Full Length Research Paper
ABSTRACT
Vancomycin-resistant enterococci (VRE) have been reported as a major hazard to human and animal health, causing problems like clinical mastitis in dairy cattle and nosocomial infections in humans. The aim of this study was to determine the in vitro microbial susceptibility profile of enterococci isolated from dairy farms in São Paulo and Minas Gerais states, Brazil. A total of 310 samples of Enterococcus species from bovine mastitis (52) and bulk tanks (258) were analyzed. The sensibility profile was studied by the disk diffusion method, and the isolates intermediately resistant to vancomycin were submitted to the minimum inhibitory concentration (MIC) test. The enterococci also were classified according to their multiresistance profile. All isolates were sensitive to vancomycin. Most of the isolates from the bulk tanks were resistant to cephalexin (93.8%), novobiocin (98.8%), cefoxitin (91.9%) and oxacillin (91.9%), while those isolated from mastitis presented a high resistance to trimethoprim/sulfamethoxazole (84.7%), novobiocin (100%), cefoxitin (88.5%) and oxacillin (80.8%). All isolates were sensitive to vancomycin (VSE). The high prevalence of isolates resistant to multiple antimicrobials emphasizes the risks existing in the use of ineffective antibiotics.
Key words: Enterococci, mastitis, multiresistence, milk, vancomycin.
INTRODUCTION
Enterococci are Gram-positive cocci and opportunistic pathogens frequently isolated from hospital environments (Arias and Murray, 2012; Prieto et al., 2016). These bacteria are considered highly multi-drug resistant, with special regards to vancomycin (Raza et al., 2018), indicating an increased risk of nosocomial infections in humans (De Kraker et al., 2013; Raza et al. 2018). Glycopeptide antibiotics (e.g. vancomycin) are the main drugs used against Gram-positive bacteria causing severe human infections unresponsive to other antibiotics. Indeed, glycopeptides provide an alternative therapy against bacteria such as methicillin- or oxacillin-resistant Staphylococcus aureus (MRSA and ORS, respectively). However, some enterococci isolates have been described as resistant to glycopeptides, which can be an obstacle for the control and treatment of enterococci infections (Tavares, 2014). A study developed in Estonia observed two Enterococcus faecalis isolates from humans with vanB genes highly resistant to vancomycin. These molecularly characterized resistance genes are present in strains with diverse origins, suggesting the occurrence of plasmid transfer events (Aun et al., 2021).
Although enterococci are considered sensitive to vancomycin, a rise in strains with resistance genes of potential transmission has been recorded (Tavares, 2014; Raza et al., 2018). These strains are termed Vancomycin-Resistant Enterococci (VRE) and are considered ‘superbacteria’ as determined by genotypic and phenotypic features (Werner, 2012; Tavares, 2014).
The importance of determining these genotypic and phenotypic profiles lies in the understanding of the movement of resistance genes, a phenomenon produced by plasmids and transposons in which genes are transferred across enterococci and other Gram-positive bacteria (Aun et al., 2021; Van Schaik et al., 2010; Werner, 2012).
Moreover, enterococci present relevance as an etiological agent of bovine mastitis. In a recent study performed in Poland with 2,000 mastitic milk samples from confirmed cases of bovine mastitis, 21.3% were caused by Enterococcus species (Ró?a?ska et al., 2019). Such inflammatory disease represents a major issue in dairy farming, especially when caused by VREs, which are more difficult to treat and can represent a huge risk of dissemination of resistance genes to other pathogens that causes mastitis, like S. aureus (Werner, 2012). Besides, VRE-induced mastitis can be a risk to public health as a foodborne pathogen, since VREs may be present in subclinical cases of mastitis and, therefore, infect humans through consumption of contaminated milk from apparently healthy cows (Werner, 2012; Kateete et al., 2013).
In Brazil, the most studied and prevalent bacterium in cattle is S. aureus. A recent study analyzed 400 strains isolated from mastitic animals in four Brazilian states, and showed that these strains have important resistance genes in almost all classes of antimicrobials currently used for human and animal treatment in the country (Pérez et al., 2020). In the case of Klebiseila pneumoniae, another very prevalent bacterium in the country, the presence of genes encoding β-lactamases was observed in several bacteria, including genes for drug resistance for commonly used drugs in the treatment of mastitis in Brazilian dairy herds (gentamicin, cephalosporins, sulfamethoxazole-trimethoprim, tetracycline), as well as antimicrobials of critical importance to human health (meropenem, ceftazidime, fluoroquinolones) (Nobrega et al., 2021). A recent study, observed the species diversity and antimicrobial resistance patterns of Enterococcus spp. isolated from mastitis cases, milking machine and dairy cow environment. As a result, of the 365 isolates studied from several Brazilian municipalities, 1.9, 0.3 and 0.6% were resistant to penicillin, vancomycin and teicoplanin, respectively. This is a recent data that demonstrates the current situation (Juliano et al., 2022).
The relevance in assessing the multiresistance of enterococci isolated from bovine milk is in four main premises: (1) human transmission through ingestion of contaminated milk, which has paramount importance in Brazil where 20 to 30% of milk and dairy products are sold without inspection or heat treatment (IBGE, 2015); (2) significant gaps are found in the literature with respect to the multiresistant profile of enterococci isolated from bovine milk in Brazil; (3) Enterococcus spp. are neglected etiological agents of cattle mastitis (Kateete et al., 2013); and (4) Enterococcus spp. can transmit resistance genes to S. aureus, the most important pathogen of mastitis in cattle (Werner, 2012).
Therefore, the aims of this study were to determine the in vitro antimicrobial susceptibility profile of Enterococcus spp. isolated from bulk tanks and mastitic milk samples from dairy farms in the states of São Paulo and Minas Gerais (Brazil).
MATERIALS AND METHODS
The farm
A total of 310 species of Enterococcus spp. were studied, of which 52 were from confirmed cases of bovine clinical mastitis and 258 from bulk tanks. The milk samples positive for enterococci were obtained between 2018 and 2019 from four dairy farms in São Paulo State and six in Minas Gerais State, Brazil.
All ten cattle herds had mastitis control programs, including computer software for data record, a somatic cell count (SCC) in bulk tanks up to 400,000 cs mL-1, a minimum of 200 lactating cows, and an automatic milking machine. In addition, the milk from the dairy farms was supplied by a high-production Holstein Friesian cattle (> 20 L each animal per day).
Animals with mastitis were treated with different classes of antibiotics, depending on the farm of origin. The most used antibiotics, in descending order, were: ceftiofur (third generation cephalosporin), tetracycline hydrochloride (tetracycline class), neomycin (aminoglycoside class), bacitracin (aminoglycoside class), cefquinoma (cephalosporin class), cefoperazone (cephamycins class) and enrofloxacin (quinolones class).
Isolation
The cases of clinical mastitis were based upon detection of macroscopic changes common to mastitic milk (e.g. clumps, pus or blood) and the systemic signs of mastitis in dairy cows (e.g. fever, loss of appetite or behavior changes) (Radostits et al., 2007). Mastitic milk samples from these animals were collected in sterile vials following teat disinfection with 70% alcohol and were immediately kept refrigerated (maximum of 7°C) during transfer to the Research Nucleus on Mastitis (NUPEMAS) laboratory of the São Paulo State University (UNESP), School of Veterinary Medicine and Animal Science, Botucatu, São Paulo State, Brazil. At the same time, monthly bulk tanks milk samples of each farm were transferred to the laboratory with the same sterility and refrigeration conditions.
In the laboratory, mastitic milk samples were spread on MacConkey agar and blood agar plates, with the latter being supplemented with 5% defibrinated ovine blood. Samples were incubated at 37°C under aerobic conditions, and examined every 24 h for three days. The bacteria were classified according to the criteria adopted by the National Mastitis Council (NMC, 1999). The colonies that were morphologically compatible with enterococci were analyzed after Gram stain by microscopy, as well as by catalase and hemolysins tests. The samples were assayed by the following biochemical tests as described by Facklam and Collins (1989) and updated by Facklam (2007): growth in halophyte broth (6.5% NaCl), esculin hydrolysis, hydrolysis of L-pyrrolidonyl-beta-naphthylamide (PYR), arginine decarboxylase test, pigment production, motility, tetrazolium reduction, mannitol, arabinose, raffinose and sorbitol fermentation test.
From the bulk tanks, 1 mL of sample was pipetted into test tubes along with 9 mL of 0.1% sterile peptone water (CLSI, 2014). Later, dilutions were carried out in a serial fashion from 10-1 to 10-3. A total volume of 1 mL from each dilution was spread onto Enterococcosel agar, and incubated at 37°C for 24 h to identify the enterococci colonies based on their morphological characteristics.
In vitro antimicrobial susceptibility profile
As proposed by Bauer et al. (1966), the antimicrobial susceptibility was evaluated by the disk diffusion method, where the bacteria were submitted to the following antimicrobials in Mueller-Hinton agar: Ampicillin - 10 µg (AMP), Cephalexin - 30 µg (CL), Cefoxitin - 10 µg (CFO), Ciprofloxacin - 10 µg (CIP), Enrofloxacin - 10 µg (ENR), Gentamicin -10 µg (GEN), Marbofloxacin - 5 µg (MRB), Neomycin - 30 µg (N), Novobiocin - 5 µg (NOV), Oxacillin - 10 µg (OXA), Penicillin G - 10 U.I. (PEN), Sulfamethoxazole/Trimethoprim - 25 µg (SXT), Tetracycline - 30 µg (TE), Teicoplanin - 30 µg (TEC) and Vancomycin - 10 µg (VAN). International reference strains were used with a positive control (E. faecium BM 4147 vanA genotype) and a negative control (S. aureus ATCC 25923). The zone of inhibition was interpreted according to CLSI (2014), and ascribing the isolates the categories susceptible (S), intermediate (I) and resistant (R).
MIC test of milk samples
The intermediate enterococci samples to VAN were tested with the minimum inhibitory concentration (MIC) method, which exhibits a higher accuracy than the disk diffusion method (Bauer et al., 1966). The commercial kit E-testTM was used in Mueller-Hinton agar, following the manufacturer's recommendations (Bauer et al., 1966; CLSI, 2014).
Multiresistance classification
The enterococci isolates that were simultaneously resistant to at least three different groups/classes of antimicrobials were designated multi-resistant.
Statistics
Descriptive statistics were used through the distribution of absolute frequencies relative to the results obtained in the in vitro microbial sensitivity tests. Fisher´s exact test was performed to verify if there were differences between the percentage of multiresistant strains from bulk tanks and mastitis samples. Kruskal Wallis test was used to verify if the median number of antimicrobial compounds to which the isolates were resistant differed between bulk or mastitis isolates. The significance level of 0.05 was considered for the analyses.
RESULTS AND DISCUSSION
None of the isolates were resistant to VAN, while 53 isolates were intermediate resistant (all bulk). These 53 intermediate resistant enterococci showed no resistance by the minimum inhibitory concentration (MIC) method.
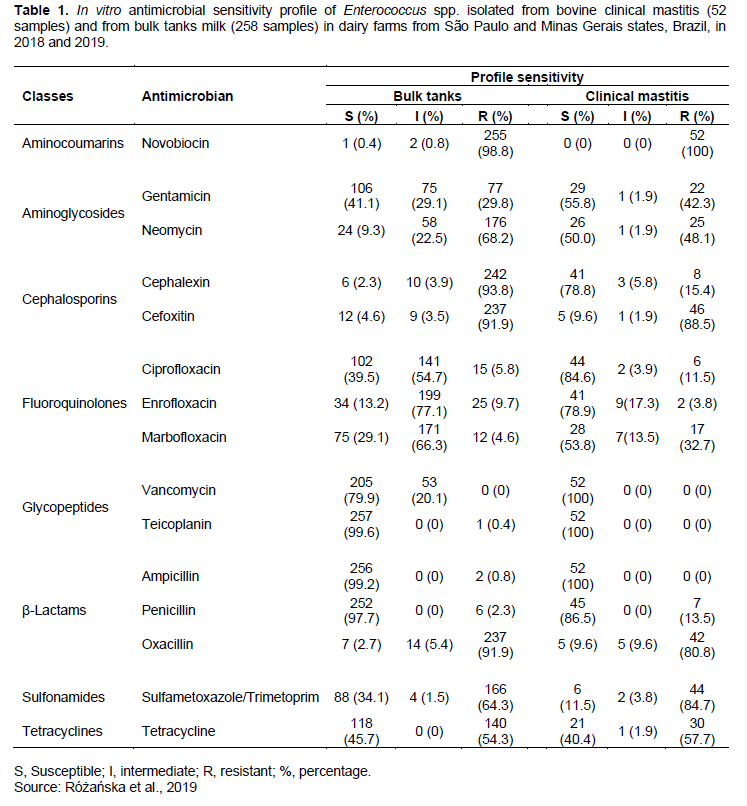
VRE are frequently isolated in hospital environments, and the studies related to in vitro antimicrobial sensitivity of enterococci isolated from bovine clinical mastitis have shown a low prevalence of resistant to VAN (Ró?a?ska et al., 2019), corroborating our findings. In Turkey, enterococci isolated from clinical mastitis showed 1.1% of isolates resistant to VAN (Erbas et al., 2016). In Poland, 426 enterococci from clinical mastitis showed 0.94% of resistance to this drug (Ró?a?ska et al., 2019). However, a significant prevalence was observed in Slovakia, with 15.2% of enterococci from bulk tanks resistant to VAN (Fabianová et al., 2010). The same study also found a high prevalence of enterococci susceptible to TEC, as was detected in isolates from bulk tanks (99.6%) and in all isolates from mastitis (Table 1).
Regarding the multiresistant strains, 245 isolates were isolated from bulk tanks (95%) and 47 from clinical mastitis (90.4%). Of these, 74.6% (bulk tanks) and 72.3% (mastitis) were resistant for at least one drug among five or more classes of antibiotics tested (Table 2).
The isolates showed high percentages of resistance to the antibiotics most commonly used on farms for the treatment of animals: ceftiofur (88.5%), tetracycline (57.7%) and neomycin (48.1%). This result is an indication of the difficulty in treating clinical mastitis on farms and the recurrence of cases annually.
In Table 1, the multiresistance profile of bulk tanks isolates are shown. More than 90% of the isolates were resistant to CL (93.8%), NOV (98.8%), CFO (91.9%), and OXA (91.9%). Among isolates from bovine mastitis, the highest resistance was identified to SXT (84.7%), NOV (100%), CFO (88.5%), and OXA (80.8%).
Drugs like PEN, TE, Cephalosporins (CL and CFO), and Fluoroquinolones (ENR, MRB and CIP) are frequently used in dairy farms, therefore, study of the susceptible profile of these antibiotics is very important (Beuron et al., 2014; Stevens et al., 2016). A low prevalence of resistance to PEN was identified, which corresponded to 13% of isolates from clinical mastitis and 2% of samples from bulk tanks. Regarding TE, 54% of isolates from bulk tanks and 57% from clinical mastitis were resistant. Ró?a?ska et al. (2019) also showed a low percentage of resistant strains from clinical mastitis to PEN (2.58%). The high prevalence of resistant strains to cephalosporins in this study should be noted (Table 1).
Resistance to fluorquinolones was low in strains from bulk tanks and clinical mastitis (less than 10% of isolates). However, there was a high percentage of intermediate resistance in the isolates from bulk tanks, 54.7, 77.1, and 66.3%, respectively to CIP, ENR, and MRB. Of the clinical mastitis isolates, 11.5 and 32.7% were resistant to CIP and MRB, respectively (Table 1). And for CIP, Ró?a?ska et al. (2019) noticed 0.47% of enterococci resistant from clinical mastitis.
Fabianová et al. (2010) reported 8.7 and 4.7% of resistant enterococci from bulk tanks to GEN and AMP, respectively. In this study, a low percentage of resistant strains were observed to AMP, only 0.8%. For GEN, 29.8% were resistant, while 29% presented an intermediate resistance (Table 1).
Erbas et al. (2016) noticed a high prevalence of resistant enterococci to TE, 81% of isolates isolated from clinical mastitis, while Ró?a?ska et al. (2019) observed 61.5%. In this study 57.7% of resistant strains were identified as shown in Table 1.
.png)
According to Fisher's test, the percentage of multidrug-resistant strains in the tanks was 94.96% and in the animals was 90.38%. There was no statistical difference, with a p value of 0.1986. According to the Kruskal Wallis test, the median number of resistant compounds of the tank was equal to 6, and the median number of resistant compounds of the animals with mastitis was 5. There was no statistical difference in the median of the two groups, and the p value was 0.1350. In summary, there was no considerable statistical difference between isolates originating from bulk tanks and from animals with mastitis. We suggest that milk samples collected from the tanks may be adequate for antimicrobial sensitivity profiling research, as they are simpler and less expensive for farmers.
Data about antimicrobial sensitivity of Enterococcus spp. from milk from cattle, in bulk tanks or clinical mastitis, are scarce. Studies related to this topic indicate that there are many factors involved in the emergence of resistant strains, and that this will depend on the farm, the management performed, and the antimicrobial treatment applied (Ró?a?ska et al., 2019; Erbas et al., 2016; Fabianová et al., 2010).
In Brazil, about 20 to 30% of milk and dairy products are sold without any heat treatment (IBGE, 2015). Our results show that more than 90% of enterococci from bulk tanks, which are milk ready for use are multiresistant, indicating risks to public health through ingestion of contaminated milk. In addition, the presence of these enterococci infecting cows could lead to horizontal transfer of resistance genes to other Gram-positive bacteria present in the mammary glands.
The treatment and the preventive use of antimicrobials in bovine mastitis cases are associated with the indiscriminate use of these drugs, and probably this is the main reason for the increase of resistant strains in cattle
farms (Krömker and Leimbach, 2017). Many studies connect the indiscriminate use of antibiotics in animal production with the dissemination of multiresistant Enterococcus spp. Therefore, the transmission of these pathogens by foods is a big risk to public health (Tavaras et al., 2012; Ali et al., 2013; Ró?a?ska et al., 2019).
More studies are needed to identify the risk factors associated to multiresistant Enterococci spp. causing bovine mastitis, including the use of genetic analyses able to characterize the enterococci species. This study might assist future researches on the impact of enterococci in animal and public health, as well as guide which treatment protocols can be used in cases of bovine mastitis.
CONCLUSION
No Enterococcus spp. resistant to vancomycin was detected. However, a high multiresistant enterococci strains were detected in bulk tanks (95%) and from clinical mastitis cases (90.4%), pointing to the risks of misuse of antibiotics in dairy cattle farming. These data highlight the need for more studies on the impacts of enterococci infections in both human and animal health, especially towards the possibility of transmission of resistance genes from Enterococcus spp. to other pathogenic bacteria.
CONFLICT OF INTERESTS
The authors have not declared any conflict of interests.
ACKNOWLEDGMENT
This study was funded by the Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP), processes number 2015/19688-8, 2017/08823-7, 2018/09103-0.
REFERENCES
|
Arias CA, Murray BE (2012). The rise of the Enterococcus: beyond vancomycin resistance. Nature Reviews Microbiology 10(4):266-78. |
|
|
Aun E, Kisand V, Laht M, Telling K, Kalmus P, Väli U, Brauer A, Remm M, Tenson T (2021). Molecular Characterization of Enterococcus Isolates From Different Sources in Estonia Reveals Potential Transmission of Resistance Genes Among Different Reservoirs. Frontiers 26(12):601490. |
|
|
Bauer AW. Kirby WM, Sherris JC, Turcket M (1966). Antibiotic susceptibility testing by a standardized single disk method. American Journal of Clinical Pathology 45(4):493-96. |
|
|
Beuron DC, Cortinhas CS, Botaro BG, Macedo SN, Gonçalves JL, Brito MAVP, Santos MV (2014). Risk factors associated with the antimicrobial resistance of Staphylococcus aureus isolated from bovine mastitis. Pesquisa Veterinária Brasileira 34(10):947-952. |
|
|
Clinical and Laboratory Standards Institute (CLSI) (2014). Performance Standards for Antimicrobial Susceptibility Testing. United States of America: M100-S24, 199 p. |
|
|
De Kraker MEA, Jarlier V, Monen JCM, Heuer OE, Sande N, Grundmann H (2013). The changing epidemiology of bacteraemias in Europe: Trends from the European antimicrobial resistance surveillance system. Clinical Microbiology and Infection 19(9):860-868. |
|
|
Erbas G, Parin U, Turkyilmaz S, Ucan N, Ozturk M, Kaya O (2016). Distribution of antibiotic resistance genes in Enterococcus spp. isolated from mastitis bovine milk. Acta Veterinaria 66(3):336-346. |
|
|
Fabianová J, Ducková V, ?anigová M, Kro?koet M (2010). Presence of enterococci in cow milk and their antibiotic resistance. Potravinárstwo 4(2):17-21. |
|
|
Facklam RR (2007). Enterococcus. In: Murray PR, Baron EJ, Manual of Clinical Microbiology. Washington, DC: American Society for Microbiology pp. 430-442. |
|
|
Facklam RR, Collins M (1989). Identification of Enterococcus species isolated from human infections by a conventional test scheme. Journal of Clinical Microbiology 27(4):731-34. |
|
|
Prieto GAM, Schaik W, Rogers MRC, Coque TM, Baquero F, Corander J, Willems RJL (2016). Global emergence and dissemination of enterococci as nosocomial pathogens: Attack of the clones? Frontiers in Microbiology 7(5):1-15. |
|
|
Instituto Brasileiro de Geografia e Estatística (IBGE) (2015). Produção da pecuária municipal. v. 29. |
|
|
Juliano LCB, Gouvêa FLR, Latosinski GS, Fabri FHH, Salvador TB, Guimaraes FF, Ribeiro MG, Langoni H, Rall VLM, Hernandes RT, Leite DS, Pantoja JCF (2022). Species diversity and antimicrobial resistance patterns of Enterococcus spp. isolated from mastitis cases, milking machine and the environment of dairy cows. Letters in Applied Microbiology. |
|
|
Kateete DP, Kabugo U, Baluku H, Nyakarahuka L, Kyobe S, Okee M, Najjuka CF, Joloba ML (2013). Prevalence and Antimicrobial Susceptibility Patterns of Bacteria from Milkmen and Cows with Clinical Mastitis in and around Kampala, Uganda. Plos One 8(5):1-12. |
|
|
Krömker V. Leimbach S (2017). Mastitis treatment-Reduction in antibiotic usage in dairy cows. Reproduction in Domestic Animals 52(3):21-9. |
|
|
National Mastitis Council (NMC) (1999). Laboratory Handbook on Bovine Mastitis: National Mastitis Council. Madison: WI, 222 p. |
|
|
Nobrega DB, Calarga AP, Nascimento LC, Chande Vasconcelos CG, de Lima EM, Langoni H, Brocchi M (2021). Molecular characterization of antimicrobial resistance in Klebsiella pneumoniae isolated from Brazilian dairy herds. Journal of Dairy Science 104(6):7210-7224. |
|
|
Pérez VKC, Custódio DAC, Silva EMM, de Oliveira J, Guimarães AS, Brito MAVP, Souza-Filho AF, Heinemann MB, Lage AP, Dorneles EMS (2020). Virulence factors and antimicrobial resistance in Staphylococcus aureus isolated from bovine mastitis in Brazil. Brazilian Journal of Microbiology 51(4):2111-22. |
|
|
Radostits OM, Gay CC, Hinchcliff KW, Constable PD (2007). Veterinary medicine: A textbook of the dieases of cattle, horses, sheep, pigs and goats. Spain: Saunders. 2065p. |
|
|
Raza T, Ullah SR , Mehmood K, Andleeb S (2018). Vancomycin resistant enterococci: A brief review. Journal of the Pakistan Medical Association 68(5):768-72. |
|
|
Ró?a?ska H, Lewtak-Pi?at A, Kubajka M, Weiner M (2019). Occurrence of Enterococci in Mastitic Cow's Milk and their Antimicrobial Resistance. Journal of Veterinary Research 63(1):93-97. |
|
|
Stevens M, Piepers S, Vliegher S (2016). Mastitis prevention and control practices and mastitis treatment strategies associated with the consumption of (critically important) antimicrobials on dairy herds in Flanders, Belgium. Journal of Dairy Science 99(4)2896-903. |
|
|
Tavares W (2014). Antibióticos e Quimioterápicos para o Clínico. 3. ed. São Paulo: Atheneu, 746 p. |
|
|
Van Schaik W, Top J, Riley DR, Boekhorst J, Vrijenhoek JEP, Schapendonk CME, Hendrickx APA, Nijman IJ, Bonten MJM, Tettelin H, Willems RJL (2010). Pyrosequencing-based comparative genome analysis of the nosocomial pathogen Enterococcus faecium and identification of a large transferable pathogenicity island. BMC Genomics 11(1):1-18. |
|
|
Werner G (2012). Current Trends of Emergence and Spread of Vancomycin-Resistant Enterococciby. In: PENA M. Antibiotic resistant bacteria: a continuous challenge in the new millennium. Croácia: Intech pp. 303-354. |
|
Copyright © 2024 Author(s) retain the copyright of this article.
This article is published under the terms of the Creative Commons Attribution License 4.0