ABSTRACT
In this study we assayed on antimicrobial activity of peel, pulp, waxy coating of Benincasa hispida. Various parts of the plant are reported as antibacterial agents worldwide. Leaves, flower, root, seed are the most studied elements as antibacterial agent. Some fruits are also proved as antibacterial agent and used as herbal medicine as well as nutritional supplements during disease. Different parts of mature and immature winter melon fruits were extracted with different organic solvents viz., methanol, ethyl acetate and chloroform. These extracts were subjected to test against selected pathogenic bacterial (Bacillus subtilis IFO 3026, Sarcina lutea IFO 3232, Xanthomonas campestris IAM 1671, Escherichia coli iw 3310 IFO 3007, Pseudomonas denitrificans KACC 32026, and fungal (Fusarium oxysporum, Aspergillus niger KTCC 1700, Collectotrichum melongenae) strains using the well diffusion method. The waxy coating and peel of mature fruit of B. hispida extracts has strong antibacterial activities than immature fruit. In these cases, the minimum inhibitory concentration was 128 μg/ml. Ethyl acetate and chloroform extracts of waxy coating of B. hispida showed inhibition rate against fungal infection 43% and 33%, respectively against F. oxysporum but there is no inhibition against A. niger, C. melongenae. These results suggested that. So, the B. hispida would be a potential source that may help to develop natural antimicrobial agents.
Key words: Antibacterial, a fruit, winter melon, infectious, extracts, minimum inhibitory concentration (MIC).
The common agricultural products are rice, wheat, jute, tea, cotton, sugarcane, flower, vegetables, fishes and seed development, livestock, horticulture are main agricultural sectors . Microbial contamination is one of the most alarming causes of agricultural production.
According to the Food and Agriculture Organization (FAO), pests and diseases are responsible for about 25% of crop loss (Sheahan and Barrett, 2017). To solve this issue, new methods are needed to detect diseases and pests early such as novel sensors that detect plant odours, and spectroscopy and biophotonics that are able to diagnostic plant health and metabolism (Martinelli et al., 2015). Globally, plant extracts are employed for their antibacterial, antifungal and antiviral activities. It has been reported that more than 400,000 species of tropical flowering plant showed medicinal properties and the usages of medicinal flowering plant make the folk medicine cheaper the other medicine (Yuan et al., 2016). Benincasa hispida (Thunb.) which commonly called as winter melon belongs to the cucurbitaceae family. It is a popular vegetable crop, especially among Asian communities both for nutritional and medicinal purposes (Al-Snafi, 2013; Arora and Kaushik, 2016). Phytochemical screening of various extracts of fruits indicate the major constituents of the fruit are triterpenoids, flavonoids, glycosides, saccharides, carotenes, vitamins, ß-sitosterin, and uronic acid (Mandal et al., 2012).
Extracts of winter melon may be a natural drug with anti-ulcer activity and as well as antioxidant property (Lee et al., 2005). It contains terpenes, flavonoids, glycosides and sterols which have antioxidant effects (Shetty et al., 2008). Seed extract of winter melon howed anti-angiogenic property through inhibition of endothelial cell proliferation (Wen et al., 2008). The methanolic extracts of B. hispida showed excellent protection against histamine-induced bronchospasm probably through an antihistamine activity that is H1 receptor-antagonism (Simons and Simons, 2008). The ethanol extract of B. hispida have antinociceptive and antipyretic activity and used as herbal medicine against fever and pain conditions (Rahmatullah et al., 2012; Sharma et al., 2014). The antioxidant property of winter melon may be beneficial in the management of colchicene-induced rat model of Alzheimer's disease (Yagnik et al., 2009). The hypoglycemic effects of B. hispida waxy coating may be used to prevent diabetes mellitus (Gu et al., 2013).The methanol extract of winter melon fruit showed significant inhibition against fungus namely Candida albicans (Xing et al., 2012)also methanolic and petroleum ether extracts showed significant inhibition of carrageenan-induced paw edema, histamine induced paw edema and cotton pellet-induced granuloma in a rat model (Ashok et al., 2010; Elhassan et al., 2020; Patil and Patil, 2017). The ethanol extract of seeds showed an anti-urolithiatic effect with reduction in stone forming constituents in the urine and decreased kidney retention that reduced the solubility product of crystallizing salts (Juliana et al., 2018; Meshram et al., 2016; Tatiya et al., 2017). The seeds of B. hispida having bioactive peptide, Hispidalin showed inhibitory effects against human bacterial and fungal pathogens (Salas et al., 2015; Sharma et al., 2014).The aqueous extract of stem of winter melon for hypoglycemic effect in alloxan-induced diabetic rabbits showed significant dose-dependent reduction in blood glucose levels (Jhonatas et al., 2019; Khan et al., 2012).
In this study the authors tried to analyse antimicrobial activity of the different parts of mature and immature winter melon fruits. The different parts were extracted with different organic solvents viz, methanol, ethyl acetate and chloroform. These extracts were subjected to test against selected pathogenic bacterial and fungal strains using the well diffusion method. The potentially of the different organic extract of B. hispida will be assayed to find strong antimicrobial activities. If they found B. hispida would be a potential source that may help to develop natural antimicrobial agents for the control of plant pathogens.
Materials
The phytochemical analysis showed that different solvents are responsible for extracting different components from the plants. So, several solvents of different polarity are used to extract different plant components. Chloroform, Ethyl Acetate and Methanol were selected according to their degree of polarity. The different types of media were used during experiment Mueller-Hinton Agar (MHA), Lactose Broth, Potato Dextrose Agar (PDA) (Mahboob et al., 2019).
Microorganisms
Five different pathogenic bacteria and three fungi were collected from the microbiology laboratory of the department of Biotechnology and Genetic Engineering, Islamic University, Kushtia. The following strains were used as test strain Bacillus subtilis IFO 3026, Sarcina lutea IFO 3232, Xanthomonas campestris IAM 1671, Escherichia coli iw 3310 IFO 3007, Pseudomonas denitrificans KACC 32026, Fusarium oxysporum, Aspergillus niger KTCC 1700 and Collectotrichum melongenae.
Sample collection
The healthy, disease free, fresh immature and mature fruits of B. hispida were safely collected from farmers land. Various parts of B. hispida waxy coating, peel of mature and immature fruits were used.
Plant extract preparation
The fleshy surface, wax gourd of B. hispida dried under the shade and then powdered with mechanical grinder. It was later stored in air tight containers protected from direct sunlight for further experiments. The fine grinded powder was mixed (10 gm/100 ml) separately with chloroform, ethyl acetate and methanol. The samples with solvent were placed in rotatory shaker for 24 h at (30-36ºC) (Elnaggar et al., 2019; Ikeda et al., 2007; Sasidharan et al., 2011).The extracts of plant material were filtered more than three times and performed by passing the extracts through filter paper. The extracts with different solvents then allowed to air dry after filtration to concentrate.
Antimicrobial Assay
The crude organic extracts of selected solvents were used In vitro screening of antibacterial and antifungal activity. The screening of antibacterial and antifungal activity is a typical microbiological assay which is performed with culture of microorganisms. One of the most commonly used methods of bacterial assay is the well diffusion method (Mohsenipour and Hassanshahian, 2016; Valgas et al., 2007). The antimicrobial activity was evaluated by measuring the zone of inhibition expressed as mm against test organisms.
The antibacterial activity of plant extract was carried out by well diffusion assay using 100 µl of inoculum suspension of bacteria. Then each well was loaded with 100 µl of the different concentrations of extracts. Negative controls were used to screen the activity of solvents which may have effect on antimicrobial activity, for this the same solvents which were used to dissolve the plant extracts employed as negative control. Positive control was used to measure the antibacterial sensitivity of the test strain as well as it can confirm the effectiveness of the methods. Streptomycin (10µg/disc from Banex Ltd., USA) was used as positive reference standard to determine the sensitivity of the tested strain in each bacterial species.
The crude sample which showed the best potentiality against the selected organisms used In vitro screening of minimum inhibitory concentration (MIC). In microbiological research, minimum inhibitory concentration (MIC) is the lowest concentration in which the extract showed inhibition of the visible growth of a microorganism after incubation. In this study, it was determined by the serial dilution technique (Meenakshi and Anoop, 2019). The process of serial dilution was continued up to nine tubes. It contained 16384 µg ml-1 in the first tube and 64 µg ml-1 in the last test tube.
The antifungal activity of plant extract was carried out by well diffusion assay using the mycelia from a 24-h culture. Each of them was well loaded with 100 µl of the different concentrations of extracts. Negative controls were used for the same solvents as was discussed earlier. The plates were incubated at 27 ± 2 ?C for 48-72 h. The antifungal activity was evaluated by calculating Growth Inhibition Rate (%) against test organisms (Bhalodia and Shukla, 2011). Each test in this experiment was replicated four times.
Data analysis
Analysis of variance of antimicrobial activities of extracts from winter melon was assayed using SPSS-16 Program. Total antimicrobial activities were expressed as the mean ± S.D. (n=4). Significance of difference was calculated by Duncan’s new multiple range test and results with P<0.05 were considered statistically significant.
Agar well diffusion method was used to test antibacterial efficacy of crude aqueous extracts of waxy coating, peel (mature fruit) and immature fruit of winter melon. In this case 100 µl aqueous methanol extract of different sample of B. hispida were applied in each well. The wells of 9 mm diameter were prepared in pre-inoculated Mueller-Hinton Agar (MHA) plates with the test organism.
Methanol extract
At the concentration of 4.096 µg/µl (applied 100 µl), the methanol extract of waxy coating showed zone of inhibition against B. subtilis IFO 3026, S. lutea IFO 3232, X. campestris IAM 1671, E. coli iw 3310 IFO 3007, P. denitrificans KACC 32026 were 15.6 mm, 16.4 mm, 15.6 mm, 16.8 mm, 15.7 mm, respectively. Peel extract of mature fruit produced zone of inhibition against these strains 15.3 mm, 14.9 mm, 13.2 mm, 16.2 mm and 13.5 mm, respectively whereas immature fruit extract showed inhibition in the diameter 11.5 mm, 11.2 mm and 12.1 mm against S. lutea IFO 3232, X. campestris IAM 1671, E. coli iw 3310 IFO 3007 but B. subtilis, P. denitrificans were not inhibited by methanol extract of immature fruit (Figures 1 and 2).
Ethyl acetate extract
At the concentration 4.096 µg/µl (applied 100 µl), ethyl acetate extract of waxy coating of winter melon, the zone of inhibition against B. subtilis IFO 3026, S. lutea IFO 3232, E. coli iw 3310 IFO 3007, and P. denitrificans KACC 32026 produced 15.7 mm, 14.4 mm, 16.5 mm, and 16.9 mm, respectively. In case of mature fruit extract, the zone of inhibition showed 14.8mm, 13.2 mm, 15.6 mm, and 15.2 mm against tested bacterial strains B. subtilis IFO 3026, S. lutea IFO 3232, E. coli iw 3310 IFO 3007, and P. denitrificans KACC 32026, respectively. X. campestris IAM 1671 did not shown inhibition zone when the waxy coating and peel extract were applied in the well. Immature fruit extract exhibited inhibition zone in the diameter of 11.5 mm and 12.1 mm against S. lutea IFO 3232, and E. coli iw 3310 IFO 3007, respectively. In this study, the most sensitive strain was P. denitrificans KACC 32026 with the inhibition zone of 16.9 mm produced by waxy coating of B. hispida (Figures 3 and 4).
Chloroform extract
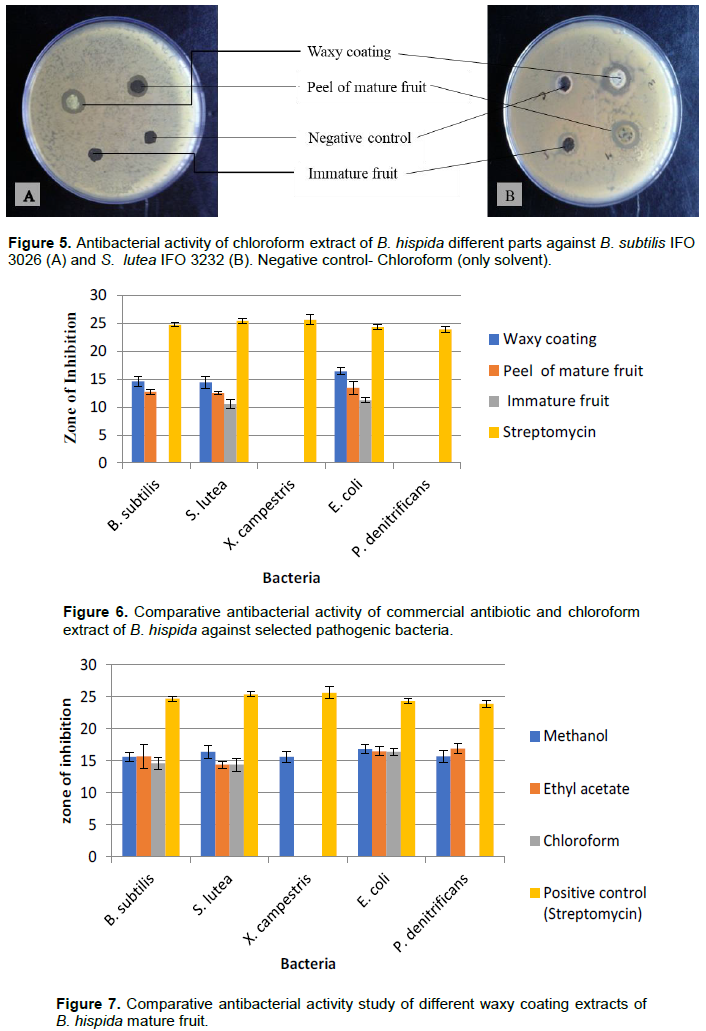
Chloroform extract of waxy coating of B. hispida produced inhibition against B. subtilis IFO 3026, S. lutea IFO 3232, and E. coli iw 3310 IFO 3007. At the concentration 4.096 µg/µl (applied 100 µl), the diameter of zone of inhibitions were found 14.6 mm, 14.4 mm, and 16.4 mm, respectively. Peel extract of chloroform also produced inhibition zones against these strains in the diameter of 12.7 mm, 12.5 mm, and 13.4 mm, respectively. X. campestris IAM 1671 and P. denitrificans KACC 32026 did not show any sensitivity with these extracts. Immature fruit extracts showed small zone of inhibition in the diameter of 10.2 mm, and 10.1 mm against S. lutea IFO 3232, and E. coli iw 3310 IFO 3007, respectively but did not produce any inhibitions against other bacterial strains (Figures 5 and 6).
Comparative study of different crude extracts of waxy coating of B. hispida against tested pathogenic bacteria
Different concentration of crude extracts of waxy coating of B. hispida was examined. It is found that methanol and ethyl acetate extract of waxy coating showed better antibacterial activity than chloroform extract. It was found that E. coli iw 3310 IFO 3007 showed minimum inhibitory concentration (MIC) of Methanol and ethyl acetate extract at lower extent than other bacteria (Figures 7 and 8).
Comparative study of different crude extracts of mature fruit (peel) of B. hispida against tested pathogenic bacteria
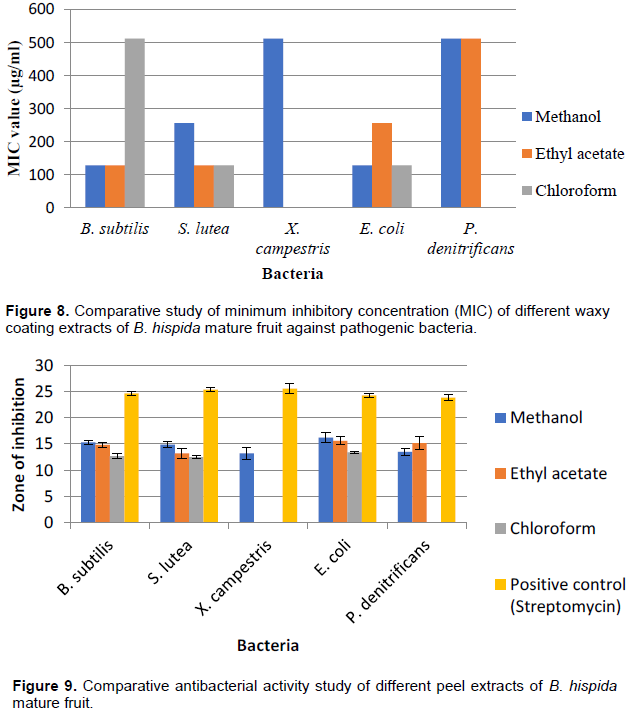
The comparative study of peel extracts of B. hispida mature fruit were evaluated against different test strains. It is indicated that methanol and ethyl acetate extract of mature fruit (peel) showed better antibacterial activity against E. coli iw 3310 IFO 3007 than chloroform extract. Ethyl acetate extract produced better inhibition zone against P. denitrificans KACC 32026 than methanol extract, whereas chloroform extract did not show inhibition zone against this bacteria and it was found that E. coli iw 3310 IFO 3007 showed minimum inhibitory concentration (MIC) of different extract at lower extent than other bacteria. Methanol extracts showed good results in case of MIC (Figures 9 and 10).
Comparative study of different crude extracts of immature fruit of B. hispida against tested pathogenic bacteria
The comparative study of immature fruit extracts of B. hispida along with standard antibiotic streptomycin. It is indicated that methanol, ethyl acetate and chloroform extract of immature fruit didn’t show better antibacterial activity against B. subtilis IFO 3026, X. campestris IAM 1671 and P. denitrificans KACC 32026, whereas showed small inhibition zone against S. lutea IFO 3232, E. coli iw 3310 IFO 3007. It was found that methanol extract showed good results in case of MIC than ethyl acetate and chloroform extracts (Figures 11 and 12).
Antifungal activity study
Agar well diffusion method was used to test antifungal efficacy of crude aqueous extracts of waxy coating, peel (mature fruit) and immature fruit of winter melon. Ethyl acetate extract of waxy coating and peel of mature fruit showed more positive results against Fusarium oxysporum than methanol or chloroform extract. Immature fruit extract did not show any considerable inhibition against this fungus (Figures 13 and 14).
The importance on the investigations of natural antimicrobials from plant extracts which can substitute synthetic chemicals may reduce harmful side effects due to potential toxicity of these commercially available drugs.
Now days multiple drug resistance become great threat of public health due to indiscriminate use of commercially available antimicrobial drugs. There is a significant role of phytochemical to mitigate societal health issues and to avoid drug resistance (Zhang et al., 2015; Karuppiah and Rajaram, 2012). Humans from the pre-historical times have used the herbs, spices, and plants as a natural sources of antimicrobial agents, although the levels and range of activity were not fully defined. Studies have pointed out that many drugs that are used in commerce have come from folk-use and use of plants by indigenous cultures (Cowan, 1999). The antimicrobial activity of the leaves of some wild Cucurbitaceae species against some human pathogenic microorganisms has investigated recently (Anyanwu and Okoye, 2017). The present study was conducted to find out the antimicrobial effects of different extracts of B. hispida belongs to Cucurbitaceae family.
In this study, different samples that are the waxy coating, peel of mature fruit, and immature fruit of B. hispida were extracted with methanol, ethyl acetate and chloroform. These extracts were evaluated againsttwo species of Gram-positive bacteria B. subtilis IFO 3026, S. lutea IFO 3232 and three species of Gram-negative bacteria, X. campestris IAM 1671, Escherichia coli iw 3310 IFO 3007, P. denitrificans KACC 32026. These organic extracts were also investigated against three pathogenic fungal species such as F. oxysporum, A. niger KTCC 1700, C. melongenae. The inhibition zone was the highest in case of standard antibiotic but methanol extract inhibited all bacterial strain efficiently than ethyl acetate or chloroform extract. Methanol extract has been reported effectiveness in many studies (Atef et al., 2019). This study explored a query about the reason of the effectiveness of methanol extract comparing other solvent extracts. Further study is required to find out the chemical composition of methanol extract and other extracts to search the responsible components of antimicrobial activity. B. hispida known as water melon is a common and popular fruits all over the world. These findings explore the health benefit of B. hispida which the authors take very frequently. So, B. hispida can be recommended antimicrobial agent.
Winter melon is used for the management of various diseases and also used as antioxidant, styptic, anti-inflammatory, astringent, anthelmintic, aphrodisiac, demulcent, diuretic, febrifuge, and tonic agents. The results of the study revealed that the different extracts of waxy coating and peel of mature of B. hispida possess many active ingredients that inhibited the growth of selected pathogenic bacteria (B. subtilis IFO 3026, S. lutea IFO 3232, X. campestris IAM 1671, E. coli iw 3310 IFO 3007, P. denitrificans KACC 32026) . On the other hand, ethyl acetate extracts of waxy coating and peel of mature winter melon fruit showed maximum antifungal activity against F. oxysporum. So, it is concluded that waxy coating and peel of mature fruit has better antibacterial and antifungals activities. If it is possible to find out the reason of self-protective mechanism of mature fruits of B. hispida, there is enormous possibility to prevent immature fruit-rot of winter melon from various infections and also will help to develop antifungal and antibacterial agents.
The authors have not declared any conflict of interests.
The entire work was funded by Islamic University research fund Ref.-141/Edu./IU-2019/385 Date: 16-11-2019.
REFERENCES
|
Al-Snafi A (2013). The Pharmacological Importance of Benincasa hispida. A review. International Journal of Pharmaceutical Sciences and Research 4(12):165-170.
|
|
|
|
Anyanwu MU, Okoye RC (2017). Antimicrobial activity of Nigerian medicinal plants. Journal of Intercultural Ethnopharmacology 6(2):240-259.
Crossref
|
|
|
|
Arora P, Kaushik D (2016). Therapeutic potential of Benincasa cerifera: A review. Chinese Journal of Integrative Medicine, pp. 1-14.
Crossref
|
|
|
|
Ashok P, Koti BC, Thippeswamy AHM, Tikare VP, Dabadi P, Viswanathaswamy AHM (2010). Evaluation of Antiinflammatory Activity of Centratherum anthelminticum (L) Kuntze Seed. Indian Journal of Pharmaceutical Sciences 72(6):697.
Crossref
|
|
|
|
Atef NM, Shanab SM, Negm SI (2019). Evaluation of antimicrobial activity of some plant extracts against antibiotic susceptible and resistant bacterial strains causing wound infection. Bulletin of the National Research Centre 43(1):1-11.
Crossref
|
|
|
|
Bhalodia NR, Shukla VJ (2011). Antibacterial and antifungal activities from leaf extracts of Cassia fistula l. An ethnomedicinal plant. Journal of Advanced Pharmaceutical Technology and Research 2:104-109.
Crossref
|
|
|
|
Cowan MM (1999). Plant products as antimicrobial agents. Clinical microbiology reviews 12(4):564-582.
Crossref
|
|
|
|
Elhassan B, Nouria N, Amal D (2020). Antimicrobial Effect of Quercus robur L Leaves Selective Extracts from the Mezi Mountain of Djeniene Bourezg (West of Algeria). Current Bioactive Compounds 16(8):1181-1190.
Crossref
|
|
|
|
Elnaggar M, Abdulla G, El-Shourbagy G, El-Badawi A, El-Sohaimy S (2019). Antimicrobial and antioxidant activities of some plant extracts. Polymers 44:1061-1071.
Crossref
|
|
|
|
Gu M, Fan S, Liu G, Guo L, Ding X, Lu Y, Zhang Y, Ji G, Huang C (2013). Extract of Wax Gourd Peel Prevents High-Fat Diet-Induced Hyperlipidemia in C57BL/6 Mice via the Inhibition of the PPARγ Pathway. Evidence-Based Complementary and Alternative Medicine P 342561.
Crossref
|
|
|
|
Ikeda UP, Silva M, Di Stasi L, Barbosa L, Junior A (2007). Antibacterial activity of medicinal plant extracts. Brazilian Journal of Microbiology 38:717-719.
Crossref
|
|
|
|
Jhonatas ERC, Joyce FCG, Marcos SG, Guilherme ROF, Enyara RM (2019). Phytochemical Analysis and Evaluation of Antimicrobial Activity of Peumus boldus, Psidium guajava, Vernonia polysphaera, Persea Americana, Eucalyptus citriodora Leaf Extracts and Jatropha multifida Raw Sap. Current Pharmaceutical Biotechnology 20(5):433-444.
Crossref
|
|
|
|
Juliana MP, Priscilla CV, Grazielle NN, Meireles MAA (2018). Extraction Methods for Obtaining Natural Blue Colorants. Current Analytical Chemistry 16(5):504-532.
Crossref
|
|
|
|
Karuppiah P, Rajaram S (2012). Antibacterial effect of Allium sativum cloves and Zingiber officinale rhizomes against multiple-drug resistant clinical pathogens. Asian Pacific journal of tropical biomedicine 2(8):597-601.
Crossref
|
|
|
|
Khan V, Najmi AK, Akhtar M, Aqil M, Mujeeb M, Pillai KK (2012). A pharmacological appraisal of medicinal plants with antidiabetic potential. Journal Of Pharmacy and Bioallied Sciences 4(1):27.
Crossref
|
|
|
|
Lee K, Choi HR, Kim CH (2005). Anti-angiogenic effect of the seed extract of Benincasa hispida Cogniaux. Journal of Ethnopharmacology 97(3):509-513.
Crossref
|
|
|
|
Mahboob N, Iqbal H, Ahmed M, Magnet MMH, Mamun K (2019). Disk diffusion method in enriched mueller hinton agar for determining susceptibility of candida isolates from various clinical specimens Journal of Dhaka Medical College 28(1):28-33.
Crossref
|
|
|
|
Mandal U, De D, Ali K, Biswas A, Ghosh D (2012). Effect of different solvent extracts of Benincasa hispida T on experimental hypochlorhydria in rat. Journal of Advanced Pharmaceutical Technology and Research 3(1):41-46.
|
|
|
|
Martinelli F, Scalenghe R, Davino S, Panno S, Scuderi G, Ruisi P, Villa P, Stroppiana D, Boschetti M, Goulart LR (2015). Advanced methods of plant disease detection. A review. Agronomy for Sustainable Development 35(1):1-25.
Crossref
|
|
|
|
Meenakshi G, Anoop K (2019). Comparison of Minimum Inhibitory Concentration (MIC) value of statin drugs: A Systematic Review. Anti-Infective Agents 17(1):4-19.
Crossref
|
|
|
|
Meshram GG, Kumar A, Rizvi W, Tripathi CD, Khan RA (2016). Evaluation of the anti-inflammatory activity of the aqueous and ethanolic extracts of the leaves of Albizzia lebbeck in rats. Journal of Traditional and Complementary Medicine 6(2):172-175.
Crossref
|
|
|
|
Mohsenipour Z, Hassanshahian M (2016). Antibacterial Activity of Euphorbia hebecarpa Alcoholic Extracts Against Six Human Pathogenic Bacteria in Planktonic and Biofilm Forms. Jundishapur Journal of Microbiology 9(6):e34701.
Crossref
|
|
|
|
Patil KR, Patil CR (2017). Anti-inflammatory activity of bartogenic acid containing fraction of fruits of Barringtonia racemosa Roxb. in acute and chronic animal models of inflammation. Journal of Traditional and Complementary Medicine 7(1):86-93.
Crossref
|
|
|
|
Rahmatullah M, Hasan A, Parvin W, Moniruzzaman M, Khatun A, Khatun Z, Jahan FI, Jahan R (2012). Medicinal plants and formulations used by the Soren clan of the Santal tribe in Rajshahi district, Bangladesh for treatment of various ailments. African Journal of Traditional, Complementary and Alternative Medicines 9(3):350-359.
Crossref
|
|
|
|
Salas CE, Badillo-Corona JA, Ramírez-Sotelo G, Oliver-Salvador C (2015). Biologically active and antimicrobial peptides from plants. BioMed Research International pp.102-129.
Crossref
|
|
|
|
Sasidharan S, Chen Y, Saravanan D, Sundram KM, Yoga Latha L (2011). Extraction, isolation and characterization of bioactive compounds from plants' extracts. African Journal of Traditional, Complementary and Alternative Medicines 8(1).
Crossref
|
|
|
|
Sharma S, Verma HN, Sharma NK (2014). Cationic Bioactive Peptide from the Seeds of Benincasa hispida. International Journal of Peptides Article ID 156060, 12 p.
Crossref
|
|
|
|
Sheahan M, Barrett CB (2017). Food loss and waste in Sub-Saharan Africa. Food Policy 70:1-12.
Crossref
|
|
|
|
Shetty RP, Endy D, Knight TF (2008). Engineering BioBrick vectors from BioBrick parts. Journal of Biological Engineering 2:5.
Crossref
|
|
|
|
Simons FER, Simons KJ (2008). H1 antihistamines: current status and future directions. World Allergy Organization Journal 1(9):145-155.
Crossref
|
|
|
|
Tatiya AU, Saluja AK, Kalaskar MG, Surana SJ, Patil PH (2017). Evaluation of analgesic and anti-inflammatory activity of Bridelia retusa (Spreng) bark. Journal of Traditional and Complementary Medicine 7(4):441-451.
Crossref
|
|
|
|
Valgas C, Souza SMD, Smania EFA, Smania Jr A (2007). Screening methods to determine antibacterial activity of natural products. Brazilian Journal of Microbiology 38(2):369-380.
Crossref
|
|
|
|
Wen W, Lu J, Zhang K, Chen S (2008). Grape seed extract inhibits angiogenesis via suppression of the vascular endothelial growth factor receptor signaling pathway. Cancer Prevention Research 1(7):554-561.
Crossref
|
|
|
|
Xing Y, Xu Q, Li X, Che Z, Yun J (2012). Antifungal activities of clove oil against Rhizopus nigricans, Aspergillus flavus and Penicillium citrinum in vitro and in wounded fruit test. Journal of Food Safety 32(1):84-93.
Crossref
|
|
|
|
Yagnik B, Vaghasiya J, Nurudin J, Kanzariya N, Rameshwar P, Natavarlal P (2009). Antioxidant activity of Benincasa hispida on renal ischemia/reperfusion injury. Pharmacologyonline 1:44-49.
|
|
|
|
Yuan H, Ma Q, Ye L, Piao G (2016). The Traditional Medicine and Modern Medicine from Natural Products. Molecules 21:559.
Crossref
|
|
|
|
Zhang YJ, Gan RY, Li S, Zhou Y, Li AN, Xu DP, Li HB (2015) Antioxidant Phytochemicals for the Prevention and Treatment of Chronic Diseases. Molecules 20(12):21138-56.
Crossref
|