Full Length Research Paper
ABSTRACT
This study aimed to evaluate the performance of the PanbioTM Covid-19 Ag Rapid Test (Abbott) in a medical center in Ouagadougou. The PanbioTM COVID-19 Ag test was evaluated from January 26 to March 31, 2021 in symptomatic and asymptomatic patients in the medical Centre of Kossodo. A total of 268 individuals were tested by both SARS-CoV-2 RT-PCR, and antigen RDT. Of these 268 individuals, 52 were positive and 216 were negative for COVID-19 RT-PCR. The performance parameters of the test and its Kappa agreement with the RT-PCR were calculated according to the presence or absence of symptoms in the patients on one hand, and according to the time onset of symptoms on the other hand. The sensitivity of the Panbio™ COVID-19 Ag Rapid Test ranged from 29.63% (95% CI: 13.75 to 50.18) among COVID-19 asymptomatic patients, to 87.5% (95% CI: 52.91 to97.76) among symptomatic patients with symptom onset time of 1-5 days. Similarly, the Panbio™ COVID-19 Ag Rapid Test specificity was 97.3% (95% CI: 90.58 to 99.67) and 96.4% (95% CI: 91.81 to 98.82) in symptomatic and asymptomatic RT PCR negative patients. The Panbio™ COVID-19 Ag Rapid Test shows good performance in detecting COVID-19 cases in patients with a symptom onset time of less than seven (7) days. This performance is even better when the symptom onset is reduced to five (5) days. The results show that the antigen RDT is not suitable for COVID-19 detection among asymptomatic patients.
Key words: COVID-19, SARS-CoV-2, diagnosis, antigen test, rapid test, point-of-care.
INTRODUCTION
COVID-19 has been a major public health problem for countries around the world since it emerged in December 2019 in Wuhan, People's Republic of China (Wu et al., 2020). The SARS-COV-2 infection is undoubtedly one of the greatest pandemics that humanity has ever experienced. According to new estimates by the World Health Organization (WHO), as of 30 August 2022, the global epidemiology estimates 599,071,265 confirmed cases of COVID-19 and 6,467,023 deaths (WHO, 2021b). The African region, particularly Burkina Faso, seems to be relatively spared by the pandemic compared to the rest of the world (Wamai et al., 2021). In Burkina Faso, as of 31 July 2022, the number of COVID-19 officially reported cases was 21,204, including 387 deaths (https://covid19.who.int/table).
The current reference method for COVID-19 diagnosis is Real-Time Polymerase Chain Reaction (RT-PCR), which is specific for the detection parts of the SARS-CoV-2 genome and the virus responsible for COVID-19 (Carter et al., 2020; La Marca et al., 2020; Zhai et al., 2020). This diagnostic method is only available in laboratories equipped with a molecular biology technical platform such as RT-PCR thermocyclers. It is performed on nasopharyngeal or oropharyngeal swabs, sputum or bronchoalveolar lavage samples (Zhai et al., 2020). According to standard protocols, RNA (ribonucleic acid) must be extracted and its presence confirmed by RT-PCR (Carter et al., 2020; Zhai et al., 2020).
This requires several steps and sometimes about 48 to 72 h for the return of the results to the care staff, with the potential risk for further spread of the virus meanwhile. RT-PCR is the gold standard for detection of SARS-CoV-2 virus. The application of a rapid antigen detection kit is limited by its sensitivity (Mak et al., 2021)
Rapid tests for the antigen diagnosis of SARS-CoV-2 have been developed (Carter et al., 2020; Deeks et al., 2020; Dinnes et al., 2020; La Marca et al., 2020). They are easy to use outside the laboratory and provide results in less than 30 min. Rapid tests for the detection of SARS-CoV-2 antigens are recommended for use particularly in the diagnosis of COVID-19 in symptomatic cases, contacts of confirmed cases, outbreaks, and screening of high-risk workers such as health care workers. (Loho and Widodo, 2021; Sumita et al., 2018; Thakur et al., 2021; WHO, 2021a, 2021c; Yamamoto et al., 2021). It is expected that antigen tests with good clinical performance could be an alternative in the triage of symptomatic patients in health care settings, especially when access to RT-PCR is limited (WHO, 2021a). With the decentralization of SARS-CoV-2 diagnosis and infection control at land border crossings, West African countries, including Burkina Faso, are using RT-PCR (Sagna et al., 2021; Zoure et al., 2022)and increasingly the antigen testing in health centers and the surveillance of SARS-CoV-2 among travelers at land entry points. However, the performance of these tests evaluation has not been conducted in our context. This study proposed to evaluate the performance of the PanbioTM Covid-19 Ag Rapid Test (Abbott) to contribute to the strengthening of access to biological diagnosis of COVID-19.
MATERIALS AND METHODS
Study site
The study was conducted in Ouagadougou, prior to the introduction of the vaccine against SARS-CoV-2 infection in Burkina Faso. Participants were recruited at the “Centre Médical avec Antenne Chirurgicale” (CMA) in Kossodo, Ouagadougou.
Study type, period, and population
A cross-sectional evaluation of the PanbioTM COVID-19 Ag test was conducted between January 26 and March 31, 2021.
The study population consisted of males and females of all ages who were seen at the COVID-19 screening center of the Kossodo Medical Center with Surgical Branch (CMA) with or without symptoms who consented to participate in the study. Participants were selected according to the following criteria: (i) male or female of any age, (ii) voluntary and willing to be diagnosed with COVID-19, (iii) clinically suspected (symptomatic) or not of COVID-19. A subject suspected of having COVID-19 in an epidemic setting is defined as one with an acute onset of fever, cough or an acute onset of three or more of the following signs or symptoms: fever, cough, general weakness, fatigue, headache, muscle pain, sore throat, runny nose, difficulty breathing, lack of appetite, nausea, vomiting, loss of smell, diarrhea, mental disturbance and severe acute respiratory infection (SARI): with a history of fever (T°≥ 38°C) and cough; occurring within the last 7 days requiring hospitalization.
Not included in the study were (i) patients with active nose bleeds, or with facial injuries and trauma or a condition that creates a mechanical barrier to safely obtaining samples; (ii) patients enrolled in a study to evaluate an investigational drug or vaccine; (iii) patients with nasopharyngeal specimens collected within the last 24 h of enrollment and (iv) nasopharyngeal specimens collected more than 2 hours after patient enrollment.
Sampling and sample size
The authors enumerated patients meeting the above criteria (suspected COVID-19 disease cases) during the study period until the desired numbers of positive and negative tests were reached. A total of 268 individuals (symptomatic or not) were tested by both RT-PCR and antigen RDT. Of these 268 individuals, 52 were positive and 216 were negative for COVID-19 RT-PCR.
Recruitment of participants and on-site testing procedure
Recruiting
Patients’ recruitment was carried out by the providers (an investigator, a sampling agent, and a laboratory technician) of the COVID-19 disease screening site at the Kossodo medical center. At the site, participants were examined for COVID-19 disease symptoms using the national checklist for COVID-19 disease screening. For individuals who consented to participate to the study, two nasopharyngeal swab samples were simultaneously collected, (i) for the on-site Panbio COVID-19 Antigen RDT, (ii) in Viral Transport Medium (VTM) for the SARS-COV2 RT-PCR reference testing in the laboratory.
Nasopharyngeal swabs
Two nasopharyngeal swabs were taken from each patient at inclusion. One of the swabs collection material was provided in the Panbio COVID-19 Antigen kit (for on-site antigen testing) and the other using the regular swab and viral transport medium (VTM) for routine RT-PCR reference testing in the laboratory.
Samples intended for RT-PCR were transported to the laboratory at the end of the day by the specialized service of the post office and stored at +4°C before being analyzed the same day following collection.
PanbioTM COVID-19 Ag
The PanbioTM COVID-19 Ag rapid test device is a lateral flow immunochromatographic test. It is a rapid in-vitro diagnostic test for the qualitative detection of SARS-CoV-2 antigen (Ag) in human nasopharyngeal swab specimens from individuals meeting the clinical or epidemiological criteria for COVID-19. The Panbio™ COVID-19 Ag Rapid Test Device is intended for professional use only and is intended to be used as an aid in the diagnosis of SARS-CoV-2 infection. The product may be used in any laboratory and non-laboratory environment that meets the requirements specified in the instructions for use and local regulations. The PanbioTM COVID-19 Ag Rapid Test is supplied as a cassette containing a lateral flow test strip and can be stored at 2°C to 30°C.
The PanbioTM COVID-19 Ag rapid test was used according to the manufacturer's instructions. Direct swab specimens were tested immediately at the health facility after collection. PanbioTM COVID-19 Ag external control swabs (positive and negative) were tested with a PanbioTM COVID-19 Antigen test each time a new kit was opened, for use.
RT-PCR of SARS-COV-2 in the laboratory
Nasopharyngeal samples taken in VTM tubes were used for routine RT-PCR of SARS-CoV-2, the reference method for confirmation of COVID-19 cases. RNA extraction with QIAamp Viral RNA Mini Kit (QIAGEN®) and amplification performed with kits made available to the laboratory by the Ministry of Health for the diagnosis of COVID-19 in Burkina Faso. The amplification and testing interpretation of SARS-CoV2 results were done using the STANDARD nCoV Real-Time Detection kit (SD BIOSENSOR, Inc. following the manufacturer’s instructions, blinded to the RDT results. The presence of SARS-CoV-2 RNA indicates an ongoing COVID-19 infection. The Cycle Threshold for each sample was also collected to establish the viral load.
Origin of the tests
The PanbioTM COVID-19 Ag Rapid Test was provided by Abbott Diagnostics for evaluation and the RT-PCR test was provided by the Ministry of Health of Burkina Faso as part for the routine diagnosis of COVID-19 disease in Burkina Faso.
Data processing and analysis
Data were entered into Excel and analyzed using Open-Epi software ((http://www.openepi.com ). For the PanbioTM COVID-19 Ag rapid test, the results obtained were compared with those of the RT-PCR, and its main performance characteristics were determined. For this purpose, the results of the PanbioTM COVID-19 Ag rapid test were classified into 2 categories (positive or negative results). Regarding, the known results of the RT-PCR method (a reference to the antigen RDT), the Ag-RDT results were classified into true positives (TP), false positives (FP), true negatives (TN), and false negatives (FN) on a double-entry contingency table (Table 2). Test sensitivity was calculated according to the formula (TP)/(TP+FN) and diagnostic specificity according to the formula (TN)/(TN+FP). In addition to the two main characteristics (Sensitivity and Specificity) of the diagnostic performance of the test, other test-specific parameters such as positive predictive value (PPV) and negative predictive value (NPV): PPV = TP/TP+FP and NPV=TN/TN+FN); the positive and negative likelihood ratios (LR+ and LR-); and the Kappa Coefficient of agreement between the antigen RDT and the RT-PCR tests. These characteristics were calculated with their 95% confidence intervals. The results of these calculations were expressed as a percentage. The Kappa coefficient of agreement was interpreted according to the criteria of Landis and Koch (1977) (Landis & Koch, 1977)as follows: Kappa <0, no agreement; 0 < kappa≤ 0.2 = slight agreement; 0.2< kappa < 0.4 = moderate agreement; 0.4 <kappa≤ 0.6; moderate agreement; 0.6<kappa≤0.8 = substantial agreement; 0.8<kappa≤1, near-perfect agreement.
The results of antigen RDT evaluation are presented according to several situations: (i) The first according to the use of the Panbio™ COVID-19 Ag Rapid Test at any time regardless of symptoms, (ii) the second taking into account symptoms and time of onset in the diagnosis of suspected COVID-19 disease cases, and (iii) the third in COVID-19 disease asymptomatic patients.
RESULTS
A total of 268 participants were tested with both the RT-PCR and the Panbio COVID-19 Rapid Antigen Test. The mean age was 39.7 years with ranges from 9 to 86 years. Males represented 62.3% (167/268) of the participants. Among the participants, 61.9% were asymptomatic (166/268), while 36.9% (99/268) had symptoms. The presence or absence of symptoms was not reported in 1.2% (3/268) of the participants. For 29 of the 99 participants with symptoms, the reported symptom onset periods were 1 to 5 days, for 47 participants the period was 1 to 7 days. Finally, 9 symptomatic participants had a symptom onset time greater than 7 days, while for 14 symptomatic participants, the symptom onset time was unknown (Table 1).
Performance of Panbio™ COVID-19 Ag Rapid Tests
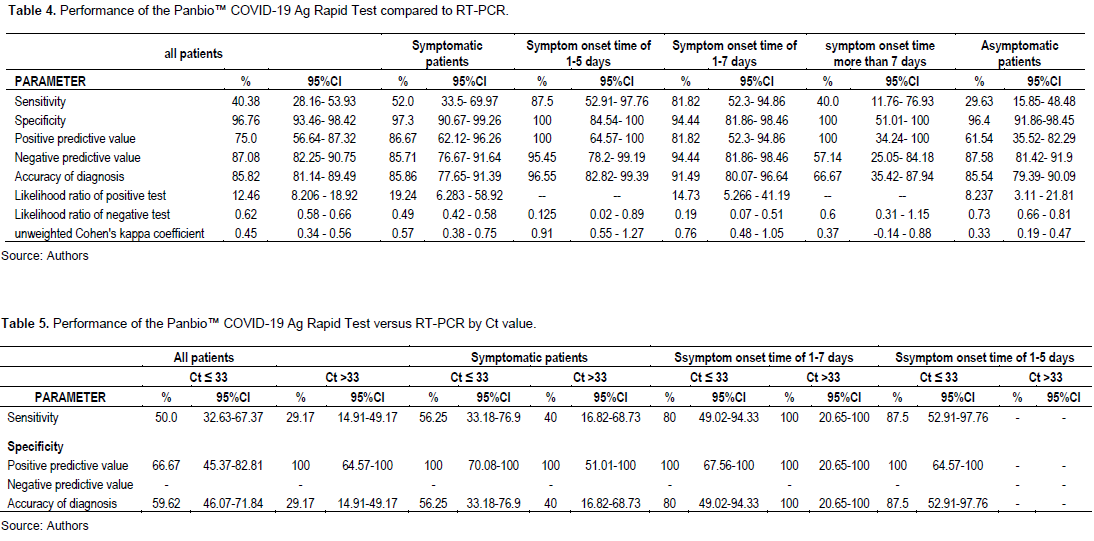
Tables 2, 3, 4, and 5 show the raw results and performance of the antigen tests in several situations compared with RT-PCR. It is shown that the sensitivity of the Panbio™ COVID-19 Ag Rapid Test ranges from 29.63% (95% CI: 13.75 to 50.18) among patients asymptomatic for COVID-19 disease, to 81.82% (95% CI: 52.3 to 94.86) in symptomatic patients with a symptom onset time of 1 to 7 days. Among patients with a symptom onset time of 1 to 5 days, and detected positive for SARS-CoV-2 by RT-PCR with a Ct value ≤ 33, the sensitivity of the Panbio™ COVID-19 Ag Rapid Test is estimated to be 87.5% (95% CI:52.91-97.76), compared to 80.0% in those with a symptom onset time of 1 to 7 days with a Ct ≤ 33. Overall, regardless of symptoms, the sensitivity is 50.0% (95% CI: 29.45 to 67.47) for Ct values ≤ 33, compared with 29.2% (95% CI: 10.69 to 44.87) when the Ct value was greater than 33 (Table 5).
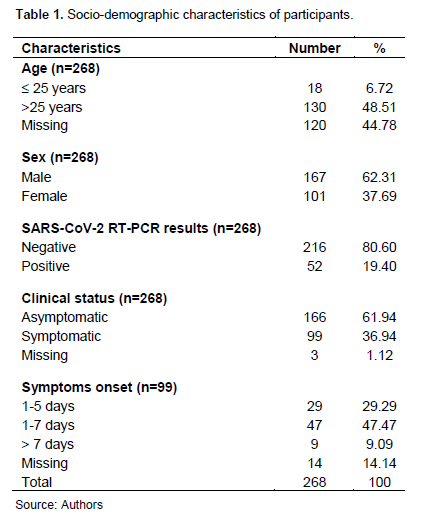
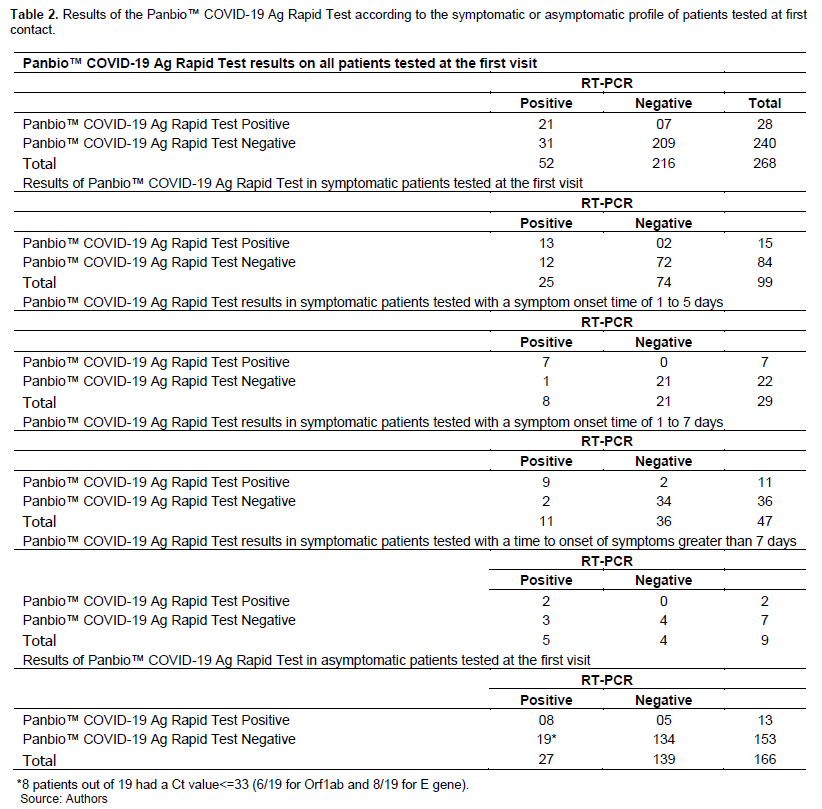
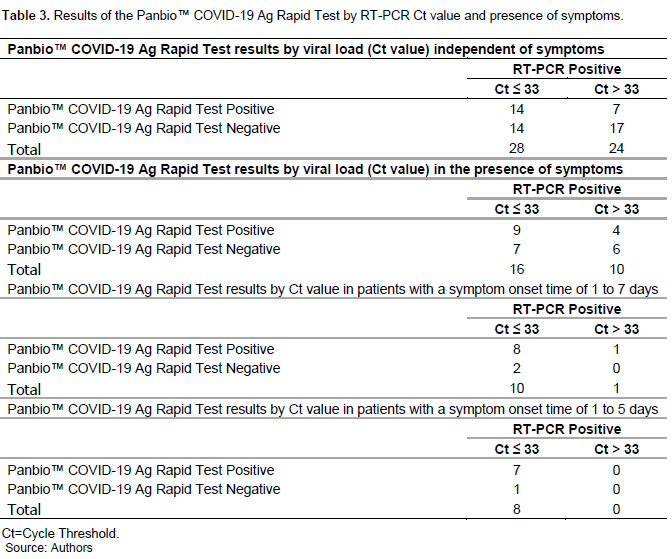
Of 27 asymptomatic patients who tested positive by RT-PCR, eight returned positive by Panbio™ COVID-19 Ag Rapid Test (29.6%). Among the 19 symptomatic patients who tested negative for Panbio™ COVID-19 Ag, 12 had a Ct value greater than 33, which is considered low contagious according to the literature (Al Bayat et al., 2021; Platten et al., 2021).
As for the specificity of the Panbio™ COVID-19 Ag Rapid Test, it was 97.3% (CI95%: 90.58 to 99.67) in symptomatic patients and 96.4% (95% CI: 91.81- 98.82) in RT-PCR negative asymptomatic patients
DISCUSSION
Several rapid antigenic diagnostic tests for COVID-19 disease have been developed since the appearance of SARS-CoV-2. Our study evaluated the performance of a Panbio™ COVID-19 test in a health care setting to guide their use in a local context. The Panbio™ COVID-19 test was commercially available in Hong Kong at the end of October 2020. In previous, study, the overall sensitivity of the Panbio kit ranged from 73.3% to 75.5% (Mak et al., 2021). This study shows that the sensitivity of the Panbio™ COVID-19 Ag Rapid Test is 82% (95% CI: 48.22 to 97.72) in symptomatic patients with a time to symptom onset ≤7 days. Among patients with a time to symptom onset ≤ 5 days, positive for SARS-CoV-2 RT-PCR, and with a Ct value ≤ 33, the sensitivity of the Panbio™ COVID-19 Ag Rapid Test is estimated to be 87.5% (95% CI:52.91 to 97.76). Some authors had previously shown that the SARS-CoV-2 virus viral load in throat or nasopharyngeal swabs peaks before the 5th day of symptom onset, and progressively decreases after this
period (Wölfel et al., 2020).
Regarding specificity, it was estimated to be 97.3% among symptomatic patients, and 96.4% among RT-PCR negative asymptomatic patients. These results agree with those provided by the manufacturer and confirm that the Panbio™ COVID-19 Ag Rapid Test can be an alternative to RT-PCR in the diagnosis of COVID-19 disease in symptomatic patients with a symptom onset time of fewer than 7 days, as suggested by the manufacturer. Indeed, according to the manufacturer, the sensitivity of the Panbio™ COVID-19 Ag Rapid Test is 93.3% among symptomatic patients with a time to symptom onset of fewer than 7 days according to the user's manual. Like the sensitivity provided by the manufacturer, which is comparable to our results (confidence interval 95% CI: 52.91-97.76), the specificity of the Panbio antigen among symptomatic patients in our study also confirms that provided by the manufacturer (99.4%).
Our results agree other evaluations done on Panbio around the world. Indeed, during the second wave in Switzerland, for the Panbio COVID- 19 test, the clinical sensitivity was 81% and clinical specificity was 99.1%. Based on their findings, the diagnostic performance ofthe Panbio™Covid-19 test meet the criteria required by the WHO for Ag-RDTs (sensitivity ≥80% and specificity ≥97%) in a high incidence setting in symptomatic individuals (Nsoga et al., 2021).
In Spain, a multicenter evaluation of the Panbio™ COVID-19 test showed an overall sensitivity and specificity for the Panbio™ COVID-19 test were 90.5% and 98.8% respectively (Merino et al., 2021). Still in Spain, overall sensitivity was 60.0 % for the Panbio COVID- 19 test (Pérez-García et al., 2021). In the Netherlands, a prospective cohort study for SARS- CoV- 2 infection in asymptomatic individuals using the Panbio COVID- 19 antigen rapid test (Abbott) compared with RT- PCR, showed the sensitivity of Panbio ranged from 80.0 to 86.67% and specificity from 99.53 to 100% (Winkel et al., 2021). Also, in asymptomatic Canadians, an evaluation of the Abbott PanbioTM COVID-19 Ag rapid antigen test showed a low sensitivity (54.5%), but it allowed for faster identification of infected individuals (Shaw et al., 2021).
In contrast, the Panbio COVID- 19 test displays low sensitivity (35 to 50%) in asymptomatic close contacts of COVID-19 patients (Torres et al., 2021). Also, clinical performance of the Panbio COVID- 19 test depends on the nature of the sample. Collection of throat (sensitivity 57.7%) and saliva (sensitivity 2.6%) was stopped early due to poorer.
Nasopharyngeal swab was the best one (sensitivity 87.7%). The Panbio COVID- 19 test is suitable for patients presenting within 7 days of symptom onset using nasopharyngeal swabs. Throat and saliva swabs are not reliable specimens for the Panbio COVID- 19 test (Stokes et al., 2021). Sensitivity for samples within the first 5 days after the onset of symptoms was 91.3 % for the Panbio COVID- 19 test (Pérez-García et al., 2021). Also, (Merino et al., 2021)found in patients with threshold cycle (CT) < 25 a sensitivity was 99.5% and in participants with symptoms onset ≤5 days, it was 91.8%. Thus, the Panbio™ COVID-19 test could be easily recommended for early symptom detection (≤5 days).
In our study, the Kappa concordance between the Panbio™ COVID-19 Ag Rapid Test and RT-PCR is highly variable depending on the type of subject tested. Indeed, the best concordances between the antigenic RDT and RT-PCR are respectively observed in patients with symptoms dating from 1 to 5 days (0.91), and 01 to 07 days (0.76). This agreement is 0.37 among patients with a delay in the onset of symptoms of more than 07 days, and 0.57 among asymptomatic patients.
These results show that the use of the Panbio™ COVID-19 Ag Rapid in asymptomatic patients or beyond the first 07 days of symptom onset significantly reduces the diagnostic sensitivity of the test. Indeed, this sensitivity is 29.63% among patients with asymptomatic COVID-19 disease and 40.0% when the time to symptom onset is beyond 07 days. This seems logical especially since the SARS-CoV-2 viral load is generally at a low level among asymptomatic patients, as well as among patients with symptoms dating back more than 7 days, taking into account the kinetics of antigens in the infected subject (Loho and Widodo, 2021; Thakur et al., 2021; Zhang and Guo, 2020). Therefore, it is not advisable to use the antigen test alone in the detection of COVID-19 disease among asymptomatic patients, especially when with a low viral load (Ct>33) or in the diagnosis of patients consulting more than 07 days after the onset of symptoms. This confirms the manufacturer's recommendation that Panbio antigen is indicated for use among symptomatic patients with less than seven days of symptom onset.
According to (Nsoga et al., 2021), a presumed cut-off for infectious virus was Ct ≤26.7 corresponding ≥ 1E6 SARS-CoV-2 genomes copies/mL. Indeed, for samples with Ct ≤ 25, sensitivity was 96.4 % for Panbio test and with Ct>25, sensitivity was 24.4 %. The Panbio COVID-19 Ag showed excellent performance and agreement results for samples with high viral loads (Ct ≤ 25) or samples taken within the first 5 days after the onset of symptoms (Pérez-García et al., 2021). Aslo, (Nordgren et al., 2021) found that The Panbio COVID-19 Ag test had high sensitivity for samples with Ct-values <25 (>88%) and no sample with a Ct-value >27 was shown to contain infectious virus with Panbio COVID-19 Ag test.in conclusion, the Panbio COVID-19 Ag test performs well clinically, with even more reliable results for patients with a shorter clinical course of the disease or a higher viral load (Merino et al., 2021).
This study is not without its limitations, among which we could mention the low number of positive cases, with the consequence of widening the confidence intervals of the different estimated parameters. Despite these difficulties and limitations, the study was able to provide useful information for assessing the performance of the test evaluated, which could guide its use in the local context.
CONCLUSION
In conclusion, the Panbio™ COVID-19 Ag Rapid Test showed good performance in detecting COVID-19 cases in patients with a symptom onset time of fewer than seven (7) days. This performance is even better when this delay is reduced to fewer than 5 days. The results show that the antigenic RDT is not suitable for the detection of COVID-19 in asymptomatic patients such as travelers, or patients with a delay of more than 7 days since the onset of suspected symptoms.
ACKNOWLEDGEMENTS
The authors would like to thank the staff of the medical centre of Kossodo, and the Laboratoire de Recherche Biomédicale (LaReBio), the Ministry of Health of Burkina Faso, and Abbott Diagnostics for providing tests and lab consumables for the evaluation.
FUNDING
This study was supported by Abbott Diagnostics and the Ministry of Health of Burkina Faso.
CONFLICT OF INTERESTS
The authors have not declared any conflict of interests.
REFERENCES
|
Al Bayat S, Mundodan J, Hasnain S, Sallam M, Khogali H, Ali D, Alateeg S, Osama M, Elberdiny A, Al-Romaihi H, Al-Thani MHJ (2021). Can the cycle threshold (Ct) value of RT-PCR test for SARS CoV2 predict infectivity among close contacts? Journal of Infection and Public Health 14(9):1201-1205. |
|
|
Carter LJ, Garner LV, Smoot JW, Li Y, Zhou Q, Saveson CJ, Sasso JM, Gregg AC, Soares DJ, Beskid TR, Jervey SR, Liu C (2020). Assay Techniques and Test Development for COVID-19 Diagnosis. ACS Central Science 6(5):591-605. |
|
|
Deeks JJ, Dinnes J, Takwoingi Y, Davenport C, Spijker R, Taylor- Phillips S, Adriano, A, Beese S, Dretzke J, Ferrante di Ruffano L, Harris IM, Price MJ, Dittrich S, Emperador D, Hooft L, Leeflang MM, Van den Bruel A, Cochrane COVID-19 Diagnostic Test Accuracy Group (2020). Antibody tests for identification of current and past infection with SARS-CoV-2. The Cochrane Database of Systematic Reviews 6, CD013652. |
|
|
Dinnes J, Deeks JJ, Adriano A, Berhane S, Davenport C, Dittrich S, Emperador D, Takwoingi Y, Cunningham J, Beese S, Dretzke J, Ferrante di Ruffano L, Harris IM, Price MJ, Taylor-Phillips S, Hooft L, Leeflang MM, Spijker R, Van den Bruel A, Cochrane COVID-19 Diagnostic Test Accuracy Group (2020). Rapid, point-of-care antigen and molecular-based tests for diagnosis of SARS-CoV-2 infection. The Cochrane Database of Systematic Reviews 8, CD013705. |
|
|
La Marca A, Capuzzo M, Paglia T, Roli L, Trenti T, Nelson SM (2020). Testing for SARS-CoV-2 (COVID-19): A systematic review and clinical guide to molecular and serological in-vitro diagnostic assays. Reproductive Biomedicine Online 41(3):483-499. |
|
|
Landis JR, Koch GG (1977). The measurement of observer agreement for categorical data. Biometrics 33(1):159-174. |
|
|
Loho T, Widodo D (2021). Rapid Antigen Detection Test for Severe Acute Respiratory Syndrome Coronavirus 2: How to Use It Properly? Acta Medica Indonesiana 53(1): 119-131. |
|
|
Mak GCK, Lau SSY, Wong KKY, Chow NLS, Lau CS, Lam ETK, Chan RCW, Tsang, DNC (2021). Evaluation of rapid antigen detection kit from the WHO Emergency Use List for detecting SARS-CoV-2. Journal of Clinical Virology 134(December 2020):104712. |
|
|
Merino P, Guinea J, Muñoz-Gallego I, González-Donapetry P, Galán JC, Antona N, Cilla G, Hernáez-Crespo S, Díaz-de Tuesta JL, Gual-de Torrella A, González-Romo F, Escribano P, Sánchez-Castellano MÁ, Sota-Busselo M, Delgado-Iribarren A, García J, Cantón R, Muñoz P, Folgueira MD, Montes M (2021). Multicenter evaluation of the PanbioTM COVID-19 rapid antigen-detection test for the diagnosis of SARS-CoV-2 infection. Clinical Microbiology and Infection 27(5):758-761. |
|
|
Nordgren J, Sharma S, Olsson H, Jämtberg M, Falkeborn T, Svensson L, Hagbom M. (2021). SARS-CoV-2 rapid antigen test: High sensitivity to detect infectious virus. Journal of Clinical Virology 140:2-5. |
|
|
Nsoga MTN, Kronig I, Rodriguez FJP, Sattonnet-Roche P, Silva DD, Helbling, J, Sacks JA, de Vos M, Boehm E, Gayet- Ageron A, Berger A, Jacquerioz-Bausch F, Chappuis F, Kaiser L, Schibler M, Renzoni A, Eckerle I (2021). Diagnostic accuracy of Panbio rapid antigen tests on oropharyngeal swabs for detection of SARS-CoV-2 PLoS ONE 16:1-7. |
|
|
Pérez-García F, Romanyk J, Gómez-Herruz P, Arroyo T, Pérez-Tanoira R, Linares M, Pérez Ranz I, Labrador BA, Moya GH, Ruiz-Álvarez MJ, Cuadros-González J (2021). Diagnostic performance of CerTest and Panbio antigen rapid diagnostic tests to diagnose SARS-CoV-2 infection. Journal of Clinical Virology 137(February). |
|
|
Platten M, Hoffmann D, Grosser R, Wisplinghoff F, Wisplinghoff H, Wiesmüller G, Schildgen O, Schildgen V (2021). SARS-CoV-2, CT-Values, and Infectivity-Conclusions to Be Drawn from Side Observations. Viruses 13(8):1459. |
|
|
Sagna T, Ouedraogo HG, Zouré AA, Zida S, Compaore RT, Kambire D, Joseph KZ (2021). Le Laboratoire à l'épreuve de la pandémie de la COVID-19 au Burkina Faso: Quels défis pour la régularité de l'offre de diagnostic Revue Malienne d'Infectiologie et de Microbiologie 16(1):32-37. |
|
|
Shaw JLV, Deslandes V, Smith J, Desjardins M (2021). Evaluation of the Abbott PanbioTM COVID-19 Ag rapid antigen test for the detection of SARS-CoV-2 in asymptomatic Canadians. Diagnostic Microbiology and Infectious Disease 101(4):115514. |
|
|
Stokes W, Berenger BM, Portnoy D, Scott B, Szelewicki J, Singh T, Venner AA, Turnbull LA, Pabbaraju K, Shokoples S, Wong AA, Gill K, Guttridge T, Proctor D, Hu J, Tipples G (2021). Clinical performance of the Abbott Panbio with nasopharyngeal, throat, and saliva swabs among symptomatic individuals with COVID-19. European Journal of Clinical Microbiology and Infectious Diseases 40(8):1721-1726. |
|
|
Sumita NM, Ferreira CES, Martino, MDV, Franca CN, Faulhaber ACL, Scartezini M, Pinho JRR, Dias CM, Cesar KR, Pariz VM, Guerra JCC, Barbosa IV, Faulhaber MH W, Batista MC, Andriolo A, Mendes ME, Machado AMO, Colombini MP, Slhessarenko N, Galoro CA (2018). Clinical Applications of Point-of-Care Testing in Different Conditions Clinical Laboratory 64(7):1105-1112. |
|
|
Thakur P, Saxena S, Manchanda V, Rana N, Goel R, Arora R (2021). Utility of Antigen-Based Rapid Diagnostic Test for Detection of SARS-CoV-2 Virus in Routine Hospital Settings. Laboratory Medicine 52(6):e154-e158. |
|
|
Torres I, Poujois S, Albert E, Colomina J, Navarro D (2021). Evaluation of a rapid antigen test (PanbioTM COVID-19 Ag rapid test device) for SARS-CoV-2 detection in asymptomatic close contacts of COVID-19 patients. Clinical Microbiology and Infection 27(4):636e1-636e4. |
|
|
Wamai RG, Hirsch JL, Van Damme W, Alnwick D, Bailey RC, Hodgins S, Alam U, Anyona M (2021). What Could Explain the Lower COVID-19 Burden in Africa despite Considerable Circulation of the SARS-CoV-2 Virus? International Journal of Environmental Research and Public Health 18(16):8638. |
|
|
World Health Organization (WHO) (2021a). Antigen-detection in the diagnosis of SARS-CoV-2 infection. |
|
|
World Health Organization (WHO) (2021b). WHO Coronavirus (COVID-19) Dashboard. |
|
|
World Health Organization (WHO) (2021c). Use of antigen detection rapid diagnostic testing: |
|
|
Winkel B, Schram E, Gremmels H, Debast S, Schuurman R, Wensing A, Bonten M, Goedhart E, Hofstra M (2021). Screening for SARS-CoV-2 infection in asymptomatic individuals using the Panbio COVID-19 antigen rapid test (Abbott) compared with RT-PCR: A prospective cohort study. BMJ Open 11(10):1-6. |
|
|
Wölfel R, Corman VM, Guggemos W, Seilmaier M, Zange S, Müller MA, Niemeyer D, Jones TC, Vollmar P, Rothe C, Hoelscher M, Bleicker T, Brünink S, Schneider J, Ehmann R, Zwirglmaier K, Drosten C, Wendtner C (2020). Virological assessment of hospitalized patients with COVID-2019 Nature 581(7809):465-469. |
|
|
Wu F, Zhao S, Yu B, Chen YM, Wang W, Song ZG, Hu Y, Tao ZW, Tian JH, Pei YY, Yuan ML, Zhang YL, Dai FH, Liu Y, Wang QM, Zheng JJ, Xu L, Holmes EC, Zhang Y-Z (2020). A new coronavirus associated with human respiratory disease in China Nature 579(7798):265-269. |
|
|
Yamamoto K, Suzuki M, Yamada G, Sudo T, Nomoto H, Kinoshita N, Nakamura K, Tsujimoto Y, Kusaba Y, Morita C, Moriya A, Maeda K, Yagi S, Kimura M, Ohmagari, N (2021). Utility of the antigen test for coronavirus disease 2019: Factors influencing the prediction of the possibility of disease transmission International Journal of Infectious Diseases: IJID: Official Publication of the International Society for Infectious Diseases 104: 65-72. |
|
|
Zhai P, Ding Y, Wu X, Long J, Zhong Y, Li Y (2020). The epidemiology, diagnosis and treatment of COVID-19. International Journal of Antimicrobial Agents 55(5):105955. |
|
|
Zhang L, Guo H (2020). Biomarkers of COVID-19 and technologies to combat SARS-CoV-2. Advances in Biomarker Sciences and Technology 2:1-23. |
|
|
Zoure AA, Ouedraogo HG, Sagna T, Compaore TR, Soubeiga ST, Cisse K, Kambire D, Ouedraogo O, Zida S, Dabire C, Zongo D, Savadogo B, Yonli AT, Kouanda S, Simpore J (2022). Molecular diagnosis of COVID-19 in Burkina Faso: Successful challenge. International Journal of Biological and Chemical Sciences 16(1):440-463. |
|
Copyright © 2024 Author(s) retain the copyright of this article.
This article is published under the terms of the Creative Commons Attribution License 4.0