ABSTRACT
The root lesion nematode (Pratylenchus brachyurus) is one of the main phytosanitary problems of cotton plants in Brazil. Searching for alternatives that minimize the damages in the crop, several methods are performed aiming to manage these damages. Among them, is the use of vegetal extracts. In this sense, the aim of this study was to evaluate the potential of black angico extract (Anadenanthera macrocarpa) in the management of P. brachyurus in cotton crop. The experiment was conducted in a greenhouse at the Phytopathology Laboratory of the Federal University of Piauí in Bom Jesus-PI. The experimental design was completely randomized, in a factorial scheme (2×6), composed of two sources of extracts (leaf and bark) of black angico under six concentrations (0, 20, 40, 60, 80 and 100 g L-1), with five replications per treatment. The plants were inoculated with 1900 specimen/juveniles and eggs, 96 h after the transplanting. Sixty days after the application of extracts, some agronomic variables of the cotton and P. brachyurus were evaluated. The volume and fresh root mass showed considerable gains for all concentrations with the leaf extract. The plant height was negatively influenced by concentrations above 60.83 g L-1 for both extracts. Regarding the parasitism, all the extract concentrations, regardless of the source (leaf or bark), showed suppressiveness to P. brachyurus. Therefore, the aqueous extracts of black angico present nematicidal action and favor the development of cotton plants.
Key words: Gossypium hirsutum, root lesion nematode, alternative management.
The cotton crop (Gossypium hirsutum L.) represents one of the most important activities of Brazilian agribusiness in a vigorous expansion process and with exceptional technical and economic results for the use of its seed and
mainly its fiber (Ribeiro et al., 2012). In the 2015/2016 season, the cotton planted are decreased by 2% compared to the previous harvest, with an area of 956.2 thousand hectares (CONAB, 2016).
Cotton is grown in more than 60 countries. Anobg, China, India and the United States are the largest producers and together they produce 64% of the world production. Despite having larger planted area, India produces a volume of fibers almost equal to the United States due to the low yield of its crops. The list of the top five producers is completed by Pakistan and Brazil. In recent years, Brazil has improved its ranking in producing countries. Currently, it is the fifth largest producer in the world (Abrapa, 2015).
Among the barriers to cotton crop management, phytosanitary problems caused by fungi, bacteria, viruses and mainly nematodes are often associated with reduced low crop yield (Ribeiro et al., 2012). Among the key nematodes of this crop, there are approximately five species responsible for causing severe damage worldwide. Three of them are considered as causing significant damages to Brazilian cotton production: Meloidogyne incognita, Rotylenchulus reniformis, Pratylenchus brachyurus (Starr et al., 2007; Jones et al., 2013).
Currently, the nematode P. brachyurus, which is responsible for root lesions, has been considered the most frequent in Brazil and is widespread in the main agricultural regions of the country (Severino et al., 2010). Because it is a polyphagous species and is extremely common in regions of tropical climate (Arieira et al., 2009), and has become a concern to cotton producers in the Northeast region. It is the third most important regarding the global economic impacts caused to crops, being exceeded only by root-knot and cyst nematodes (Heterodera and Globodera) (Jones et al., 2013). The symptoms associated with this species in cotton include darkened injuries on the roots, causing atrophy, and may even compromise the absorption of water and nutrients (Dinardo-Miranda et al., 2003) and consequently, reduction in the shoot part development of plants with a sharp drop in production (Ribeiro et al., 2012).
Considering the great importance of phytonematodes management in commercial production areas, chemical control has always stood out because of its fast and efficient results (Oliveira et al., 2005). However, numerous problems are encountered due to their high toxicity, risk of environmental contamination, high cost, or low control effectiveness after repeated applications (Dong and Hang, 2006). In an attempt to reduce these effects, different control methods such as genetic, biological, crop rotation and alternative control have been studied.
Within this context, vegetable extracts represent a viable alternative to alleviate economic and social conditions of most of the farmer. In addition, their use reduces or replaces chemical application (Ferraz et al., 2010). Several studies have demonstrated the nematicidal effect of extracts of different plants on different species of phytonematodes when applied directly to the soil or by air (Cetintas and Yarba, 2010). Among these species, the black angico (Anadenanthera macrocarpa) is worth mentioning because it has potential to manage several diseases in the human, animal and plant area, with emphasis to phytonematodes. Black angico is a tree that can reach 13 to 20 m of height and trunk with 40 to 60 cm of diameter, when adult, occurring from Maranhão and Brazilian Northeast to São Paulo, Minas Gerais and Mato Grosso (Gonçalves et al., 2012).
Thus, the aim of this study was to evaluate the potential of plant extracts based on black angico (A. macrocarpa) on the management of P. brachyurus in cotton.
Location of the experimental area and soil treatment
The experiment was performed under greenhouse conditions at the Phytopathology Laboratory, at the Universidade Federal do Piauí, Prof Cinobelina Elvas campus, Bom Jesus city, from October to December, 2014.
To evaluate the treatments, the substrate was composed of soil-sand-manure in a proportion of 3:2:1, respectively. It was autoclaved, at a temperature of 120°C and pressure of 1.05 kg/cm2 for 2 h. The substrate was fertilized according to the previous analysis and distributed into plastic containers with capacity of 4 dm -3.
Origin and multiplication of inoculum
The inoculum was obtained from a population of P. brachyurus from soybean crops in Bom Jesus-PI. The extraction was carried out by liquefaction and centrifugation in sucrose with kaolin solution, according to Coolen and D'Herde's (1972) methodology. Soon after, the specimens were isolated and inoculated in corn hybrid plants Pioneer 30F53 grown in pots and kept in a greenhouse for 30 days for multiplication. The pre-identification of the specimen was done with semi-permanent blades in formalin, examined under an optical microscope, comparing the characteristics observed with the literature (Handoo and Golden, 1989).
Experimental procedures
The experimental design was completely randomized, in a factorial scheme (2×6), composed of two sources of extracts (leaf and bark) of black angico under six concentrations (0, 20, 40, 60, 80 and 100 g L-1), with five replications per treatment.
The seedlings were prepared in trays of expanded polystyrene with 128 cells, with substrate consisting of sand, manure and earthworm humus (in the same ratio), sterilized by autoclaving, at a temperature of 120°C and pressure of 1.05 kg/cm2 for 2 h. Transplanting of seedlings was done on the thirteenth day after emergence, and two seedlings were maintained per pot. Thinning was done 28 days after transplanting, keeping a single plant which corresponded to the experimental unit.
Subsequently, at 4 days after transplanting, a suspension with 2000 specimens/juveniles and inoculum eggs was used for inoculation with the aid of a pipette and distributed in three holes of 5.0 cm deep, spaced 2.0 cm from the hypocotyl of cotton plants to facilitate the development of nematode action in the soil.
The botanical material (leaves and bark) of the black angico plant species was collected in the region of Bom Jesus – PI. The dehydration process was done in the laboratory at room temperature during 5 days, then subjected to a mechanical mill pulverization process, reduced to powder, and stored in a 1000 ml beaker until the preparation of the fractionated aqueous extracts.
One day prior to application of the treatments, the bark and leaf powder of Angico at concentrations 0, 20, 40, 60, 80 and 100 g/L was subjected to cold extraction with distilled water for 24 h to obtain the maximum extraction of the chemical constituents. The resulting extractive solution was filtered and then applied in the treatment through the soil.
A solution of 100 ml was applied in each pot. It was divided into 4 aliquots of 25 ml each, at intervals of 15 days. The concentrations used throughout the intervals were prepared only 24 h before the applications.
Analyzed variables
The evaluations were performed sixty days after application of the extracts. Agronomic variables of the cotton plant were evaluated: plant height and root length using a graduated ruler; fresh shoot mass and the fresh root mass, obtained with the aid of a semi-analytical balance. Root volume was measured using a 1000 ml test tube, considering a fixed volume of 800 ml and immersing the root in this volume, calculating the difference to obtain the final volume.
The variables on parasitism were estimation of the number of specimens in the soil of each treatment, extracted in 100 cm3 of soil by centrifugation and flotation (Jenkins, 1964) and estimation of the root nematodes (Coolen and D'herde, 1972).
Statistical analysis
Data on agronomic variables and parasitism were analyzed by the Shapiro-Wilk test and the analysis of variance (ANOVA) by the F test (p <0.05), using the statistical program "R" version 3.1.2. When significant, the mean were adjusted in regression equations using the software SigmaPlot 10.0.
Influence of aqueous extracts of black angico on cotton plants
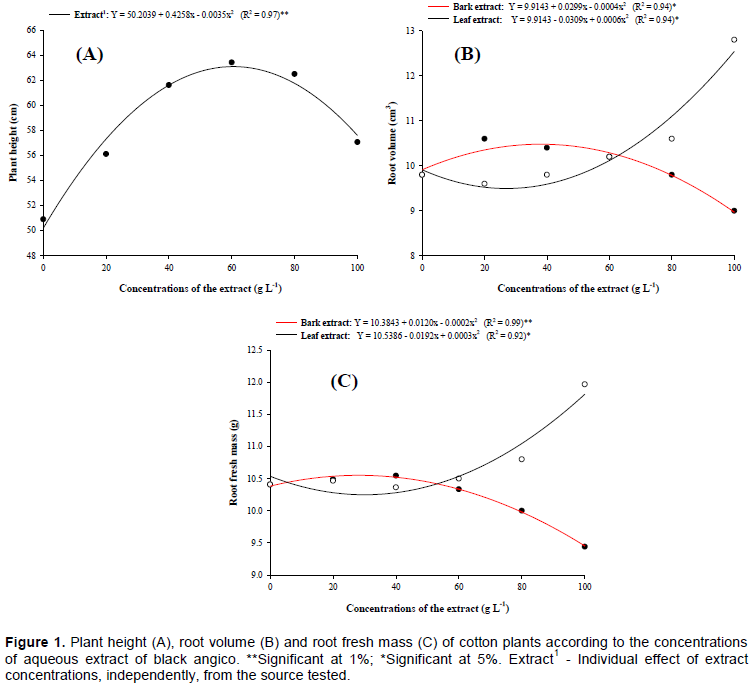
By the analysis of variance, it was observed that there was an interaction between sources and concentrations of the black angico extract, with significant effect only for volume (P< 0.01) and fresh root mass (P<0.05). At the same time, only the plant height (P<0.01) was affected by the individual performance of the extract concentrations.
The height of cotton plants was positively influenced by the extracts of black angico. Regardless of the source tested (bark or leaf), there were quadratic responses as a function of the concentrations applied (Figure 1A). Thus, the plants showed greater heights when they received 60.83 g L-1 of the extract, reaching an increase of 25.80% (Figure 1B). However, the plants showed a reduction in growth at concentrations above 60.83 g L-1. The harmful effect of the black angico extract on the plant in high concentrations could be related to the presence of tannin in this species, which is considered an allelopathic agent, due to its ability to act directly on the cytological characteristics, phytohormones, membranes, mineral absorption, respiration and enzymatic activity (King and Ambika, 2002).
Root volume and root fresh mass of cotton plants presented positive gains after the extracts application as means adjustment was made in the quadratic polynomial regression model (Figure 1B and C). The highest averages of these variables were observed with a leaf extract concentration of 60 g L-1, reaching respective maximum increases at 30.61 and 15.01%, at the highest concentration tested (100 g L-1). The increase in the root system with leaf extract can be attributed to the reduction of the parasitism of P. brachyurus (Figure 2), which is also associated with other factors such as the presence of some allelochemicals stimulating the roots development, as well as an allelopathic effect (Carvalho et al., 2002). Abreu (1997), reported a possible presence of an allelochemical in the aqueous extract of red angico (Anadenanthera peregrina (L) Speg), acting as a phytohormone in the roots development.
For the bark extract, the best results for volume and fresh root mass occurred at low concentrations of 37.38 and 30 g L-1, respectively. However, at concentrations above these, there was a reduction of root development due to allelopathic effects demonstrated by the bark extract (Figure 1B and C). As previously mentioned, tannin is the main chemical constituent present in the leaves and bark of black angico, which may interfere with the physiological activity of plants, harming or stimulating growth and development. Thus, the divergent results between leaf and bark extract may be related to the pronounced presence of tannin in the barks (around 15 to 20%) (Lorenzi and Matos, 2002).
Influence of aqueous extracts of black angico on P. brachyurus parasitism
For the variables of P. brachyurus parasitism, there was no significant interaction (P>0.05) between sources and concentrations of black angico extracts (Table 1). However, there was a significant effect (P<0.01) of extract concentrations on juvenile variables in the root and juveniles in the soil.
The extracts of black angico influenced negatively the number of juveniles of P. brachyurus in the root, with exponential reduction according to the tested concentrations (Figure 2A). These results demonstrate that the lowest applied concentration (20 g L-1), regardless of the source (bark or leaf), was efficient in reducing root nematodes by 71.43%. These results of P. brachyurus control may be related to tannins presence in black angico bark (Lorenzi and Matos, 2002), and may present antimicrobial activity (Djipa et al., 2000). Tannins have action on microorganisms' cell membranes and modify their metabolism (Scalbert, 1991). Wilted black angico leaves are popularly toxic and can be used as natural defenses (Silva Filho, 2007).
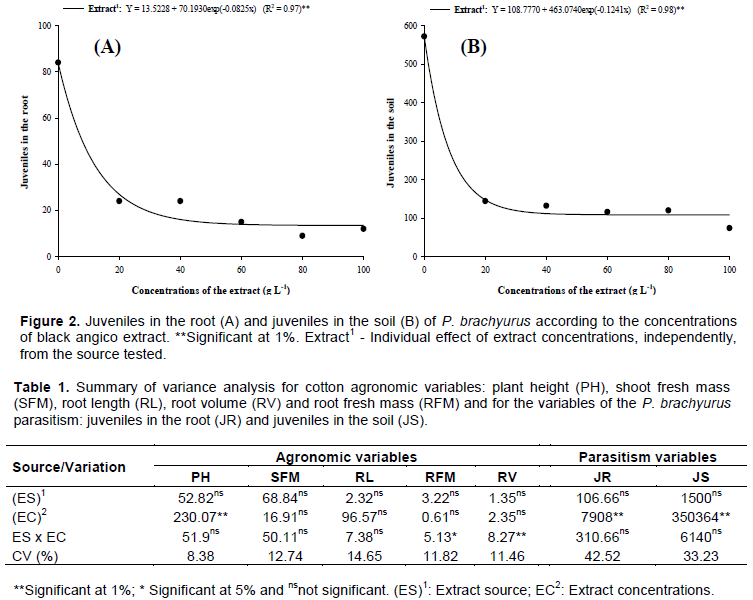
Leaf and bark extracts of black angico reduced the number of juveniles of P. brachyurus in soil, with exponential reduction as a function of the applied concentrations (Figure 1B). The lowest number of nematodes in the soil was observed in the lowest extract concentration applied (20 g L-1), with a reduction of 74.83%. The nematicidal action of black angico is attributed to compounds involved in chemical defense, which include lectins, protease and amylase inhibitors, toxins, and low molecular mass secondary metabolites (Xavier-Filho, 1993). As the extracts in this research were directly applied on the soil, possibly the compounds present in the leaf and bark of the black angico have acted directly by contact on the nematodes, promoting a population decrease. Maistrello et al. (2010), demonstrated the nematicidal action of tannin in preventing hatching and development of phytonematodes of the Meloidogyne genus.
Several natural substances of different plant species have been isolated and chemically characterized, and some are promising for field application. Martinez (2002) demonstrated the nematicidal effect of neem on several species of phytonematodes such as Pratylenchus species, R. reniformis, and M. incognita. Franzener et al. (2007) verified the nematicidal effect of the aqueous extract at 0.05 g ml-1 of Tagetes patulae flowers when applied to the soil, observing a reduction of 62.2, 61.5 and 52.8%, in the number of galls, number of juveniles in the soil and number of eggs for M. incognita in tomato roots, respectively. Aqueous extracts obtained from crotalaria leaves (Crotalaria mucronata L.), at a concentration of 0.2 g ml-1, when applied via soil in tomato plants, reduced the number of galls caused by Meloidogyne javanica by 33% compared to the control, in which only water was applied (Gardiano et al., 2010).
Thus, the use of secondary metabolites of plants with nematicidal properties represents an economically viable option, since it presents a lower risk of environmental contamination due to its biodegradable characteristics. However, it is necessary to carry out new studies to characterize the active ingredients in the extracts so as to pinpoint the mode of action of the extracts on cotton plants. In addition, this could pave the way for possible synthesis of botanical nematicides based on the extracts.
The aqueous extracts of black angico present nematicidal potential and promote plant growth and development.
The leaf aqueous extract contributed to an increase in root volume and root fresh mass of cotton plants.
Leaf and bark extracts of black angico negatively influenced plant height was at concentrations higher than 60.83 g L-1. Root volume and root fresh mass decreased when exposed to concentrations above 37.38 and 30 g L-1, respectively of bark extract.
All concentrations for leaf and bark extracts showed some nematicidal action, mainly in the lower concentrations (20 and 40 g L-1).
The authors have not declared any conflict of interests.
REFERENCES
|
Abrapa – Associação Brasileira dos Produtores de Algodão. 2016, 381p. Disponível em:
View Acessado em: 26 de setembro. 2016.
|
|
|
|
Abreu JC (1997). Potencial alelopático do angico vermelho (Anadenanthera peregrina (L.) Speg): efeito sobre a germinação de sementes e ciclo mitótico de plântulas de alface (Lactuca sativa L.) e canafístula (Peltophorum dubium (Spreng.) Taub.). Dissertação (Mestrado) - Universidade Federal de Lavras, Lavras. 55 p.
|
|
|
|
|
Arieira CRD, Ferraz S, Ribeiro RCF (2009). Reação de gramíneas forrageiras a Pratylenchus brachyurus. Nematologia Brasileira 33(1): 90-93.
|
|
|
|
|
Carvalho GJ, Fontanétti AA, Cançado CT (2002). Potencial alelopático do feijão de porco (Canavalia ensiformes) e da mucuna preta (Stilozobium aterrimum) no controle da tiririca (Cyperus rotundus). Ciência e Agrotecnologia. 26:647-651.
|
|
|
|
|
Cetintas R, Yarba MM (2010). Nematicidal effects of five plant essential oils on the Southern root-knot nematode, Meloidogyne incognita race 2. Journal of Animal and Veterinary Advances 9(2):222-225.
Crossref
|
|
|
|
|
Companhia nacional de Abastecimento- CONAB (2016). Acompanhamento de safra brasileira: grãos, décimo segundo levantamento, setembro 2016. Brasília: CONAB, 2016. Pp. 1-127.
|
|
|
|
|
Coolen WA, D'Herde CJ (1972). A method for the quantitative extraction of nematodes from plant tissue. Ghent, Belgian: State of Nematology and Entomology Research Station, 77p.
|
|
|
|
|
Dinardo-Miranda LL, Gil MA, Garcia V, Menegati CC (2003). Efeito da torta de filtro e de nematicida sobre as infestações de nematoide e a produtividade da cana-de-açúcar. Nematologia Brasileira 27:61-67.
|
|
|
|
|
Djipa CD, Delmée C, Leclercq JQ (2000). Antimicrobial activity of bark extrats of Syzygium jambos (L.) Alston (Myrtaceae). Journal of Ethnopharmacology 71(1-2):307-331.
Crossref
|
|
|
|
|
Dong LQ, Zhang KQ (2006). Microbial control of plant parasitic nematodes: a five-party interaction. Plant Soil 288(1):31-45.
Crossref
|
|
|
|
|
Ferraz S, Freitas LG, Lopes EA, Dias-arieira CR (2010). (eds). Manejo sustentável de fitonematoides. Viçosa, MG, ed. UFV.
|
|
|
|
|
Franzener G, Martinez-Franzener AS, Stangarlin JR, Furlanetto C, Schwan-estrada KRF (2007.) Proteção de tomateiro a Meloidogyne incognita pelo extrato aquoso de Tagetes patula. Nematologia Brasileira 31(1):27-37.
|
|
|
|
|
Gardiano CG, Dallemole-Giaretta R, Lopes EA, Zooca RJF, Ferraz S, Freitas LG (2010). Atividade nematicida de extratos de sementes de espécies de Crotalaria sobre Meloidogyne javanica. Revista Trópica: Ciências Agrárias e Biológicas 4(1):3-7.
|
|
|
|
|
Gonçalves EO, Paiva HN, Neves JCL, Gomes JM (2012). Nutrição de mudas de angico-vermelho (Anadenanthera macrocarpa (benth.) brenan) submetidas a doses de N, P, K, Ca e Mg. Revista Árvore 36(2):219-228.
Crossref
|
|
|
|
|
Handoo ZA, Golden MAA (1989). A key and diagnostic compendium to the species of the genus Pratylenchus Filipjev. Journal of Nematology 21(2):202-218.
|
|
|
|
|
Jenkins WRA (1964). A rapid centrifugal-flotation technnique for separating nematodes from soil. Plant Disease Report P 48.
|
|
|
|
|
Jones JT, Haegeman A, Danchin EGJ, Gaur HS, Helder J, Jones MGK, Kikuchi T, Manzanilla-López R, Palomares-Rius JE, Wesemael WML, Perry R (2013). Top 10 plant-parasitic nematodes in molecular plant pathology. Molecular Plant Pathology 14(9):946-961.
Crossref
|
|
|
|
|
King SR, Ambika R (2002) Allelopathic plants. 5. Chromolaena odorata (L.). Allelopathy Journal 9:35-41.
|
|
|
|
|
Lorenzi H, Matos FJA (2002). Plantas Medicinais do Brasil Nativas e Exóticas. São Paulo: Instituto Plantarum. P 296.
|
|
|
|
|
Maistrello L, Vaccari G, Sasanelli N (2010). Effect of chestnut tannins on the root-knot nematode Meloidogyne javanica. Helminthologica 47:48-57.
Crossref
|
|
|
|
|
Martinez MM (2002). Ação do nim sobre nematoides. In: O Nim- Azadirachta indica, natureza, usos múltiplos e produção. Instituto Agronômico do Paraná, Londrina-PR. pp. 65-68.
|
|
|
|
|
Oliveira FS, Rocha MR, Reis AJS, Machado VOF, Soares RAB (2005). Efeito de produtos químicos e naturais sobre a população de nematoide Pratylenchus brachyurus na cultura da cana-de-açúcar. Pesquisa Agropecuária Tropical 35(3):171-178.
|
|
|
|
|
Ribeiro LM, Campos HD, Ribeiro GC, Neves DL, Dias-Arieira CR (2012). Efeito do tratamento de sementes de algodão na dinâmica populacional de Pratylenchus brachyurus em condições de estresse hídrico. Nematropica 42(1):84-90.
|
|
|
|
|
Scalbert A (1991). Antimicrobial properties of taninis. Phytochemistry 30(12):3875-3883.
Crossref
|
|
|
|
|
Severino JJ, Dias-Arieira CR, Tessmann DJ (2010). Nematodes associated with sugarcane in sandy soils in Paraná, Brazil. Nematropica 40:111-119.
|
|
|
|
|
Starr JL, Koenning SR, Kirkpatrick TL, Robinson AF, Roberts PA, Nichols RL (2007). The future of nematode management in cotton. Journal of Nematology 39(4):283-294.
|
|
|
|
|
Silva Filho, ML (2007). Avaliação in vitro da ação antiparasitária do extrato aquoso e etanólico do angico preto (Anadenanthera macrocarpa) (Benth.) brenan sobre o carrapato Rhipicephalus (Boophilus) microplus (Canestrini, 1887). Dissertação (Mestrado) – Univerdidade Federal do Piauí, Teresina. 62 p.
|
|
|
|
|
Xavier - Filho J (1993). Sementes e suas defesas contra insetos. Projeto multinacional de biotecnologia e alimentos. Organização dos Estados Americanos. Edições UFC, Fortaleza.
|
|